Abstract
OBJECTIVE
Adipose tissue may contain few large adipocytes (hypertrophy) or many small adipocytes (hyperplasia). We investigated factors of putative importance for adipose tissue morphology.
RESEARCH DESIGN AND METHODS
Subcutaneous adipocyte size and total fat mass were compared in 764 subjects with BMI 18–60 kg/m2. A morphology value was defined as the difference between the measured adipocyte volume and the expected volume given by a curved-line fit for a given body fat mass and was related to insulin values. In 35 subjects, in vivo adipocyte turnover was measured by exploiting incorporation of atmospheric 14C into DNA.
RESULTS
Occurrence of hyperplasia (negative morphology value) or hypertrophy (positive morphology value) was independent of sex and body weight but correlated with fasting plasma insulin levels and insulin sensitivity, independent of adipocyte volume (β-coefficient = 0.3, P < 0.0001). Total adipocyte number and morphology were negatively related (r = −0.66); i.e., the total adipocyte number was greatest in pronounced hyperplasia and smallest in pronounced hypertrophy. The absolute number of new adipocytes generated each year was 70% lower (P < 0.001) in hypertrophy than in hyperplasia, and individual values for adipocyte generation and morphology were strongly related (r = 0.7, P < 0.001). The relative death rate (∼10% per year) or mean age of adipocytes (∼10 years) was not correlated with morphology.
CONCLUSIONS
Adipose tissue morphology correlates with insulin measures and is linked to the total adipocyte number independently of sex and body fat level. Low generation rates of adipocytes associate with adipose tissue hypertrophy, whereas high generation rates associate with adipose hyperplasia.
Adipose tissue expands by increasing the volume of preexisting adipocytes (adipose hypertrophy), by generating new small adipocytes (hyperplasia), or by both. Although the amount and distribution of adipose tissue associate independently with insulin resistance, type 2 diabetes, and other metabolic disorders (1), the size of adipocytes within the adipose tissue is also important (2). Increased adipocyte size correlates with serum insulin concentrations, insulin resistance, and increased risk of developing type 2 diabetes (3–10). Obese subjects with few large adipocytes are more glucose intolerant and hyperinsulinemic than those having the same degree of obesity and many small fat cells (5,7,9–14). Furthermore, adipocyte hypertrophy may impair adipose tissue function by inducing local inflammation, mechanical stress, and altered metabolism (15–17). There is, however, a large interindividual variation in adipocyte size among lean and obese individuals (10,18,19). Lean individuals can have larger adipocytes than obese individuals and the other way around. Hitherto there is no straightforward method to assess adipose morphology. It is not valid to merely adjust fat cell size for BMI by linear regression because the relationship between BMI or fat mass and adipocyte size is curve-linear (10,18,19).
The mechanisms responsible for the development of different forms of adipose morphology are unknown; however, adipocyte turnover may be involved. The turnover rate of adipocytes is high at all adult ages and body fat levels (18). Approximately one-tenth of the total fat cell pool is renewed every year by ongoing adipogenesis and adipocyte death.
We presently investigated whether adipocyte turnover was involved in the different morphologies of subcutaneous adipose tissue (the body's dominant fat depot). A method to quantitatively assess adipose morphology was developed. Based on the relationship between adipocyte size and total body fat, the subjects were categorized as having different degrees of either adipose hypertrophy or hyperplasia. Thereafter, we set the different forms of adipose morphology in relation to adipocyte turnover in vivo using previously generated data on the incorporation of atmospheric 14C into adipocyte DNA (18). Finally, we correlated adipose morphology with fasting plasma insulin and insulin sensitivity in vivo.
RESEARCH DESIGN AND METHODS
In a methodological study, 207 men and 557 women (aged 18–77 years) were recruited. Seventy-four men and 172 women were lean (BMI <25 kg/m2), and 86 men and 318 women were obese (BMI ≥30 kg/m2). Total body fat was determined by a formula based on age, sex, and BMI (20). In 555 of the subjects, body fat was also determined by directly using bioimpedance as previously described (18). Fasting plasma levels of glucose and insulin were determined in 716 of the subjects to assess in vivo sensitivity by the homeostasis model assessment (HOMA) index (21). The relation between adipose tissue morphology and adipocyte turnover was determined in 35 subjects who were previously investigated (18) and who were not part of the methodological study. The studies were approved by the regional ethics committee and explained in detail to each subject. Written informed consent was obtained.
