Abstract
OBJECTIVE
The contribution of antecedent viral infection to the development of type 1 diabetes in humans is controversial. Using a newer rat model of the disease, we sought to 1) identify viruses capable of modulating diabetes penetrance, 2) identify conditions that increase or decrease the diabetogenicity of infection, and 3) determine whether maternal immunization would prevent diabetes.
RESEARCH DESIGN AND METHODS
About 2% of LEW.1WR1 rats develop spontaneous autoimmune diabetes, but disease penetrance is much higher if weanling rats are exposed to environmental perturbants including Kilham rat virus (KRV). We compared KRV with other viruses for diabetogenic activity.
RESULTS
Both KRV and rat cytomegalovirus (RCMV) induced diabetes in up to 60% of LEW.1WR1 rats, whereas H-1, vaccinia, and Coxsackie B4 viruses did not. Simultaneous inoculation of KRV and RCMV induced diabetes in 100% of animals. Pretreatment of rats with an activator of innate immunity increased the diabetogenicity of KRV but not RCMV and was associated with a moderate rate of diabetes after Coxsackie B4 and vaccinia virus infection. Inoculation of LEW.1WR1 dams with both KRV and RCMV prior to pregnancy protected weanling progeny from virus-induced diabetes in a virus-specific manner.
CONCLUSIONS
Exposure to viruses can affect the penetrance of autoimmune diabetes in genetically susceptible animals. The diabetogenicity of infection is virus specific and is modified by immunomodulation prior to inoculation. Maternal immunization protects weanlings from virus-induced diabetes, suggesting that modification of immune responses to infection could provide a means of preventing islet autoimmunity.
Type 1 diabetes results from inflammatory infiltration of pancreatic islets (insulitis), leading to destruction of insulin-producing β-cells (1). Much evidence suggests that the disease is caused by nongenetic environmental factors operating in a genetically susceptible host (2). Environmental factors thought most likely to modulate its pathogenesis include toxins, vaccination, diet, and infection.
An association between type 1 diabetes and viral infection was first noted in epidemiological studies (3) and continues to attract attention (4–6). Viruses have been invoked to explain the increasing prevalence of diabetes (7), seasonal variation in onset (8), and enhanced susceptibility of transmigratory populations (9). Viruses associated with human diabetes include measles, congenital rubella, mumps, and influenza B (10–13). Coxsackie B virus RNA sequences have been found in the blood of patients early in the disease (14), and Coxsackie antigens can be recovered from children with recent diabetes onset (15). Human cytomegalovirus (HCMV) major DNA-binding protein encodes a peptide that stimulates clonal CD4+ T-cells that recognize the human autoantigen GAD (16). In addition, a molecular hybridization study of lymphocytes using a HCMV-specific probe found evidence of viral genome in 22% of diabetic patients but only 2.6% of control subjects (17).
There are, however, no mechanistic data that link human diabetes with infection, and, on the contrary, it is plausibly argued that virus infections may prevent the disease (4,18). The “hygiene hypothesis” posits an inverse correlation between viral exposure and the prevalence of both autoimmunity and allergy (19). The role of viruses in diabetes induction has been studied in nonobese diabetic (NOD) mice, but in this model infections are associated with disease prevention. Coxsackie B virus accelerates diabetes in NOD mice but only after autoimmunity has already been initiated (20); it is also associated with exocrine pancreatitis (21), which is not a feature of human type 1 diabetes. Other studies have used transgenic mice that express viral antigen on β-cells (18), but these are inherently artificial.
In rats with the high-risk major histocompatibility complex (MHC) class II RT1B/Du haplotype infection with Kilham rat parvovirus (KRV) can lead to autoimmune diabetes (22). KRV in rats has been advocated as a model for studying the problem of viral triggering (23). Our objective was to investigate rat models as platforms for evaluating the role(s) of virus infection in autoimmunity. We report that in addition to KRV, infection with rat CMV (RCMV) leads to diabetes in LEW.1WR1 rats, only 2% of which develop spontaneous disease (24). The diabetogenicity of infection is shown to be dependent on age and the preexisting state of the immune system, and innate immune activation renders Coxsackie B4 and vaccinia viruses diabetogenic. Finally, we demonstrate that inoculation of LEW.1WR1 dams with diabetogenic viruses prevents induction of diabetes in weanling progeny. Protection is both complete and virus specific.
RESEARCH DESIGN AND METHODS
Inbred LEW.1WR1 rats (RT1u/u/a) and BBDR rats (RT1u/u/u) were obtained from BioMedical Research Models (Worcester, MA). BBDR rats develop autoimmune diabetes after immunological perturbation but not spontaneously (25). Animals were housed in a shower-in viral antibody–free (VAF) facility, and periodic testing of sentinel rats documented the absence of common rodent pathogens (listed in the online appendix Table 1 [available at http://diabetes.diabetesjournals.org/cgi/content/full/db09–0255/DC1]). Animals were maintained in accordance with guidelines of the institutional animal care and use committees of the University of Massachusetts Medical School and BioMedical Research Models and the Guide for the Care and Use of Laboratory Animals (26). Rats were provided with autoclaved laboratory chow (Purina 7012) and acidified water ad libitum.
TABLE 1.
Frequency of induced diabetes in LEW0.1WR1 rats
| Treatment type | Group | Poly I:C | Virus | n | n (%) diabetic |
|---|---|---|---|---|---|
| Culture medium alone | 1 | No | None | 16 | 0 (0) |
| Single viral inoculations* | 2 | No | KRV alone | 8 | 3 (38) |
| 3 | No | RCMV alone†‡ | 38 | 14 (37) | |
| 4 | No | Coxsackie B4 alone§ | 18 | 0 (0) | |
| 5 | No | Vaccinia alone‖ | 14 | 1 (7) | |
| 6 | No | H-1 alone | 20 | 0 (0) | |
| 7 | Yes | None | 18 | 0 (0) | |
| 8 | No | RCMV + KRV | 45 | 34 (76) | |
| Simultaneous viral inoculations¶ | 9 | No | RCMV + CoxB4 | 8 | 1 (13) |
| 10 | Yes | KRV | 6 | 6 (100) | |
| Sequential treatments** | 11 | Yes | RCMV | 29 | 3 (10) |
| 12 | Yes | Coxsackie B4 | 18 | 3 (17) | |
| 13 | Yes | Vaccinia | 10 | 4 (40) | |
| 14 | Yes | H-1 | 6 | 0 (0) |
Twenty- to 25-day-old male and female LEW0.1WR1 rats were inoculated intraperitoneally with the indicated viral agents with or without pretreatment with poly I:C as described in research design and methods. Doses were as follows: KRV, 107 PFU; H-1, 107 PFU; CoxB4, 108 PFU; VV, 106 PFU; or RCMV, 104 PFU.
