Abstract
OBJECTIVE
In vitro models suggest that free fatty acid–induced apoptotic β-cell death is mediated through protein kinase C (PKC)δ. To examine the role of PKCδ signaling in vivo, transgenic mice overexpressing a kinase-negative PKCδ (PKCδKN) selectively in β-cells were generated and analyzed for glucose homeostasis and β-cell survival.
RESEARCH DESIGN AND METHODS
Mice were fed a standard or high-fat diet (HFD). Blood glucose and insulin levels were determined after glucose loads. Islet size, cleaved caspase-3, and PKCδ expression were estimated by immunohistochemistry. In isolated islet cells apoptosis was assessed with TUNEL/TO-PRO3 DNA staining and the mitochondrial potential by rhodamine-123 staining. Changes in phosphorylation and subcellular distribution of forkhead box class O1 (FOXO1) were analyzed by Western blotting and immunohistochemistry.
RESULTS
PKCδKN mice were protected from HFD-induced glucose intolerance. This was accompanied by increased insulin levels in vivo, by an increased islet size, and by a reduced staining of β-cells for cleaved caspase-3 compared with wild-type littermates. In accordance, long-term treatment with palmitate increased apoptotic cell death of isolated islet cells from wild-type but not from PKCδKN mice. PKCδKN overexpression protected islet cells from palmitate-induced mitochondrial dysfunction and inhibited nuclear accumulation of FOXO1 in mouse islet and INS-1E cells. The inhibition of nuclear accumulation of FOXO1 by PKCδKN was accompanied by an increased phosphorylation of FOXO1 at Ser256 and a significant reduction of FOXO1 protein.
CONCLUSIONS
Overexpression of PKCδKN in β-cells protects from HFD-induced β-cell failure in vivo by a mechanism that involves inhibition of fatty acid–mediated apoptosis, inhibition of mitochondrial dysfunction, and inhibition of FOXO1 activation.
Obesity is associated with high plasma concentrations of free fatty acids (FFAs). Especially, saturated FFAs like palmitate have been described to induce apoptotic cell death in insulin-secreting cells (1–4). Previous data suggest that the protein kinase C (PKC)δ is activated by FFAs and plays a crucial role in β-cell survival (5). In particular, overexpression of kinase-negative PKCδ (PKCδKN) in insulin-secreting RINm5F cells protected cells from palmitate-induced cell death by a mechanism involving nuclear translocation of PKCδ and probably stimulation of a phospholipase C (6). Overexpression of PKCδKN in INS-1 cells inhibited interleukin-1β–induced cell death (7). In contrast, downregulation of PKCδ by long-term treatment with phorbol myristate acetate (PMA), a synthetic analog of diacylglycerol, did not protect against palmitate-induced cell death (8). Furthermore, PKCδKO mice displayed reduced glucose-induced insulin secretion and developed glucose intolerance as they aged (9).
Due to these controversial observations, our previous study showing that PKCδ mediates FFA-induced apoptotic cell death needs further in vivo evidence (6). For this purpose, we generated a transgenic mouse model overexpressing PKCδKN selectively in insulin-secreting β-cells. This mouse model was used to test whether PKCδKN protects against high-fat diet (HFD)-induced glucose intolerance in vivo and to analyze molecular changes due to PKCδKN overexpression in comparison with wild-type littermate controls.
RESEARCH DESIGN AND METHODS
Generation of PKCδKN transgenic mice and stably transfected INS-1E cells.
To generate β-cell–specific PKCδKN transgenic mouse lines, the RIP-I/PKCδKN chimeric gene containing the K376R mutation was excised from the plasmid, purified, and microinjected into fertilized eggs as described previously (10,11). Two transgenic mouse lines (#179 and #162) on a C57BL/6 background were selected and analyzed. They displayed normal fertility and growth. For in vivo experiments control mice (wild type) were littermates of transgenic mice. All animal experiments were done in accordance with the accepted standard of human care of animals and were approved by the local Animal Care and Use Committee. INS-1E cells were infected with a retrovirus containing the wild-type or PKCδKN construct. Transfected cells were selected by geneticin (G418) and subcloned by separation of single cells.