Adipose tissue studies.
In the methodological study, an abdominal subcutaneous fat specimen was obtained by needle biopsy as previously described (22). Adipocytes were collagenase isolated, and mean volume and total number of adipocytes in the body were determined as previously described (18). The total adipocyte number in the body was obtained by dividing total weight of body fat by mean adipocyte weight (23). A curve fit of the relationship between adipocyte volume and body fat mass was performed as previously described (18). The difference between observed and expected adipocyte volume (as obtained from the fitted curve) at the corresponding level of total body fat mass was calculated for each subject. The subjects then were classified as having either hyperplasia (negative deviation) or hypertrophy (positive deviation) relative to the estimated average for their value of body fat. The renewal of adipocytes in vivo was estimated using our recently developed method to measure 14C in DNA (18,24) and set it in relation to the atmospheric radioactivity. Relative adipocyte turnover rate and adipocyte age were estimated using a mathematical model for cell birth and death (18). The total number of adipocytes replaced each year was calculated based on the relative turnover, total cell number, and age of each subject (18).
Statistical methods.
Values are means ± SD or box plots. They were compared by ANCOVA, unpaired t test, single or multiple regression, and χ2. Values for adipocyte generation rate (in 1010 cellsper year) and HOMA index were log transformed to obtain a normal distribution. A likelihood ratio test and Akaike information test were used as previously described (18). Power calculations on means and SD for the entire group of subjects with 14C data showed that for age, relative death rate, and generation rate of adipocytes, we could detect between-group difference of 20% with 80% power at P <0.05.
Additional information.
Further details about the research design and methods can be found in the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0942/DC1.
RESULTS
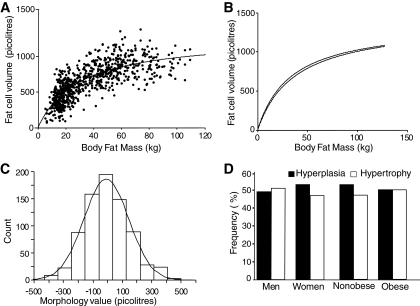
The relationship between adipocyte volume and fat mass is shown in Fig. 1A. All subjects with a total body fat mass <13 kg were lean, and those with a fat mass >36 kg were obese. The relationship between fat mass and adipocyte volume fits a curve-linear model, which postulates that adipose tissue development is due to a combination of an increase in volume of preexisting adipocytes and the generation of new adipocytes (P < 0.001 by likelihood ratio test and Akaike information criterion as compared with a linear model). The positions of the curved-lines for women and men were slightly different (P < 0.05 by unpaired t test). Therefore, sex-specific relationships were used to classify subjects according to morphology. When bioimpedance was used on a subgroup, the relationship between body fat and adipocytes size did not differ significantly from the formula-based calculation of fat mass (Fig. 1B), indicating that the formula-derived fat mass is valid for determining adipose morphology. A morphology value was defined as the difference between measured and expected adipocyte volume given by the curved-line fit for a given body fat mass. A positive morphology value indicates an adipocyte volume larger than expected, while a negative morphology value indicates an adipocyte volume smaller than expected. In the remaining analyses, subjects with positive morphology values were classified as hypertrophic and those with negative morphology values were classified as hyperplastic. Large absolute morphology values indicate pronounced hypertrophy or hyperplasia. The morphology values were normally distributed around zero (Fig. 1C). The curve fit does not deviate from the mean (P = 0.82). This was also true when data were subdivided according to sex or obesity status. Each form of morphology was almost equally common (394 negative and 370 positive values). Sex and obesity had no influence on the frequency of hypertrophy or hyperplasia (Fig. 1D).
FIG. 1.