*Overall χ2 for single virus inoculation category = 25.48, df = 5, P = 0.001.
†Group 3 vs. group 8, Fisher exact statistic, P = 0.0007.
‡Group 3 vs. group 11: Fisher exact statistic, P = 0.02.
§Group 4 vs. group 12, P = NS.
‖Group 5 vs. group 13, Fisher exact statistic, P = NS.
¶Group 8 vs. group 9, Fisher exact statistic, P = 0.001.
**Overall χ2 for sequential treatments category = 25.58, df = 3, P < 0.001. In all experiments in which diabetes occurred, both male and female rats were affected. In groups 3 and 8 in which there were larger numbers of diabetic animals, both sexes were affected in approximately equal proportions.
Reagents.
Polyinosinic:polycytidylic acid (poly I:C; Sigma, St. Louis, MO) was dissolved in Dulbecco's PBS, sterile filtered, and stored at −20°C. The concentration of contaminating endotoxin (LPS) was <50 units/mg (Charles River Endosafe). Monoclonal antibody to the RCMV early antigen (mAb8) was provided by Dr. Jan-Luuk Hillebrands (University of Groningen, The Netherlands). KRV (UMass strain, KRV) and Toolan's H-1 virus (H-1) were obtained from stocks maintained in our laboratories as described (27). RCMV was provided by Dr. Jan-Luuk Hillebrands and maintained as described (28). Vaccinia virus (VV) was provided by Dr. Raymond Welsh (University of Massachusetts Medical School) (29) and Coxsackie B4 virus (CoxB4, Edwards strain) by Dr. Charles Gauntt (University of Texas, San Antonio). CoxB4 was maintained as described (30).
Diabetes induction.
For diabetes induction studies, litters of male and female LEW.1WR1 or BBDR rats were randomized and injected intraperitoneally with KRV, H-1, CoxB4, VV, or RCMV at doses (in plaque forming units [PFU]) that are indicated in the table and figure legends. Rats were either 20–25 days old (weanling), 40–45 days old, or 48–50 days old when inoculated. In certain experiments, poly I:C at a dose of 1.0 μg/g body wt was injected three times on days −3, −2, and −1 relative to virus inoculation. In one experiment, KRV and RCMV were inoculated simultaneously. In another experiment, sequential inoculations of KRV and RCMV were performed by injecting with one virus 4 days, 3 weeks, or 6 weeks before the other.
Detection of diabetes.
Animals were screened twice weekly for glycosuria (CliniStix; Bayer HealthCare, Elkhart, IN) beginning 1 week after the start of each diabetes induction protocol. Diabetes in glycosuric rats was diagnosed on the basis of a capillary glucose concentration >250 mg/dl (Accu-Chek; Roche Diagnostics, Indianapolis, IN). Screening for diabetes was performed until onset or day 40 after starting the induction protocol.
Histology and serology.
After diagnosis of diabetes or at the end of 40 days of observation, rats were killed and pancreata were removed and fixed in 10% buffered formalin. Paraffin-embedded sections of pancreas were sectioned at 4-μm intervals and stained with hematoxylin and eosin. A pathologist (B.A.W.) who was not informed of the donor's glycemic status scored the tissues for intensity of insulitis as follows: 0, no inflammatory mononuclear cell infiltration; 1+, small numbers of mononuclear cells infiltrating islets with preservation of islet architecture; 2+, moderate numbers of infiltrating mononuclear cells with preservation of islet architecture; 3+, many mononuclear cells with most islets affected and distortion of islet architecture; and 4+, florid infiltration and distorted islet architecture or end-stage islets with or without residual inflammation.
In some experiments, RCMV antigens were visualized on 4-μm formalin-fixed, paraffin-embedded sections of pancreas and salivary glands by reacting with RCMV early antigen monoclonal antibody (mAb8) as described (31). In all experiments involving RCMV, serum was obtained from diabetic animals to confirm the absence of KRV serologically (Charles River Laboratories, Wilmington, MA). Random samples of serum from KRV-inoculated rats uniformly confirmed the presence of anti-KRV antibodies. We were unable to test for the presence of anti-RCMV antibodies due our inability to locate or develop a suitable assay.
Statistics.
Diabetes-free survival was analyzed using the method of Kaplan and Meier, and the equality of nondiabetic survival distributions was tested by log-rank statistic (32). Parametric data are given as arithmetic means ± 1 SD. Latency to onset of diabetes is given as the median. Fisher's exact statistic was used for analyzing 2 × 2 tables and the χ2 test for larger tables. Two-tailed P values <0.05 were considered statistically significant.
RESULTS
Both KRV and RCMV induce diabetes in weanling LEW.1WR1 rats.
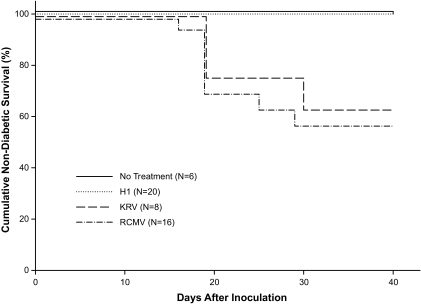
KRV is known to induce diabetes in BBDR, PVG.RT1u, and LEW.1WR1 rats, all of which express the class II B/Du MHC haplotype (22). We first confirmed that KRV induces diabetes in LEW.1WR1 rats inoculated at 20–25 days of age (Fig. 1 and Table 1, group 2). The percentage of diabetic animals (38%) and median latency to onset (19 days) were similar to those observed in BBDR rats (27). Among virus-inoculated animals, mean insulitis scores were 3.7 ± 0.7 (n = 3) in diabetic rats and 0.4 ± 0.9 in nondiabetic rats (n = 5). We also observed that H-1 parvovirus, which is highly homologous to KRV (33), does not induce diabetes in LEW.1WR1 rats, all of which were entirely free of insulitis (n = 20) (Fig. 1 and Table 1, group 6).