Glucose and insulin tolerance test and insulin secretion in vivo.
Mice were fed either a standard diet or HFD and kept under a light/dark cycle of 12/12 h. HFD consisting of 45% kcal fat from lard was fed to 4-week-old mice for 8 weeks (D12451; Research Diet, New Brunswick, NJ). Blood glucose was determined in overnight-fasted mice using a Glucometer Elite (Bayer, Elkhart, IN). Plasma insulin concentrations were measured by radioimmunoassay (Linco Research, St. Charles, MO). Glucose tolerance tests (intraperitoneal glucose tolerance tests [ipGTTs]) were performed in overnight-fasted mice. Animals were injected intraperitoneally with a single dose of d-glucose (2 g/kg body wt), and blood glucose concentrations were detected at the indicated times. For glucose-stimulated insulin release, a d-glucose dose of 3 g /kg body wt was used. To determine insulin tolerance, a bolus of human insulin (1 unit/kg body wt) was injected intraperitoneally into fed mice and glucose concentrations were determined. The results were expressed as percent of the initial glucose levels.
In vitro insulin secretion.
Mouse islets were isolated and cultured as described previously (12). In brief, after culture islets were preincubated for 1 h at 37°C in modified Krebs-Ringer Bicarbonate buffer containing (in mmol/l) 140 NaCl, 5.6 KCl, 1.2 MgCl2, 2.6 CaCl2, 10 HEPES, 2.8 glucose, and 4 g/l BSA (FFA free; Sigma, Deisenhofen, Germany), pH 7.4. Thereafter, batches of 5 islets/0.5 ml were incubated for 30 min at 37°C in the presence of test substances as indicated for each experiment. Insulin released into the supernatant and insulin content after extraction with acid ethanol (HCl 1.5% [vol/vol]/ethanol 75% [vol/vol]) were measured by radioimmunoassay.
Apoptosis.
Activation of caspase-3 was examined by immunohistochemical staining against cleaved caspase-3 in pancreatic slices of mice fed standard diet or HFD. Sections of frozen pancreatic tissue were fixed with 4% paraformaldehyde in PBS for 20 min, permeabilized with 0.2% Triton X-100 for 2 min on ice, and then blocked with 10% FCS in PBS for 45 min. Primary antibody against cleaved caspase-3 (1:200; Cell Signaling Technology, Danvers, MA) was applied overnight in PBS supplemented with 10% FCS. After washing with PBS supplemented with 10% FCS, the slices were incubated for 1 h with an anti-rabbit secondary antibody (1:400; Alexa-Fluor546 IgG, Invitrogen, Paisley, U.K.).
Transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed in isolated islet cells that were prepared as described previously and cultured in RPMI-1640 supplemented with 10% FCS, 10 mmol/l HEPES, 1 mmol/l Na pyruvate, 2 mmol/l l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (13). Prior to the addition to the culture medium palmitate from a 200 mmol/l stock solution dissolved in DMSO was coupled to FCS at a concentration of 6 mmol/l. The palmitate-to-albumine ratio was ∼10:1, with the final concentration of DMSO of 0.3%. The same concentration of the solvent was added to control culture medium. After 3 days of culture in the presence of 0.6 mmol/l palmitate, apoptosis was quantified in islet cells by TUNEL staining using a commercial kit (Roche Diagnostics, Mannheim, Germany). Nuclei were stained with 1 μmol/l TO-PRO3 in PBS for 1 h (Invitrogen, Karlsruhe, Germany). The fluorescence was examined with a confocal microscope (Leica, Wetzlar, Germany) using a 40× objective and excitation wavelengths of 546 nm (for cleaved caspase-3 staining), 488 nm (for TUNEL staining), and 633 nm (for nuclei staining).
Mitochondrial potential.