Adipose morphology. A: Curve-linear relationship between fat cell volume and fat mass in all 764 subjects. B: Comparison of curves obtained from fat mass measured with a formula and by bioimpedance in a subset of 555 subjects. C: Distribution of adipose tissue morphology values. D: Frequency of different forms of morphology in male (n = 207), female (n = 557), nonobese (n = 300), and obese (n = 404) subjects. The morphology value is defined in the results section.
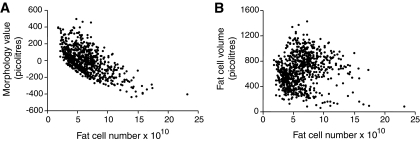
The morphology value (r = 0.61) (Fig. 2A) but not the unadjusted measured adipocyte volume (r = 0.11) (Fig. 2B) correlated (P < 0.0001) with total adipocyte number in a quantitative fashion, i.e., the higher the degree of hyperplasia, the greater the number of adipocytes and the other way around for hyperthrophy.
FIG. 2.
Relationship between total fat cell number and adipose morphology values (A) or fat cell size (B) in all 764 subjects. The morphology value is defined in the results section.
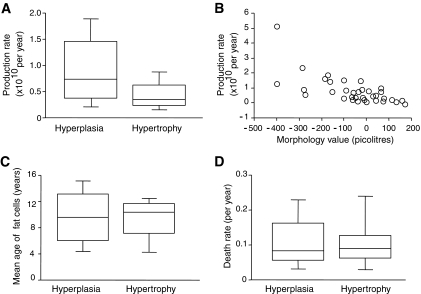
Adipocyte turnover in vivo is shown in Fig. 3. The number of adipocytes added per year was ∼70% reduced in the 12 hypertrophy subjects compared with the 23 hyperplasia subjects (Fig. 3A) (P = 0.027 by unpaired t test and P = 0.02 by ANCOVA with BMI as co-variate using 10 log-transformed data). The average number of adipocytes generated per year (1010 cells per year) was 1.2 in hyperplasia and 0.4 in hyperthrophy. The individual morphology values were inversely related to the number of adipocytes generated per year (r = −0.70, P < 0.001 by linear regression of 10 log-transformed adipocyte production data in Fig. 3B and β-coefficient = −0.65 and P < 0.001 after correction for influence of BMI by multiple regression). Age (∼10 years) and relative death rate (∼10% per year) of adipocytes were not different between hyperplasia and hypertrophy (Fig. 3C and D).
FIG. 3.
Role of adipocyte turnover for adipose tissue morphology in hypertrophy (n = 12) or hyperplasia (n = 23) subjects. Production rate of new fat cells is shown by groups (A) and in individual subjects (B). The statistical calculation used log-transformed values. Age (C) and death rates (D) of fat cells. The morphology value is defined in the results section.
Finally, we investigated the clinical impact of adipose morphology (Table 1). In all subjects, in men and women separately, or in those who were healthy and free of medicine, fasting plasma insulin and insulin sensitivity (HOMA index) correlated (P <0.001) with the morphology value independently of unadjusted adipocyte volume. Women with hypertrophy had a more unfavorable metabolic profile and body shape than women with hyperplasia (supplementary Table 1).
TABLE 1.
Relationships between insulin sensitivity (HOMA index) or fasting plasma insulin level and adipose tissue morphology (partial [i.e., β] coefficients are reported)
| Measure | All subjects | Women | Men | Subjects who were healthy and free of medicine |
|---|---|---|---|---|
| n | 716 | 526 | 190 | 629 |
| HOMA index | 0.26 | 0.26 | 0.36 | 0.27 |
| Insulin level | 0.29 | 0.30 | 0.37 | 0.29 |
Insulin values and 10 log-transformed HOMA index values were compared with adipose morphology values in multiple regression where the unadjusted fat cell volume was used as additional independent regressor. β-Coefficient values for the latter regression were 0.73–0.86 (P < 0.0001). All β-coefficient values for HOMA index and insulin gave P < 0.0001.