FIG. 1.
Frequency of diabetes in LEW.1WR1 rats. Animals 20–25 days old of either sex were randomized to groups that were untreated or inoculated intraperitoneally with H-1 virus (107 PFU), KRV (107 PFU), or RCMV (104 PFU) as described in research design and methods. Rats were observed for 40 days for onset of diabetes. Overall log-rank statistic for the dataset = 13.79, df = 3, P = 0.003. The KRV and RCMV groups are statistically similar (P = 0.7).
Only 10% of LEW.1WR1 rats inoculated with KRV at 40–45 days of age rats developed diabetes 19–30 days later (n = 20); no rats inoculated with H-1 virus at this age became diabetic (n = 12). Mean insulitis scores were 0.3 ± 0.7 among nondiabetic KRV-treated rats (n = 18) and 0 ± 0 among H-1–treated rats (n = 12). In a sample of BBDR animals inoculated with KRV at 40–45 days of age, none became diabetic (n = 8).
We then found that RCMV (104 PFU) induced diabetes in 37% of weanling male and female LEW.1WR1 rats within 30 days of inoculation (Table 1, group 3). Median latency to diabetes onset was 19 days, similar to that observed after KRV inoculation (Fig. 1). Separately, none of eight animals inoculated between 35 and 40 days of age developed diabetes or any insulitis. One of eight animals inoculated with RCMV at 48–50 days of age developed diabetes 8 days later, but this may have been a spontaneous event, as untreated LEW.1WR1 rats can become diabetic beginning at age 46 days (24). No insulitis data are available for this cohort.
We next tested different doses of RCMV for diabetogenicity. Diabetes frequency through day 40 after inoculating weanling LEW.1WR1 rats given 103, 104, or 105 PFU of virus was lower than in the previous experiment, 19% at each dosage level (n = 3 of 16 at each level, median latency 15–19 days). Among rats inoculated with 106 PFU, diabetes frequency was 31% (n = 13).
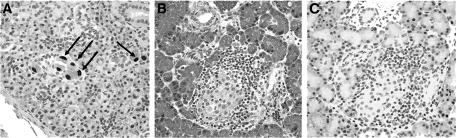
Pancreata from RCMV-inoculated diabetic weanlings uniformly showed insulitis (Fig. 2B), whereas islets from the nondiabetic animals showed minimal inflammation. Mean insulitis scores were 4.0 ± 0.0 (n = 7) among diabetic rats and 0.3 ± 0.7 (n = 9) among nondiabetic rats. To confirm that inoculation had led to infection and to exclude direct infection of islets as the cause of diabetes, immunohistochemistry for the RCMV early antigen was performed. RCMV early antigen was readily detected in salivary gland samples (Fig. 2A) but was undetectable in pancreata isolated from infected animals at the time of diabetes onset (Fig. 2C).
FIG. 2.
RCMV antigen is detected in salivary glands but not pancreata of diabetic LEW.1WR1 rats. Salivary glands and pancreata from diabetic LEW.1WR1 rats inoculated with RCMV were isolated within 2 days of onset of diabetes as described in research design and methods. Representative images from salivary glands (A) and serial pancreas sections (B and C) are shown. Tissues were either stained with hematoxylin and eosin (B) or were processed for RCMV immunohistochemistry using an antibody specific for RCMV early antigen (A and C). RCMV early antigen could be detected in salivary glands (A, arrows) but not in pancreata (C) despite the presence of insulitis (B). ×133 magnification.
Two other viruses were tested for diabetogenicity. Inoculation of VV was associated with subsequent diabetes in 1 of 14 (7%) treated rats. The diabetic animal exhibited 4+ insulitis; immunohistochemistry revealed no insulin staining with preservation of glucagon staining. The mean insulitis score in a sample of five nondiabetic VV-inoculated rats was 0.1 ± 0.2. Inoculation with CoxB4 virus did not induce diabetes in weanling LEW.1WR1 rats (n = 18) (Table 1, group 4). Histology was not available for this group.
Penetrance of diabetes is altered by concurrent infection.
We next asked if concurrent exposure to two moderately diabetogenic viruses would alter the penetrance of disease. Weanling LEW.1WR1 rats were inoculated simultaneously with KRV and RCMV (both diabetogenic) or with RCMV and CoxB4 virus (one diabetogenic, the other not). Additional cohorts were inoculated with each of the viruses alone. In four separate trials, animals (n = 45) were inoculated simultaneously with KRV and RCMV and the penetrance of diabetes ranged from 50 to 100% (mean 76%, P < 0.001 vs. RCMV alone at the same dose [38%]) (Table 1, groups 3 and 8). In contrast, simultaneous inoculation of RCMV and CoxB4 reduced the penetrance of diabetes (13%) (Table 1, group 9) when compared with inoculation with RCMV alone (37%) (Table 1, group 3), but this trend did not reach statistical significance (P = 0.1).
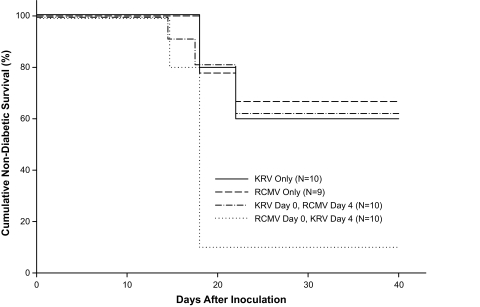
We next asked if sequential viral inoculation would affect the frequency of diabetes. Inoculation of weanling LEW.1WR1 rats with RCMV followed 4 days later by KRV increased the penetrance of diabetes to 90% (n = 10, median latency to onset 18 days) compared with RCMV alone (33%, P = 0.004, median latency 18 days) (Fig. 3). This result is consistent with the data for concurrent inoculation of KRV and RCMV (76%) (Table 1, group 8) and suggests that the two viruses act synergistically to induce disease (Fig. 3). In contrast, when LEW.1WR1 rats were inoculated first with KRV and then with RCMV, the frequency of diabetes was 40%, comparable to that observed with either virus alone (Fig. 3, n = 10). When LEW.1WR1 rats were inoculated sequentially with KRV and RCMV at intervals of 3 or 6 weeks, the second inoculation of virus was not associated with diabetes induction in any rats not diabetic after the first inoculation (n = 5–14 for each group).