Mitochondrial potential (ΔΨ) was measured in isolated and cultured islet cells loaded with rhodamine-123 as described previously (13). Briefly, cultured islet cell clusters were treated for 3 days with 0.6 mmol/l palmitate. Thereafter the cells were loaded with 10 mg/l rhodamine-123 in modified Krebs-Ringer bicarbonate buffer solution containing 0.5 mmol/l glucose for 10 min at 37°C. The fluorescence was measured using a device provided by Till Photonics (Gräfelfing, Germany). Mitochondrial hyperpolarization induced by increasing glucose concentration is expressed as percentage of maximal increase of fluorescence induced by carbonylcyanide-p-trifluoromethoxyphenylhydrazone (1 μmol/l FCCP).
Immunohistochemistry.
Sections of frozen pancreatic tissue, cultured isolated islet cells, and INS-1E cells, control or stably transfected with RIP-I/PKCδ constructs, were fixed with 4% paraformaldehyde in PBS, permeabilized with PBS containing 0.2% Triton X-100, and blocked with 10% FCS in PBS for 45 min. Primary antibodies against PKCδ (1:500; BD Transduction Laboratories, Heidelberg, Germany), insulin (1:150; Dako Denmark, Denmark), and forkhead box class O (FOXO)-1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) were applied overnight in PBS containing 10% FCS. After 30 min washing with PBS supplemented with 10% FCS, the samples were incubated for 1 h with the appropriate secondary antibodies (1:400 in 10% FCS-PBS): Alexa-Fluor488 anti-mouse IgG (for PKCδ), Alexa-Fluor546 anti-rabbit IgG (for FOXO1), and Alexa-Fluor546 anti-guinea pig IgG (for insulin). Nuclei were stained with 1 μmol/l TO-PRO3 in PBS for 1 h.
For morphometric estimation of β-cell mass, insulin antibody binding on pancreatic sections was visualized with a second antibody coupled to horseradish peroxidase. The islet sizes (insulin-stained areas) of every 10th cryosection of a mouse pancreas were measured with the AxioVision LE documentation program (AxioVs40 LE V 4.4.0.0.; Carl Zeiss Vision).
Western blotting.
Isolated islets, excised mouse tissues, and INS-1E cells were lysed in buffer containing 125 mmol/l NaCl, 1% (v/v) Triton X-100, 0.5% sodiumdeoxycholate, 0.1% SDS, 10 mmol/l EDTA, 25 mmol/l HEPES pH 7.3, 10 mmol/l NaPP, 10 mmol/l NaF, 1 mmol/l Na-vanadate, 10 μg/ml pepstatin A, 10 μg/ml aprotinin, and 0.1 mmol/l phenylmethylsulfonyl fluoride. Cytosolic and nuclear fractions of INS-1E cells were prepared using a commercial kit (#78833; Pierce Biotechnology, Rockford, IL). The 10,000 g supernatant of homogenates or the cell fractions were subjected to a SDS-PAGE (8–12%) and blotted onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). The membranes were incubated overnight with primary antibodies against FOXO1, PKCδ, PKCα, PKCε, histone H1, and glyceraldehyde-3-phosphate dehydrogenase (each 1:1,000 in Tris-buffered saline [TBS] containing 5% milk, Santa Cruz Biotechnology); P-Ser256-FOXO1 and Tubulin (each 1:1,000 in TBS containing 5% BSA, Cell Signaling Technology); followed by incubation with a secondary antibody (horseradish peroxidase–linked anti-rabbit IgG; 1:2,000 in TBS containing 5% milk).
Pancreatic insulin content.
Insulin was extracted by acid ethanol from homogenized whole pancreata of 9-month-old mice.
Statistics.
Data are expressed as means ± SEM; P < 0.05 (unpaired Student t test) was considered to be statistically significant.
RESULTS
Overexpression of PKCδKN in mice protects against HFD-induced glucose intolerance.
