Very few studies have focused on the involvement of protein kinase C (PKC) in mediating β-cell dysfunction, which is surprising because there are several hundred articles addressing a corresponding role for PKC during insulin resistance. The conventional and novel subgroups of this family of serine-threonine, protein kinases are usually activated in receptor signaling cascades by lipid second messengers. Under conditions of lipid oversupply, however, it was envisioned that nutrient fatty acids or their derivatives might also activate PKC and thereby disrupt both insulin signaling and, more speculatively, β-cell function (1). Important support for this concept in β-cells is now provided in this issue of Diabetes (2).
Hennige et al. (2) generated transgenic mice that overexpress a catalytically inactive form of PKCδ specifically in β-cells. This competes functionally with endogenous PKCδ and inhibits its activity in a relatively specific manner. This strategy improved the glucose intolerance caused by feeding mice a high-fat diet; minimal effects were seen in normal chow–fed mice. The improvement was accompanied in vivo by an enhancement of glucose-stimulated insulin secretion, an increase in mean islet size, and a reduction in markers of apoptosis. This is consistent with a generally proapoptotic role of PKCδ reported in a few prior investigations of β-cells (3–5 ) and studied more extensively in several other cell types (6). The current study also demonstrates that once the alteration in β-cell mass is taken into account, PKCδ does not appear to regulate insulin secretion directly.
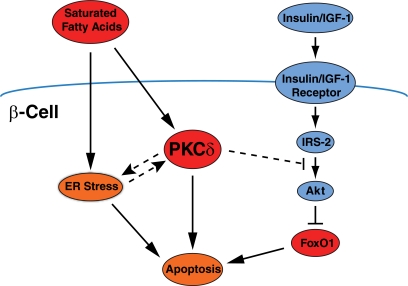
Translocation of PKCδ from the cytosol to the nucleus often accompanies apoptosis (6) and, indeed, correlates with the capacity of different fatty acids to promote β-cell death (5). The study by Hennige et al. now shows that nuclear localization of the transcription factor FOXO1 is stimulated in vitro by a saturated proapoptotic fatty acid (palmitate) and that this depends on PKCδ activation. Moreover, phosphorylation of FOXO1 on Ser256, a key event for nuclear exclusion, is inhibited by overexpression of wild-type PKCδ and augmented by the catalytically inactive mutant. Although it remains to be confirmed whether the observed regulation of apoptosis by PKCδ is causally mediated by changes in FOXO1 localization, this observation is consistent with some prior work. FOXO1 phosphorylation and apoptosis are inversely regulated in β-cells chronically exposed to fatty acids, and both features can be counteracted by the growth factor IGF in a manner dependent on the antiapoptotic protein kinase akt (7). In a further twist, fatty acids can inhibit akt, and this contributes permissively to their apoptotic potential (8). Activation of PKC has been previously implicated in this inhibition of akt (9), so it will now be important to determine whether this involves PKCδ specifically (Fig. 1).
FIG. 1.
Potential sites of action of PKCδ in regulating β-cell apoptosis due to chronic exposure to saturated fatty acids. Pathways that are well established, including those now arising from the study by Hennige et al., are shown with solid lines. More speculative links concerning how PKCδ might impact other pathways that regulate β-cell mass, including ER stress and akt signaling, are shown as dashed lines. As outlined in this article, it is possible some of these individual pathways might play greater or lesser roles depending on the duration and extent of fatty acid exposure.