FIG. 3.
Frequency of diabetes in LEW.1WR1 rats. Animals 20–25 days old of either sex were randomized to four groups. Two groups were inoculated intraperitoneally with KRV (107 PFU) or RCMV (104 PFU) alone; two other groups were inoculated sequentially with both KRV and RCMV at the same doses at an interval of 4 days in the order indicated in the figure. Rats were observed for 40 days for onset of diabetes. Overall log-rank statistic for the dataset = 13.55, df = 3, P = 0.004. The RCMV followed by KRV group was statistically significantly different from each of the three other groups (P ≤ 0.012). No other pairwise comparisons were statistically significant.
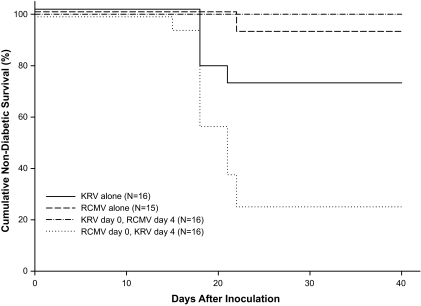
To extend the outcome of the 4-day sequential inoculation study in LEW.1WR1 rats, we performed a similar study using diabetes susceptible BBDR rats. We confirmed that KRV inoculation alone induces diabetes in the BBDR rat (Fig. 4). A single BBDR rat became diabetic 22 days after inoculation with RCMV alone (n = 15). In keeping with the data from LEW.1WR1 rats (Fig. 3), inoculation of BBDR rats with RCMV followed 4 days later by KRV increased the penetrance of diabetes to 75% (Fig. 4, n = 16, median latency 18 days) compared with RCMV alone (7%, P < 0.0001). Inverting the order of infection, in contrast, was associated with complete prevention of diabetes (Fig. 4, n = 16. P < 0.05 vs. KRV alone).
FIG. 4.
Frequency of diabetes in BBDR rats. Animals 20–25 days old of either sex were randomized to four groups. Two groups were inoculated intraperitoneally with KRV (107 PFU) or RCMV (104 PFU) alone; two other groups were inoculated sequentially with both KRV and RCMV at the same doses at an interval of 4 days in the order indicated in the figure. Rats were observed for 40 days for onset of diabetes. Overall log-rank statistic for the dataset = 31.38, df = 3, P < 0.001. The RCMV followed by KRV group was statistically significantly different from each of the three other groups (P ≤ 0.01). The KRV alone group was statistically significantly different from the KRV followed by RCMV group (P < 0.05). No other pairwise comparisons were statistically significant.
Innate immune activation selectively alters the penetrance of diabetes following viral infection.
Poly I:C is an analog of double-stranded (DS) RNA that is a ligand of both TLR3 and IFIH1 (interferon induced with helicase C domain 1). IFIH1, also known as MDA5 (melanoma differentiation–associated protein 5), is a retinoic acid–inducible gene I–like helicase (RLH) (34). Pretreatment with small doses of poly I:C enhances the diabetogenicity of KRV in BBDR rats (33), and we hypothesized that the outcome of the sequential infections may have been determined by the status of the innate immune system prior to inoculation with the second virus. We therefore injected weanling LEW.1WR1 rats with poly I:C prior to inoculation with virus. Injection of LEW.1WR1 rats with poly I:C alone did not induce diabetes (n = 18) (Table 1, group 7) but was associated with a mild degree of insulitis that was detectable as early as day 5 (0.6 ± 0.8, n = 8). Consistent with BBDR rat data (33), pretreatment of LEW.1WR1 rats with poly I:C prior to KRV inoculation increased the frequency of diabetes to 100% (Table 1, group 10). In contrast, pretreatment with poly I:C prior to RCMV inoculation decreased the frequency of disease from 37 to 10% (P = 0.02) (Table 1, groups 3 and 11).
Pretreatment with poly I:C also increased the penetrance of diabetes after VV inoculation from 7 to 40% through 40 days of observation (Table 1, groups 5 and 13). The increase in diabetes penetrance was not statistically significant, but 1+ to 3+ insulitis was present in animals that did not become diabetic (mean score 2.2 ± 0.8, n = 5).
In two separate experiments, pretreatment with poly I:C prior to CoxB4 inoculation induced diabetes in LEW.1WR1 animals at a rate of 17% (n = 3 of 18) (Table 1, group 12). Examination of pancreata from 12 of these animals revealed 4+ insulitis in 2 diabetic rats and no insulitis in any nondiabetic animals. No exocrine pancreatitis was seen in any of the 12 specimens. Immunohistochemistry revealed abundant glucagon but no insulin staining in the two diabetic specimens; both hormones were abundant in the nondiabetic specimens.
In a final experiment, pretreatment with poly I:C did not enhance the diabetogenicity of H-1 infection (n = 6) (Table 1, group 14), but pancreatic histology revealed that two of six rats had 3+ insulitis; the remainder were free of inflammation.
Maternal immunization prevents induction of diabetes.
Lastly, we hypothesized that passive transfer of antibody from dam to pup would prevent diabetes induced by viruses. Weanling LEW.1WR1 female rats were inoculated with either RCMV or both KRV and RCMV. Rats that did not subsequently become diabetic were mated to normal LEW.1WR1 males. Progeny of the virus-inoculated dams were then inoculated with KRV, RCMV, or both when weanlings. Inoculation of the progeny of naïve dams with RCMV, KRV, or RCMV plus KRV induced diabetes at the expected frequencies (62, 38, and 100%, respectively) (Table 2, groups 1, 3, and 5). In contrast, pups born to females that had been inoculated with RCMV or RCMV plus KRV before pregnancy were protected from diabetes induced by these specific infections (Table 2, groups 2 and 6). Pups born to RCMV-inoculated mothers, however, were not protected from diabetes induced by KRV (Table 2, group 4).