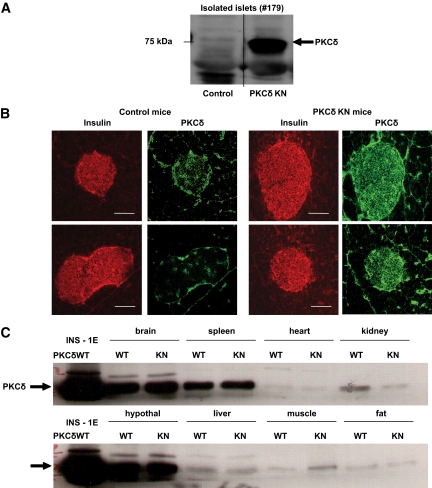
To define the physiological and molecular consequences of depleted PKCδ signaling in pancreatic β-cells, we established two transgenic mouse lines (#179 and #162) that overexpress PKCδKN in insulin-producing cells. PKCδKN overexpression was verified by specific immunoblotting of islet extracts containing equal amounts of total protein and by immunohistochemistry (Fig. 1A and B; supplemental Fig. S1A, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0512/DC1). The data revealed that PKCδKN was highly overexpressed in line #179 compared with line #162, and a low endogenous expression of PKCδ was detected in islets of wild-type controls. Immunoblotting of other tissues revealed that the overexpression of PKCδ was restricted to pancreatic β-cells (Fig. 1C; supplemental Fig. S1B).
FIG. 1.
Expression of PKCδ in islets and other tissues of control and PKCδKN-overexpressing mice. A: Detection of PKCδ by Western blotting of homogenates of isolated islets from control and PKCδKN mice (#179). B: In islet cells of pancreatic slices that stain positive for insulin (red), a low endogenous and a significant overexpression of PKCδ (green) is detected in control and PKCδKN-overexpressing mice (#179), respectively. C: Western blot analysis of tissue homogenates (50 μg mouse line #179) of control (WT) and PKCδKN-overexpressing (KN) mice reveals a high expression of PKCδ in brain, spleen, and hypothalamus (hypothal) but a low expression of PKCδ in heart, kidney, liver, muscle, and fat. As control, homogenate of INS-1E cells transfected with PKCδWT (15 μg) is blotted on the left. (A high-quality color digital representation of this figure is available in the online issue.)
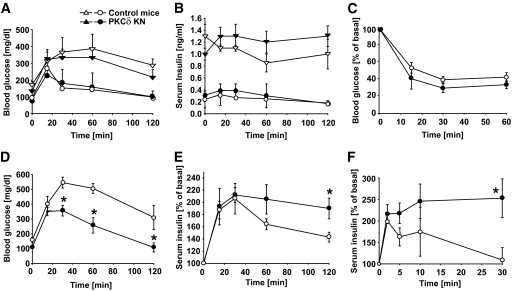
To study the impact of impaired PKCδ signaling in pancreatic β-cells on glucose homeostasis in vivo, glucose tolerance tests (ipGTT) were performed in both lines (Fig. 2; supplemental Fig. S1C and D). As depicted in Fig. 2A, control and transgenic mice (line #179) displayed the same increase in blood glucose levels at 6 weeks of age (circles). With aging, 9-month-old control and PKCδKN mice had comparable fasting glucose levels and similar body weight (Fig. 2A, triangles; supplemental Table S1) but seemed to be partly protected against age-related glucose intolerance because 120 min after a glucose load, plasma glucose levels were lower in 9-month-old PKCδKN animals compared with control mice; this was also true for the second transgenic line (Fig. 2A, triangles; supplemental Table S1 and Fig. S1C). However, the ipGTTs expressed as area under the curve (AUC glucose) were not significantly improved. Consistently, serum insulin increased up to 1.3 ± 0.13 ng/ml throughout the glucose tolerance test in PKCδKN animals, whereas aging control mice were not able to increase insulin secretion during the ipGTT (Fig. 2B, triangles).
FIG. 2.
Overexpression of PKCδKN in β-cells counteracts impaired glucose tolerance. A and B: Blood glucose and serum insulin concentrations were measured during ipGTT of control (▿, ○) and PKCδKN (▲, ●) mice at the age of 6 weeks (circles) and 9 months (triangles). C: Insulin sensitivity in control and PKCδKN-overexpressing mice fed HFD. Blood glucose concentrations were measured after injection of insulin (1 unit/kg body wt) at 0 min as described in details under research design and methods. Shown are means ± SEM of control mice (n = 3; ▿, ○) and PKCδKN mice (#179, n = 3; ▲, ●). D and E: Blood glucose and serum insulin concentrations were measured during ipGTT in mice fed HFD for 8 weeks. Results are expressed as means ± SEM of three to five mice. F: Serum insulin concentrations measured during ipGTT in mice fed HFD after injection of 3 g glucose/kg body wt; shown are the means ± SEM of n = 3 control mice (▿, ○) and n = 4 PKCδKN mice (#179, ▲, ●). *P < 0.05 against the value of control mice at the same time point.