Other questions remain to be resolved. In particular there is extensive evidence implicating endoplasmic reticulum (ER) stress in β-cell lipoapoptosis (10,11). Although not addressed directly, Hennige et al. suggest that PKCδ might link ER stress with mitochondrial apoptosis as hypothesized for other cell types (12). Alternatively, PKCδ could act upstream of ER stress, perhaps via inhibition of autophagy (13). Another possibility is that ER stress and PKCδ represent separate pathways, used differentially depending on the duration and especially extent of fatty acid exposure (Fig. 1). Although not always appreciated, the free fatty acid concentration is determined by the ratio of fatty acid to albumin, and this varies widely among various studies. Under moderate exposure (fatty acid–to–albumin ratio <4) ER stress was shown to be responsible for the majority of β-cell apoptosis (11). At the higher ratio used by Hennige et al. (∼10) it is possible that other mechanisms, perhaps including PKCδ and/or FOXO1, come into play. Interestingly, one of the genes regulated by FOXO1 is the transcription factor C/EBP homologous protein (CHOP) (14). This is a major mediator of β-cell death due to ER stress during moderate lipid exposure (15,16), but CHOP is also induced independently of ER stress. Thus, PKCδ and FOXO1 could contribute to CHOP-mediated apoptosis in a manner that becomes quantitatively more important at fatty acid concentrations higher than those required for ER stress. Conversely, use of lower effective concentrations of fatty acids might explain a previous report that PKCδ was not involved in β-cell apoptosis (17).
Whatever the mechanisms, it is now clear that inhibiting PKCδ specifically in β-cells does improve both β-cell function and glucose tolerance in mice maintained on a high-fat diet (2). This raises the potential of inhibiting of PKCδ to treat type 2 diabetes. This possibility is underscored by a recent study using whole-body deletion of PKCδ in which glucose intolerance due to high-fat feeding was also reduced (18). In this instance the major mechanism appeared to be an improvement in hepatic lipid metabolism and insulin sensitivity. Perhaps a β-cell phenotype would have been more apparent if, as with Hennige et al., a diet enriched in saturated (as opposed to polyunsaturated) fatty acids had been used (18). In any event, these two studies now suggest that inhibition of PKCδ might exert benefits at multiple sites. This would be analogous to a proposed rationale for inhibiting PKCε, which has been broadly implicated as negative regulator of insulin sensitivity (1). However, inhibition of PKCε also results in a pronounced enhancement of glucose-stimulated insulin secretion, specifically from defective or lipid-pretreated β-cells (19). This, together with an independent phenotype involving diminished hepatic clearance of insulin, greatly enhances insulin availability and accounts for most of the observed improvement of glucose tolerance, at least in long-term models of high-fat feeding. Unlike the situation with PKCδ, inhibition of PKCε did not augment β-cell mass (19) but specifically upregulated the amplification pathway for glucose-stimulated insulin secretion (20). The complementarity in function of these two PKC isoforms, and the difficulties associated with developing truly selective inhibitors, suggests that a single molecule antagonising both PKCδ and PKCε might constitute a suitable therapy for type 2 diabetes. At the very least, a better understanding of the roles of these key PKC isoforms, their substrates, and manners of activation should shed much-needed light on the molecular mechanisms underpinning type 2 diabetes.
Acknowledgments
Work discussed from the labs of C.S.-P. and T.J.B. was funded by project grants from the National Health and Medical Research Council of Australia.
Both labs have also received support from Kai Pharmaceuticals (San Francisco, CA) for evaluation of inhibitors of PKCε in the context of type 2 diabetes. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 119.