TABLE 2.
Frequency of induced diabetes in LEW.1WR1 rats
| Group | Maternal inoculum | Progeny inoculum | n | n (%) diabetic |
|---|---|---|---|---|
| 1* | None | RCMV | 13 | 8 (62) |
| 2 | RCMV | RCMV | 26 | 0 (0) |
| 3† | None | KRV | 8 | 3 (38) |
| 4 | RCMV | KRV | 13 | 8 (62) |
| 5‡ | None | RCMV + KRV | 10 | 10 (100) |
| 6 | RCMV + KRV | RCMV + KRV | 11 | 0 (0) |
Twenty- to 25-day-old female LEW.1WR1 rats were either left untreated or were inoculated intraperitoneally with RCMV alone or RCMV and KRV simultaneously as described in research design and methods. Females that did not become diabetic were mated to naïve male LEW.1WR1 rats. The resulting pups were inoculated with the indicated viruses at 20–25 days of age and followed for 40 days or until onset of diabetes. The doses of administered at each stage of the experiment were 107 PFU for KRV and 104 PFU for RCMV.
*Fisher exact statistic, P < 0.0001 vs. group 2.
†Fisher exact statistic, P = 0.39 vs. group 4.
‡Fisher exact statistic, P < 0.0001 vs. group 6.
DISCUSSION
Our data confirm and extend the hypothesis that certain viral agents have the capacity to trigger autoimmune diabetes in genetically susceptible rodent hosts that develop little (LEW.1WR1) or no (BBDR) spontaneous disease when maintained in VAF housing. Triggering of type 1 diabetes by virus alone has previously been documented only for KRV in BBDR and LEW.1WR1 rats (22). Pretreatment with poly I:C, an activator of innate immunity, increases the penetrance of KRV-induced diabetes in both strains, and it induces diabetes in a third strain, PVG.RT1u, which does not become diabetic after inoculation with KRV alone (22). Class II MHC–identical WF and PVG.R8 rats do not become diabetic in response to either KRV alone or KRV after poly I:C (22).
We now show that diabetes reliably occurs in LEW.1WR1 rats exposed to KRV (a single-stranded DNA virus) and RCMV (a double-stranded DNA virus) and to a lesser extent after exposure to VV (a double-stranded DNA virus) and CoxB4 (a single-stranded RNA virus). The data document that viral diabetogenicity is a complex phenotype with epidemiological characteristics that may help explain why it has been difficult to prove a role for infection in human diabetes pathogenesis and identify the relevant mechanisms.
First, for any given combination of diabetogenic virus and susceptible inbred host genotype, disease penetrance is incomplete; not all infected animals become diabetic and the fraction that does varies from experiment to experiment. For example, RCMV triggered diabetes in a fraction of LEW.1WR1-inoculated rats in every experiment performed, but despite the use of identical virus preparations and inbred rats in VAF housing, that fraction varied from ∼20% in the dose response study to as high as ∼60% in the maternal immunization study. In the case of CoxB4 virus, diabetes after inoculation was dependent on pretreatment with poly I:C, and even then the frequency of disease was low (∼17%). At the other extreme, H-1, a parvovirus with high homology to KRV (33) does not trigger diabetes in BBDR or LEW.1WR1 rats with or without poly I:C.
Second, the inflammatory substrate of diabetes, insulitis, also exhibited variable penetrance. LEW.1WR1 rats that became diabetic after infection with either KRV or RCMV alone, or CoxB4 after poly I:C, exhibited intense insulitis and selective loss of islet β-cells. In contrast, identically treated animals that did not become diabetic were largely free of insulitis and exhibited no detectable β-cell loss. There was essentially no intermediate phenotype in the islets. It appears that the diabetogenic immune response is difficult to initiate with these three viruses, but once started it goes to completion.
Third, the data document the importance of genetic background. The frequency of spontaneous diabetes in VAF environments is ∼2.5% in LEW.1WR1 rats and 0% in BBDR or PVG.RT1u rats (22). Noninfectious immunological perturbation of innate or adaptive immunity dramatically increases the penetrance and tempo of the disease in all three strains (22,24) but not in MHC class II–identical LOU or WF rats (35). LEW.1WR1 rats become diabetic after exposure to either KRV or RCMV, or after poly I:C to VV and CoxB4. In contrast, BBDR rats develop KRV-induced diabetes but have minimal susceptibility to RCMV-induced disease. PVG.RT1u rats become diabetic in response to KRV but only if pretreated with poly I:C (22). In their aggregate, the data indicate that susceptibility to virus-induced diabetes is dependent on specific combinations of host and virus. Background genes other than the class II MHC that may modulate the expression of type 1 diabetes after infection are under investigation. Generation of a (WF × BBDR)F2 cohort has identified a genetic locus, Iddm20, on chromosome 17 that is specifically associated with susceptibility to KRV-induced diabetes (36). A different locus on chromosome 20, Iddm37, has been identified in a (LEW.1WR1 × BBDR)F2 cohort treated with KRV (37). Fourth, susceptibility to virus-triggered diabetes in the rat declines with age. This was true both for older VAF animals and for older rats deliberately exposed 3 or 6 weeks earlier to a different virus. This age dependence suggests that, even in a VAF environment, developmental changes in immune response are determinants of viral diabetogenicity. Interestingly, genetic studies of spontaneous autoimmune diabetes in lymphopenic rats have identified loci that determine age at onset (38,39). Speculatively, these loci may harbor genes that modify the immune response over time.
Fifth, and perhaps most importantly, the data show that viruses require a favorable immunological environment—a “fertile field” (40)—to be diabetogenic. Poly I:C enhances disease induction by KRV in three rat strains (33) and, in LEW.1WR1 rats, by CoxB4 and VV. We also show, however, that poly I:C reduces the penetrance of RCMV-induced diabetes in the LEW.1WR1 rat. Similarly, antecedent RCMV infection enhances the diabetogenicity of KRV, but inverting the order of infection has little or no effect.