To examine whether PKCδ plays a decisive role in fatty acid–mediated dysfunction of pancreatic β-cells in vivo, mice were fed HFD for 8 weeks. After HFD, body weight and insulin sensitivity were comparable between control and PKCδKN mice (Fig. 2C; supplemental Table S1). However, glucose levels during ipGTT, expressed as AUC glucose, were greatly improved in transgenic mice (control mice: 46.047 ± 8.837 mg · dl−1 · min−1 vs. PKCδKN: 28.907 ± 6.085 mg · dl−1 · min−1; n = 3, P < 0.05; Fig. 2D). In parallel, basal insulin levels tend to be higher in PKCδKN mice, and insulin secretion rose approximately twofold in the first 30 min and sustained at a significantly higher level up to 120 min in PKCδKN transgenic mice, whereas insulin levels declined already after 60 min in control animals (Fig. 2E). In glucose-stimulated insulin release, already after 30 min, serum insulin levels of PKCδKN mice remained significantly higher than in control mice after HFD feeding (Fig. 2F). As expected, transgenic mice that express low levels of PKCδKN (line #162) were less protected against HFD-impaired glucose tolerance (supplemental Fig. S1C and D). Therefore, further experiments were performed with islets of line #179.
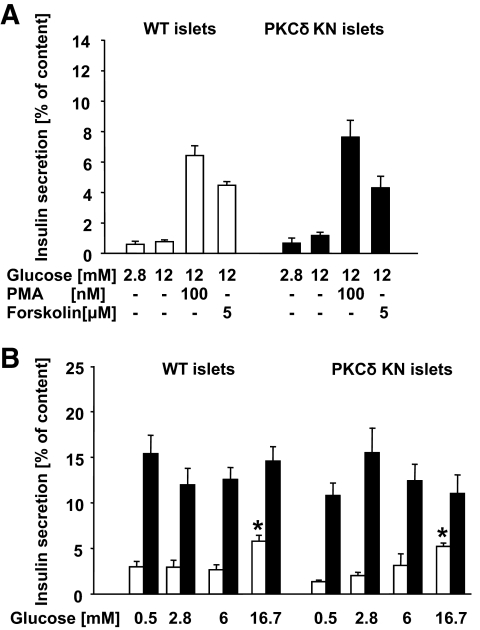
In isolated islets of wild-type and PKCδKN mice, insulin secretion was stimulated to the same extent by glucose, phorbol ester, and forskolin, and pretreatment with palmitate for 3 days resulted in the same glucose-independent hypersecretion (Fig. 3A and B). These results indicate that insulin secretion is not directly regulated by PKCδ.
FIG. 3.
Insulin secretion of isolated islets of control and PKCδKN-overexpressing mice. Islets were isolated from control (WT) and PKCδKN-overexpressing mice (#179) and treated as described in details under research design and methods. Substances were added as indicated (PMA). Results are presented as means ± SEM of n = 12 observations from three independent experiments. A: Insulin secretion expressed as perdent of content of islets of control mice (□) and of PKCδKN mice (#179, ■). B: glucose-dependent secretion after 3 days control culture (□) and after 3 days culture in the presence of 0.6 mmol/l palmitate (■). *Significant difference to the respective secretion at 0.5 mmol/l glucose.
PKCδKN overexpression augments β-cell mass and protects against apoptotic cell death induced by HFD and palmitate.