DISCUSSION
Using a biologically relevant relationship between the fat mass and adipocyte volume, we developed a novel and apparently valid method to assess quantitatively subcutaneous abdominal adipose tissue morphology. It would also be interesting to study visceral adipose tissue, which is more strongly related to type 2 diabetes and metabolic abnormalities; however, this cannot be investigated in a clinical setting. Whether the inclusion of several rather than only one subcutaneous site would result in an improvement in the accuracy to determine the morphology is not known at present. However, adipocyte sizes in other subcutaneous depots are the strongest determinants of adipocyte size in any given subcutaneous depot (19). Furthermore, it is not possible for ethical reasons to perform multiple biopsies (abdomen, buttocks, legs, arms, and neck) from lean subjects who were included in this study. There is a large variation in adipocyte size within the same subject and fat depot (25). Detailed classification requires the separation of isolated adipocytes according to size (15,17), which is not feasible using small amounts of adipose tissue.
The occurrence of adipose hypertrophy or hyperplasia was not influenced by sex or body weight (Fig. 1C). This suggests that hyperplasia and hypertrophy are equally common among men and women and evenly distributed among lean, overweight, or obese subjects in a general population. Furthermore, subcutaneous adipose morphology is tightly correlated with the total adipocyte number in the body over a large BMI range (18–60 kg/m2). This may imply that common factors regulate adipose morphology and adipocyte number.
Adipose morphology seems to be related to the generation of new adipocytes. Subjects with hypertrophy generated 70% less adipocytes per year than those with hyperplasia, and there was an inverse quantitative relationship between the residual value for adipocyte volume and adipocyte production rate (i.e., the higher the degree of hypertrophy, the lower the rate of adipocyte formation) (Fig. 3A and B). These findings suggest that in hypertrophy, the body produces few adipocytes over time, requiring existing adipocytes to accumulate more lipids in comparison with the hyperplastic state. Age and relative death rate of adipocytes were not influenced by morphology (Fig. 3C and D), indicating that the overall percentage of adipocytes replaced each year is similar in hypertrophic and hyperplastic states. Obese individuals have more adipocytes added per year than lean individuals (18), but the relationship between morphology and adipocyte production is independent of BMI. Thus, de novo adipogenesis is important for both obesity and morphology of human adipose tissue.
Obviously, the volume of adipocytes in adipose tissue is not the same as adipose tissue morphology. Subjects with hypertrophy or hyperplasia can have either small or large adipocytes dependent on their body fat content (Fig. 1A). Thus, absolute adipocyte size is strongly dependent on the degree of overweight/obesity, whereas the difference between observed and expected adipocyte volume, measuring morphology, is adjusted for the fat mass. Furthermore, the nonadjusted adipocyte volume, unlike the morphology value, was not related to adipocyte number (Fig. 2B).
We also found that adipose morphology was an independent regressor for circulating insulin and insulin sensitivity (Table 1) and that women with hypertrophy had a more adverse metabolic profile and body shape than women with hyperplasia, although BMI was similar in both groups (supplementary Table 1). The true pathophysiological role of adipose morphology must, though, be established by studies of populations selected for risk factor investigation and investigations of visceral adipose tissue.
We investigated a Swedish population. It is not known how the results model other populations. In summary, subcutaneous adipose hypertrophy and hyperplasia occur independently of sex and body fat content and are strongly related to the total adipocyte number in adults. A low generation rate of new adipocytes associates with adipose hypertrophy, which is linked to low insulin sensitivity and high circulating insulin levels. A high rate associates with the more benign adipose hyperplasia.
Supplementary Material
Acknowledgments
This study was supported by grants from the Swedish Research Council, AFA, the foundations of Swedish Heart and Lung, Swedish Diabetes, Torsten and Ragnar Söderberg, and King Gustaf V and Queen Victoria, the European Union's Seventh Framework Programme (COST action BM0602), and the Adipokines as Drug Targets to Combat Adverse Effects of Excess Adipose Tissue (ADAPT) project.