While not specifically investigated in these studies, the underlying mechanisms are likely to involve antiviral responses evoked by the production of type 1 interferons (IFNs) and other proinflammatory cytokines (34). IFNs can promote the action of β cytotoxic effector cells and, when expressed in β-cells in insulitic islets, can elicit β-cell death (41).
Acting through different signaling pathways, both RNA and DNA viruses induce these cytokine responses. At least three classes of innate pattern recognition receptors are involved: the TLRs, the RLHs (like MDA5), and nucleotide-binding oligomerization domain–like receptors (NLRs) (34). Viruses of different families differ greatly in their ability to induce type 1 IFN responses via each of these receptors, as well as in their ability to evade these responses (34). New human genome-wide association data suggest that genetic control of these responses may be critical for antiviral responses that protect against type 1 diabetes (42). Nejentsev et al. (42) have reported four variants in IFIH1 that lowered type 1 diabetes risk. These variants were predicted to alter the expression and structure of IFIH1, a cytoplasmic helicase that mediates induction of the type 1 interferon response to viral RNA and is a target of poly I:C.
We speculate that genetically determined “tuning” of innate immune responses to virus determines their diabetogenicity (43). The proinflammatory capability of poly I:C clearly enhances viral diabetogenicity for agents like KRV, but, in contrast, perhaps by impairing viral replication it reduces the diabetogenicity of RCMV. Our sequential infection data support this possibility. The data are consistent with the suggestion that innate immune system can act as a double-edged sword, having both a beneficial role in host defense while leading, in genetically susceptible individuals, to upregulation of proinflammatory autoimmune responses, islet destruction, and diabetes (4,44).
Studies to quantify the type 1 IFN responses in infected rats and to modulate diabetogenicity by manipulating these responses are underway in our laboratories. In their aggregate, the data suggest that the diabetogenicity of a given virus is dependent on both the innate immune environment in which infection occurs and on background genes that modify the immune response.
Our observation that RCMV after KRV or poly I:C reduced or had no effect on diabetes penetrance in the LEW.1WR1 also suggests that, properly timed, an innate immune response might engender the kind of protective response implicit in the “hygiene hypothesis” (18,19). Apropos of the hygiene hypothesis, it should be pointed out that spontaneous diabetes does occur in the absence of viral infection, and disease penetrance tends to increase in an increasingly clean environment. This has been reported in the BBDP (25), KDP (45), and LEW.1AR1-iddm (46) strains. BBDP and KDP rats have mutations known to affect T-cell number or function (25). LEW.1AR1-iddm rats have a normal immunophenotype and the underlying genetic mutation is not yet known (47).
Our data do provide one insight into mechanism. They demonstrate the absence of RCMV early antigen in the islets of diabetic LEW.1WR1 rats and suggest that β-cell infection and cytotoxicity are not the mechanism of diabetes induction. This observation is consistent with similar findings in BBDR rats infected with KRV (48).
Because of its relevance to human diabetes, our CoxB4 data deserve comment. Enteroviruses including CoxB4 are among the leading candidate viral triggers of human type 1 diabetes (15,18). CoxB4 did not by itself induce the disease in the rat strains we studied, but brief pretreatment with poly I:C was associated with subsequent diabetes in 17% of CoxB4-inoculated LEW.1WR1 rats. Our pretreatment protocol using three small doses of poly I:C does induce low-grade insulitis in the LEW.1WR1 rat, and this finding parallels the observation that CoxB3 accelerates diabetes in NOD mice but only in the context of preexisting insulitis (20). As is true of other viral infections in the rat, diabetes was associated with 3 to 4+ insulitis and selective destruction of insulin-producing, but not glucagon-producing, cells, whereas islet histology in animals that did not progress to diabetes was normal. In addition, there was no evidence of the exocrine pancreatitis that can occur after Coxsackie virus infection in the mouse (21). The LEW.1WR1 rat may thus provide a platform for additional investigation of the diabetogenicity of enteroviral infection.
Finally, we have shown that maternal exposure to a diabetogenic virus prior to pregnancy enables dams to produce progeny that are resistant to the diabetogenicity of viral inoculation. Our data suggest (but do not prove) that the passive transfer of maternal antibodies can prevent viral triggering of diabetes. These data suggest that it is the immune response to virus that is present in a susceptible genetic context that determines the diabetogenicity (or protectiveness) of infection.
In conclusion, exposure to viruses, including KRV, RCMV, VV, and CoxB4, can affect the penetrance of autoimmune diabetes in genetically susceptible animals. In keeping with previous analyses of both rodents and humans (49), the connection between infections and autoimmunity is multifaceted and complex. We show that low-frequency “viral footprints” (49) may be hard to detect. Such low and variable rates of penetrance by viruses of different families acting in the context of outbred genetic backgrounds may account for the lack of firm evidence that viruses trigger human type 1 diabetes. The effect of infection in target tissues may also be dependent on preinitiation of autoimmunity, as in the NOD mouse (20), and multiple infections might act in concert to precipitate clinical autoimmunity. As posited by the “fertile field hypothesis” (40), viral infection alone might not be able to induce disease in the absence of other inflammatory factors. Filippi and von Herrath (18) have written that, “based on current evidence, it…appears impossible to assess the capacity of viruses to modulate [human] type 1 diabetes without knowledge of the state of advancement of autoimmunity and infection history of affected individuals. This is no easy task …” We suggest that the task may be made more tractable by exploring rat models of virus-induced pathogenesis in which the genetic background can be dissected and the permissive cytokine milieu identified and manipulated. They provide insight into the difficulties that have impeded analysis of viral triggering and prevention of diabetes in humans and may assist in the analysis of human datasets such as those being generated by the TEDDY study. Finally, the suggestion that immunization can prevent at least some cases of autoimmune diabetes reanimates a longstanding prevention strategy (50).