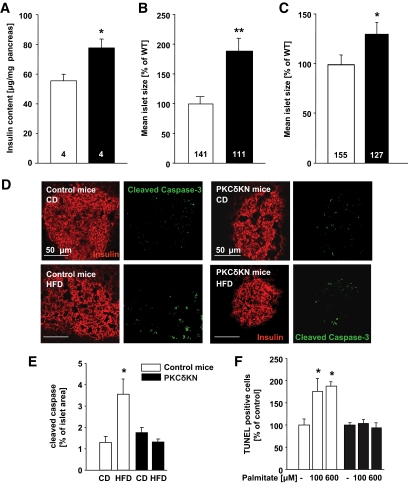
As the improvement of insulin secretion in vivo in PKCδKN-overexpressing mice may result from an increased insulin disposability due to increased β-cell mass, pancreatic insulin content and islet size were assessed. Indeed, in 9-month-old transgenic mice, total pancreatic insulin content was significantly higher than in control littermates (Fig. 4A). In addition, according to insulin staining the mean islet size was nearly doubled in old PKCδKN transgenic animals, and the islets of transgenic mice fed HFD were also significantly larger than that of control animals (Fig. 4B and C). However, BrdU and Ki67 staining suggested no increase in proliferation rates (data not shown).
FIG. 4.
Increased pancreatic insulin content and islet size and protection against HFD- and palmitate-induced cell death in PKCδKN-overexpressing mice. A: Insulin content of whole pancreatic extracts. B: Mean islet size assessed in pancreatic slices of aged (>9 months) control (□) and PKCδKN-overexpressing (#179, ■) mice fed standard chow. C: Mean islet size assessed in pancreatic slices of 3-month-old control (□) and PKCδKN-overexpressing (#179, ■) mice fed HFD. Results are presented as means ± SEM of n = 4 pancreata (A) and of the number of islets as indicated in each column from two mice (B and C). Significant difference to control mice is indicated by *P < 0.05 and **P < 0.005. D: Representative pictures of insulin (red) and cleaved caspase-3 (green) staining in pancreatic slices of control and PKCδKN mice after either chow (CD, upper panels) or HFD (lower panels) feeding. E: Means ± SEM of cleaved caspase-3–positive cells in islets (n = 30–40) of control (□) and PKCδKN (■) mice. F: Isolated islet cells were cultured for 3 days under control culture conditions and in the presence of 0.6 mmol/l palmitate as indicated. The percentage of apoptotic TUNEL-positive cells is expressed as means ± SEM of n = 3 independent experiments. (A high-quality color digital representation of this figure is available in the online issue.)
As apoptosis modulates β-cell mass, the effect of PKCδKN overexpression on cell death was examined next. In pancreatic slices from wild-type but not from PKCδKN mice, cleaved caspase-3 staining was increased almost threefold after HFD feeding (Fig. 4D and E). Inhibition of apoptosis by overexpression of PKCδKN was further confirmed in cultured isolated islet cells pretreated with palmitate. In cells of PKCδKN mice, palmitate did not change the percentage of apoptotic cells, whereas in control cells palmitate doubled the amount of TUNEL-positive cells (Fig. 4F). Therefore, the inhibition of palmitate-induced cell death in PKCδKN-overexpressing cells suggests that PKCδ indeed transmits palmitate-mediated β-cell dysfunction.
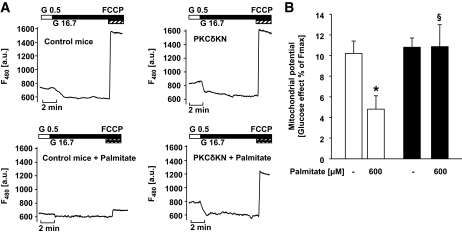
Apoptotic activation of caspase-3 implies cytochrome c release and mitochondrial depolarisation. To examine the integrity of mitochondria, glucose-induced hyperpolarization was assessed. The rise of glucose from 0.5 to 16.7 mmol/l hyperpolarized mitochondria in isolated islet cells from control and PKCδKN mice to the same extent. After pretreatment with palmitate, the effect of glucose was reduced by 60% in islet cells of control but not of PKCδKN mice (Fig. 5A and B). These results indicate that PKCδ affects mitochondrial function.
FIG. 5.