A grant from Novo Nordisk also supported this study. No other potential conflicts of interest relevant to this article were reported.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Kerstin Wåhlén, Britt-Marie Leijonhufvud, Eva Sjölin, and Katarina Hertel are acknowledged for their excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Wajchenberg BL: Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697–738 [DOI] [PubMed] [Google Scholar]
- 2.Bays HE, González-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR: Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 2008;6:343–368 [DOI] [PubMed] [Google Scholar]
- 3.Bjorntorp P, Sjostrom L: Number and size of adipose tissue fat cells in relation to metabolism in human obesity. Metabolism 1971;20:703–713 [DOI] [PubMed] [Google Scholar]
- 4.Björntorp P, Bengtsson C, Blohmé G, Jonsson A, Sjöström L, Tibblin E, Tibblin G, Wilhelmsen L: Adipose tissue fat cell size and number in relation to metabolism in randomly selected middle-aged men and women. Metabolism 1971;20:927–935 [DOI] [PubMed] [Google Scholar]
- 5.Krotkiewski M, Björntorp P, Sjöström L, Smith U: Impact of obesity on metabolism in men and women: importance of regional adipose tissue distribution. J Clin Invest 1983;72:1150–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ktotkiewski M, Sjöström L, Björntorp P, Smith U: Regional adipose tissue cellularity in relation to metabolism in young and middle-aged women. Metabolism 1975;24:703–710 [DOI] [PubMed] [Google Scholar]
- 7.Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW: Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia.’ Diabetologia 2007;50:625–633 [DOI] [PubMed] [Google Scholar]
- 8.Salans LB, Cushman SW, Weismann RE: Studies of human adipose tissue: adipose cell size and number in nonobese and obese patients. J Clin Invest 1973;52:929–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern JS, Batchelor BR, Hollander N, Cohn CK, Hirsch J: Adipose-cell size and immunoreactive insulin levels in obese and normal-weight adults. Lancet 1972;2:948–951 [DOI] [PubMed] [Google Scholar]
- 10.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE: Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000;43:1498–1506 [DOI] [PubMed] [Google Scholar]
- 11.Brook CG, Lloyd JK: Adipose cell size and glucose tolerance in obese children and effects of diet. Arch Dis Child 1973;48:301–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch J, Knittle JL: Cellularity of obese and nonobese human adipose tissue. Fed Proc 1970;29:1516–1521 [PubMed] [Google Scholar]
- 13.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW: Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982;54:254–260 [DOI] [PubMed] [Google Scholar]
- 14.Salans LB, Knittle JL, Hirsch J: The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest 1968;47:153–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jernås M, Palming J, Sjöholm K, Jennische E, Svensson PA, Gabrielsson BG, Levin M, Sjögren A, Rudemo M, Lystig TC, Carlsson B, Carlsson LM, Lönn M: Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J 2006;20:1540–1542 [DOI] [PubMed] [Google Scholar]
- 16.Monteiro R, de Castro PM, Calhau C, Azevedo I: Adipocyte size and liability to cell death. Obes Surg 2006;16:804–806 [DOI] [PubMed] [Google Scholar]
- 17.Skurk T, Alberti-Huber C, Herder C, Hauner H: Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007;92:1023–1033 [DOI] [PubMed] [Google Scholar]
- 18.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P: Dynamics of fat cell turnover in humans. Nature 2008;453:783–787 [DOI] [PubMed] [Google Scholar]
- 19.Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD: Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr 2008;87:56–63 [DOI] [PubMed] [Google Scholar]
- 20.Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB: How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 1996;143:228–239 [DOI] [PubMed] [Google Scholar]
- 21.Wahrenberg H, Hertel K, Leijonhufvud BM, Persson LG, Toft E, Arner P: Use of waist circumference to predict insulin resistance: retrospective study. BMJ 2005;330:1363–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolaczynski JW, Morales LM, Moore JH, Jr, Considine RV, Pietrzkowski Z, Noto PF, Colberg J, Caro JF: A new technique for biopsy of human abdominal fat under local anaesthesia with Lidocaine. Int J Obes Relat Metab Disord 1994;18:161–166 [PubMed] [Google Scholar]
- 23.Björntorp P: Effects of age, sex, and clinical conditions on adipose tissue cellularity in man. Metabolism 1974;23:1091–1102 [DOI] [PubMed] [Google Scholar]
- 24.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J: Retrospective birth dating of cells in humans. Cell 2005;122:133–143 [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, Cushman SW: Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 2007;50:1707–1715 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.