Supplementary Material
Acknowledgments
Supported in part by grants R43DK-60374 (to D.L.Gu.), DK49106 (to D.L.Gr., J.P.M., and E.P.B.), DK25306 (to J.P.M. and D.L.Gr.), U01 AI073871 (to D.L.Gr.), and Center Grant DK32520 from the National Institutes of Health, as well as by grants 7-08-RA-106 (to J.P.M.) and 7-06-RA-14 (to E.P.B.) from the American Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barker JM, Eisenbarth GS: The natural history of autoimmunity in type 1A diabetes mellitus. In Diabetes Mellitus: A Fundamental and Clinical Text 3rd ed.LeRoith D, Taylor SI, Olefsky JM. Eds. Philadelphia, Lippincott Williams & Wilkins, 2004, p. 471–482 [Google Scholar]
- 2.Morran MP, Omenn GS, Pietropaolo M: Immunology and genetics of type 1 diabetes. Mt Sinai J Med 2008;75:314–327 [DOI] [PubMed] [Google Scholar]
- 3.Gamble DR: Viruses and diabetes: an overview with special reference to epidemiologic studies. In Diabetes Bajaj JS. Ed. Amsterdam, Excerpta Medica, 1977, p. 1–277 [Google Scholar]
- 4.Goldberg E, Krause I: Infection and type 1 diabetes mellitus: a two edged sword? Autoimmun Rev 2009;8:682–686 [DOI] [PubMed] [Google Scholar]
- 5.Cooke A: Infection and autoimmunity. Blood Cells Mol Dis 2009;42:105–107 [DOI] [PubMed] [Google Scholar]
- 6.Richer MJ, Horwitz MS: Viral infections in the pathogenesis of autoimmune diseases: focus on type 1 diabetes. Front Biosci 2008;13:4241–4257 [DOI] [PubMed] [Google Scholar]
- 7.Laron Z: Interplay between heredity and environment in the recent explosion of type 1 childhood diabetes mellitus. Am J Med Genet 2002;115:4–7 [DOI] [PubMed] [Google Scholar]
- 8.Rosenbauer J, Herzig P, Von Kries R, Neu A, Giani G: Temporal, seasonal, and geographical incidence patterns of type I diabetes mellitus in children under 5 years of age in Germany. Diabetologia 1999;42:1055–1059 [DOI] [PubMed] [Google Scholar]
- 9.Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R: Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ 1992;304:1020–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindberg B, Ahlfors K, Carlsson A, Ericsson UB, Landin-Olsson M, Lernmark Å, Ludvigsson J, Sundkvist G, Ivarsson SA: Previous exposure to measles, mumps, and rubella, but not vaccination during adolescence, correlates to the prevalence of pancreatic and thyroid autoantibodies. Pediatrics 1999;104:E121–E125 [DOI] [PubMed] [Google Scholar]
- 11.Hyöty H, Hiltunen M, Reunanen A, Leinikki P, Vesikari T, Lounamaa R, Tuomilehto J, Åkerblom HKthe Childhood Diabetes in Finland Study Group Decline of mumps antibodies in type 1 (insulin-dependent) diabetic children and a plateau in the rising incidence of type 1 diabetes after introduction of the mumps-measles-rubella vaccine in Finland. Diabetologia 1993;36:1303–1308 [DOI] [PubMed] [Google Scholar]
- 12.Lipman TH, Chang Y, Murphy KM: The epidemiology of type 1 diabetes in children in Philadelphia 1990–1994: evidence of an epidemic. Diabetes Care 2002;25:1969–1975 [DOI] [PubMed] [Google Scholar]
- 13.Sano H, Terasaki J, Tsutsumi C, Imagawa A, Hanafusa T: A case of fulminant type 1 diabetes mellitus after influenza B infection. Diabetes Res Clin Pract 2008;79:e8–e9 [DOI] [PubMed] [Google Scholar]
- 14.Yin H, Berg AK, Tuvemo T, Frisk G: Enterovirus RNA is found in peripheral blood mononuclear cells in a majority of type 1 diabetic children at onset. Diabetes 2002;51:1964–1971 [DOI] [PubMed] [Google Scholar]
- 15.Jaidane H, Hober D: Role of coxsackievirus B4 in the pathogenesis of type 1 diabetes. Diabetes Metab 2008;34:537–548 [DOI] [PubMed] [Google Scholar]
- 16.Hiemstra HS, Schloot NC, Van Veelen PA, Willemen SJM, Franken KLMC, Van Rood JJ, De Vries RRP, Chaudhuri A, Behan PO, Drijfhout JW, Roep BO: Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc Natl Acad Sci U S A 2001;98:3988–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pak CY, Eun HM, McArthur RG, Yoon JW: Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet 1988;2:1–4 [DOI] [PubMed] [Google Scholar]
- 18.Filippi CM, Von Herrath MG: Viral trigger for type 1 diabetes: pros and cons. Diabetes 2008;57:2863–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bach JF: Mechanisms of disease: the effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–920 [DOI] [PubMed] [Google Scholar]
- 20.Serreze DV, Ottendorfer EW, Ellis TM, Gauntt CJ, Atkinson MA: Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes 2000;49:708–711 [DOI] [PubMed] [Google Scholar]
- 21.Horwitz MS, Krahl T, Fine C, Lee J, Sarvetnick N: Protection from lethal coxsackievirus-induced pancreatitis by expression of gamma interferon. J Virol 1999;73:1756–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellerman KE, Richards CA, Guberski DL, Shek WR, Like AA: Kilham rat virus triggers T-cell-dependent autoimmune diabetes in multiple strains of rat. Diabetes 1996;45:557–562 [DOI] [PubMed] [Google Scholar]
- 23.Zipris D: Epidemiology of type 1 diabetes and what animal models teach us about the role of viruses in disease mechanisms. Clin Immunol 2009;131:11–23 [DOI] [PubMed] [Google Scholar]
- 24.Mordes JP, Guberski DL, Leif JH, Woda BA, Flanagan JF, Greiner DL, Kislauskis EH, Tirabassi RS: LEW. 1WR1 rats develop autoimmune diabetes spontaneously and in response to environmental perturbation. Diabetes 2005;54:2727–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mordes JP, Poussier P, Rossini AA, Blankenhorn EP, Greiner DL: Rat models of type 1 diabetes: genetics, environment, and autoimmunity. In Animal Models of Diabetes: Frontiers in Research 2nd ed.Shafrir E. Ed. Boca Raton, CRC Press, 2007, p. 1–39 [Google Scholar]