Overexpression of PKCδKN protects against palmitate-induced mitochondrial dysfunction. Isolated islet cells were cultured for 3 days under control culture conditions and in the presence of 0.6 mmol/l palmitate as indicated. A: Shown are representative traces of measurements of mitochondrial potential after rhodamine-123 staining. B: The hyperpolarizing effect of 16.7 mmol/l glucose expressed as percent of the maximal increase in fluorescence measured after addition of carbonylcyanide-p-trifluoromethoxyphenylhydrazone (1 μmol/l FCCP) is given as means ± SEM of n = 18–30 cells of three independent experiments. *P < 0.05 against control; §P < 0.05 against islet cells of control mice cultured under the same condition.
PKCδKN overexpression interferes with FOXO1 regulation.
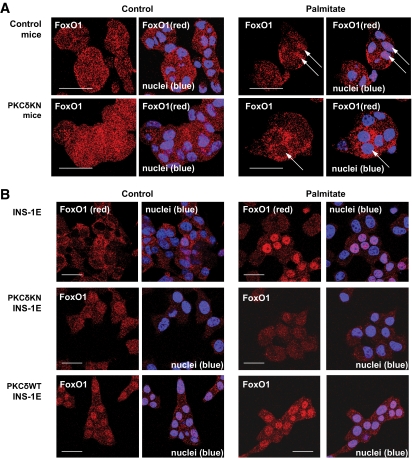
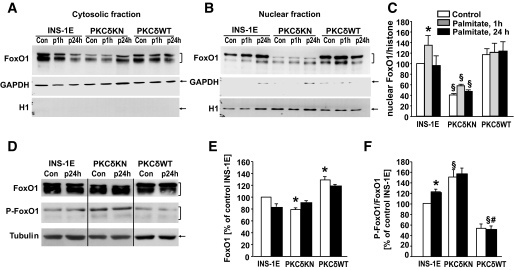
Because it has been described that the dominant-negative transcription factor FOXO1 inhibited FFA-dependent β-cell death (14), experiments were performed to examine whether nuclear translocation of FOXO1 is altered by PKCδKN. In control islet cells, immunostaining of FOXO1 was homogenously distributed throughout the cell. Palmitate triggered FOXO1 accumulation into the nucleus, and this effect was inhibited in cells overexpressing PKCδKN (Fig. 6A). That PKCδ affects nuclear translocation of FOXO1 is further confirmed by experiments performed with the insulin-secreting cell line INS-1E overexpressing either wild-type PKCδ (PKCδWT) or PKCδKN (Fig. 6B; supplemental Fig. S2). Transfection did not alter the expression of PKCε and PKCα (supplemental Fig. S2), whereas other novel PKCs (PKCθ and PKCη) remained undetectable (data not shown). In control INS-1E cells FOXO1 accumulated in the nucleus after palmitate treatment (Fig. 6B and 7A–C). Overexpression of PKCδWT resulted in an increased nuclear staining of FOXO1 already under control conditions, whereas overexpression of PKCδKN inhibited nuclear accumulation of FOXO1 (Fig. 6B and 7A–C). Because Jun NH2-terminal kinase (JNK) inhibition by SP600125 did not affect FOXO1 accumulation induced by palmitate in either control INS-1E cells or PKCδWT–INS-1E cells, it is suggested that FOXO1 accumulation does not depend on JNK activity (supplemental Fig. S3). Because phosphorylation of FOXO1 at Ser256 stimulates its nuclear extrusion and degradation, the amount of FOXO1 protein and its phosphorylation were examined in control and transfected INS-1E cells. Indeed, increased phosphorylation of FOXO1 and significantly lower protein levels were detected in PKCδKN–INS-1E cells, whereas reduced phosphorylation of FOXO1 and increased FOXO1 protein levels were found in PKCδWT–INS-1E cells (Fig. 7D–F). In summary, these data suggest that overexpression of PKCδKN in β-cells inhibits palmitate-mediated β-cell dysfunction by protecting against mitochondrial dysfunction and apoptosis while counteracting nuclear accumulation of FOXO1.
FIG. 6.