REFERENCES
- 1.Schmitz-Peiffer C, Biden TJ: Protein kinase C function in muscle, liver, and β-cells and its therapeutic implications for type 2 diabetes. Diabetes 2008;57:1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennige AM, Ranta F, Heinzelmann I, Düfer M, Michael D, Braumüller H, Lutz SZ, Lammers R, Drews G, Bosch F, Häring H-U, Ullrich S: Overexpression of kinase-negative protein kinase Cδ in pancreatic β-cells protects mice from diet-induced glucose intolerance and β-cell dysfunction. Diabetes 2010;59:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter L, Cordery D, Biden TJ: Protein kinase Cδ activation by interleukin-1β stabilizes inducible nitric-oxide synthase mRNA in pancreatic β-cells. J Biol. Chem 2001;276:5368–5374 [DOI] [PubMed] [Google Scholar]
- 4.Carpenter L, Cordery D, Biden TJ: Inhibition of protein kinase C δ protects rat INS-1 cells against interleukin-1β and streptozotocin-induced apoptosis. Diabetes 2002;51:317–324 [DOI] [PubMed] [Google Scholar]
- 5.Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, Brendel MD, Bretzel RG, Häring HU, Kellerer M: Protein kinase C δ activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes 2003;52:991–997 [DOI] [PubMed] [Google Scholar]
- 6.Reyland ME: Protein kinase Cδ and apoptosis. Biochem Soc Trans 2007;35:1001–1004 [DOI] [PubMed] [Google Scholar]
- 7.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ: Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic β-cells (INS-1). J Biol Chem 2002;277:49676–49684 [DOI] [PubMed] [Google Scholar]
- 8.Dickson LM, Rhodes CJ: Pancreatic beta-cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am J Physiol Endocrinol Metab 2004;287:E192–E198 [DOI] [PubMed] [Google Scholar]
- 9.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ: Fatty acid and phorbol ester-mediated interference of mitogenic signaling via novel protein kinase C isoforms in pancreatic beta-cells (INS-1). J Mol Endocrinol 2003;30:271–286 [DOI] [PubMed] [Google Scholar]
- 10.Eizirik DL, Cardozo AK, Cnop M: The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008;29:42–61 [DOI] [PubMed] [Google Scholar]
- 11.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ: Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 2007;50:752–763 [DOI] [PubMed] [Google Scholar]
- 12.Qi X, Mochly-Rosen D: The PKCδ-Abl complex communicates ER stress to the mitochondria: an essential step in subsequent apoptosis. J Cell Sci 2008;121:804–813 [DOI] [PubMed] [Google Scholar]
- 13.Akar U, Ozpolat B, Mehta K, Fok J, Kondo Y, Lopez-Berestein G: Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol Cancer Res 2007;5:241–249 [DOI] [PubMed] [Google Scholar]
- 14.Martinez SC, Tanabe K, Cras-Méneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA: Inhibition of Foxo1 protects pancreatic islet β-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes 2008;57:846–859 [DOI] [PubMed] [Google Scholar]
- 15.Akerfeldt MC, Howes J, Chan JY, Stevens VA, Boubenna N, McGuire HM, King C, Biden TJ, Laybutt DR: Cytokine-induced β-cell death is independent of endoplasmic reticulum stress signaling. Diabetes 2008;57:3034–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunha DA, Hekerman P, Ladrière L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, Overbergh L, Mathieu C, Lupi R, Hai T, Herchuelz A, Marchetti P, Rutter GA, Eizirik DL, Cnop M: Initiation and execution of lipotoxic ER stress in pancreatic β-cells. J Cell Sci 2008;121:2308–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welters HJ, Smith SA, Tadayyon M, Scarpello JH, Morgan NG: Evidence that protein kinase Cδ is not required for palmitate-induced cytotoxicity in BRIN-BD11 β-cells. J Mol Endocrinol 2004;32:227–235 [DOI] [PubMed] [Google Scholar]
- 18.Frangioudakis G, Burchfield JG, Narasimhan S, Cooney GJ, Leitges M, Biden TJ, Schmitz-Peiffer C: Diverse roles for protein kinase C δ and protein kinase C ε in the generation of high-fat-diet–induced glucose intolerance in mice: regulation of lipogenesis by protein kinase C δ. Diabetologia 2009;52:2616–2620 [DOI] [PubMed] [Google Scholar]
- 19.Schmitz-Peiffer C, Laybutt DR, Burchfield JG, Gurisik E, Narasimhan S, Mitchell CJ, Pedersen DJ, Braun U, Cooney GJ, Leitges M, Biden TJ: Inhibition of PKCε improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell Metab 2007;6:320–328 [DOI] [PubMed] [Google Scholar]
- 20.Cantley J, Burchfield JG, Pearson GL, Schmitz-Peiffer C, Leitges M, Biden TJ: Deletion of PKCε selectively enhances the amplifying pathways of glucose-stimulated insulin secretion via increased lipolysis in mouse β-cells. Diabetes 2009;58:1826–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]