- 26.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council Guide for the Care and Use of Laboratory Animals Washington, DC, National Academy Press, 1996, p. 1–119 [Google Scholar]
- 27.Zipris D, Hillebrands JL, Welsh RM, Rozing J, Xie JX, Mordes JP, Greiner DL, Rossini AA: Infections that induce autoimmune diabetes in BBDR rats modulate CD4+CD25+ T cell populations. J Immunol 2003;170:3592–3602 [DOI] [PubMed] [Google Scholar]
- 28.van der Strate BW, Hillebrands JL, Nijeholt SS, Beljaars L, Bruggeman CA, Van Luyn MJ, Rozing J, The TH, Meijer DK, Molema G, Harmsen MC: Dissemination of rat cytomegalovirus through infected granulocytes and monocytes in vitro and in vivo. J Virol 2003;77:11274–11278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK: Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol 2001;2:1067–1076 [DOI] [PubMed] [Google Scholar]
- 30.Martino TA, Petric M, Weingartl H, Bergelson JM, Opavsky MA, Richardson CD, Modlin JF, Finberg RW, Kain KC, Willis N, Gauntt CJ, Liu PP: The coxsackie-adenovirus receptor (CAR) is used by reference strains and clinical isolates representing all six serotypes of coxsackievirus group B and by swine vesicular disease virus. Virology 2000;271:99–108 [DOI] [PubMed] [Google Scholar]
- 31.Hillebrands JL, van der Werf N, Klatter FA, Bruggeman CA, Rozing J: Role of peritoneal macrophages in cytomegalovirus-induced acceleration of autoimmune diabetes in BB-rats. Clin Dev Immunol 2003;10:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nie NH, Hull CH, Jenkins JG, Steinbrenner K, Bent DH: Statistical Package for the Social Sciences New York, McGraw-Hill, 1975, p. 1–675 [Google Scholar]
- 33.Zipris D, Lien E, Xie JX, Greiner DL, Mordes JP, Rossini AA: TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J Immunol 2005;174:131–142 [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi O, Akira S: Recognition of viruses by innate immunity. Immunol Rev 2007;220:214–224 [DOI] [PubMed] [Google Scholar]
- 35.Ellerman KE, Like AA: Susceptibility to diabetes is widely distributed in normal class IIu haplotype rats. Diabetologia 2000;43:890–898 [DOI] [PubMed] [Google Scholar]
- 36.Blankenhorn EP, Rodemich L, Martin-Fernandez C, Leif J, Greiner DL, Mordes JP: The rat diabetes susceptibility locus Iddm4 and at least one additional gene are required for autoimmune diabetes induced by viral infection. Diabetes 2005;54:1233–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blankenhorn EP, Cort L, Greiner DL, Guberski DL, Mordes JP: Virus-induced autoimmune diabetes in the LEW.1WR1 rat requires Iddm14 and a genetic locus proximal to the major histocompatibility complex. Diabetes, 2009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallis RH, Wang K, Dabrowski D, Marandi L, Ning T, Hsieh E, Paterson AD, Mordes JP, Blankenhorn EP, Poussier P: A novel susceptibility locus on rat chromosome 8 affects spontaneous but not experimentally induced type 1 diabetes. Diabetes 2007;56:1731–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallis RH, Wang K, Marandi L, Shieh E, Ning T, Chao G, Sarmiento J, Paterson AD, Poussier P: Type 1 diabetes in the BB rat: a polygenic disease. Diabetes 2009;58:1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Herrath MG, Fujinami RS, Whitton JL: Microorganisms and autoimmunity: making the barren field fertile? Nat Rev Microbiol 2003;1:151–157 [DOI] [PubMed] [Google Scholar]
- 41.Thomas HE, Graham KL, Angstetra E, McKenzie MD, Dudek NL, Kay TW: Interferon signalling in pancreatic beta cells. Front Biosci 2009;14:644–656 [DOI] [PubMed] [Google Scholar]
- 42.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA: Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 2009;324:387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HS, Lee MS: Role of innate immunity in triggering and tuning of autoimmune diabetes. Curr Mol Med 2009;9:30–44 [DOI] [PubMed] [Google Scholar]
- 44.Lien E, Zipris D: The role of Toll-like receptor pathways in the mechanism of type 1 diabetes. Curr Mol Med 2009;9:52–68 [DOI] [PubMed] [Google Scholar]
- 45.Yokoi N, Komeda K, Wang HY, Yano H, Kitada K, Saitoh Y, Seino Y, Yasuda K, Serikawa T, Seino S: Cblb is a major susceptibility gene for rat type 1 diabetes mellitus. Nat Genet 2002;31:391–394 [DOI] [PubMed] [Google Scholar]
- 46.Lenzen S, Tiedge M, Elsner M, Lortz S, Weiss H, Jörns A, Klöppel G, Wedekind D, Prokop CM, Hedrich HJ: The LEW. 1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia 2001;44:1189–1196 [DOI] [PubMed] [Google Scholar]
- 47.Weiss H, Arndt T, Jorns A, Lenzen S, Cuppen E, Hedrich HJ, Tiedge M, Wedekind D: The mutation of the LEW. 1AR1-iddm rat maps to the telomeric end of rat chromosome 1. Mamm Genome 2008;19:292–297 [DOI] [PubMed] [Google Scholar]
- 48.Brown DW, Welsh RM, Like AA: Infection of peripancreatic lymph nodes but not islets precedes Kilham rat virus-induced diabetes in BB/Wor rats. J Virol 1993;67:5873–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Filippi C, Von Herrath M: How viral infections affect the autoimmune process leading to type 1 diabetes. Cell Immunol 2005;233:125–132 [DOI] [PubMed] [Google Scholar]
- 50.Coon B, An LL, Whitton JL, Von Herrath MG: DNA immunization to prevent autoimmune diabetes. J Clin Invest 1999;104:189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.