Palmitate-mediated nuclear accumulation of FOXO1 was inhibited by overexpression of PKCδKN in insulin secreting cells. A: Islet cells from control and PKCδKN mice and B: control INS-1E cells and INS-1E cells overexpressing PKCδWT or PKCδKN were cultured under control conditions or in the presence of 0.6 mmol/l palmitate for 1 day. Representative pictures of immunostaining of FOXO1 (A and B, red). Nuclei are stained with TO-PRO3 (A and B, blue). (A high-quality color digital representation of this figure is available in the online issue.)
FIG. 7.
Overexpression of PKCδWT and PKCδKN changes FOXO1 cellular distribution, phosphorylation, and protein concentration in insulin-secreting cells. INS-1E cells and INS-1E cells overexpressing PKCδWT or PKCδKN were cultured under control conditions and in the presence of 0.6 mmol/l palmitate for 1 h and 1 day. A: Representative Western blot for FOXO1 detection in cytosolic fractions. B and C: Representative Western blot for FOXO1 detection in nuclear fractions with means ± SEM of n = 4 independent experiments. D–F: Representative Western blot for FOXO1, P-Ser256-FOXO1, and tubulin (as loading control) of cell homogenates with means ± SEM of n = 4 independent experiments of relative amounts of FOXO1 and phosphorylation of FOXO1 (P-FOXO1/FOXO1) by setting the respective bands of control INS-1 cells to 100%. *Significant difference to INS-1E under control condition; §significant difference to the respective condition of INS-1E; #significant difference to PKCδKN at the same condition.
DISCUSSION
The observation that overexpression of PKCδKN in β-cells protects against HFD-induced glucose intolerance strongly supports the idea that PKCδ plays a central role in lipotoxicity. Indeed, significantly lower blood glucose and increased plasma insulin concentrations during HFD were observed in PKCδKN-overexpressing mice, whereas animals fed standard chow displayed no significant difference.
Our study, therefore, differs from observations in whole-body PKCδ knockout (PKCδKO) mice that displayed glucose intolerance (9). The directly opposed results may be explained first by the difference between whole-body knockout versus β-cell–specific inhibition of PKCδ because, especially in brain tissue, large amounts of PKCδ are expressed. To exclude alterations in other tissues than ß-cells, we therefore restricted expression of the transgene to insulin-secreting cells. Moreover, the most significant effect of PKCδKN overexpression was observed after high-fat feeding in our study, a condition that was not studied in PKCδKO mice.
The conclusion that PKCδKN protects against lipotoxicity in vivo was further substantiated by the fact that the percentage of islet cells that stained positive for cleaved caspase-3 after HFD was not increased and that palmitate-induced apoptotic cell death was inhibited in PKCδKN-expressing β-cells. Because we were unable to detect proliferation, i.e., BrdU incorporation or Ki67-positive cells in pancreatic slices of HFD-fed mice (data not shown), it is anticipated that PKCδKN inhibits cell death in insulin-secreting cells; this effect contributes to the increase in islet size and the increase in pancreatic insulin content. This is in contrast to studies that demonstrated that PKCδ exerts stimulatory or inhibitory effects on cell proliferation. These opposing effects of PKCδ may depend on the cell cycle status of the respective cell (15).
Because β-cell mass depends on proper insulin/IGF-1 receptor signaling, interference of PKCδ with these cascades may belong to its proapoptotic effect. Indeed, Wrede et al. (5) reported that PKC stimulation by PMA inhibits protein kinase B (PKB)/Akt activation in insulin-secreting cells and described a reduced binding of insulin receptor substrate (IRS)-2 to p85 after PMA stimulation of the cells. This observation may be explained by putative PKCδ-mediated serine/threonine phosphorylations of IRS-2 that inhibit IRS-2 activation in parallel to known PKCδ phosphorylation sites in IRS-1 (16). Further experiments are needed to clarify whether PKCδ mediates changes of IRS-2 activity via serine phosphorylation that reduce PKB/Akt activation.
In β-cells, the mitochondrial potential plays a central role in normal glucose responsiveness of insulin secretion, whereas the collapse of mitochondrial potential is an essential step of the intrinsic pathways of apoptotic cell death (17,18). The protective effect of PKCδKN on mitochondrial potential strengthens the idea that PKCδ interferes with mitochondrial function. In line with this concept, salivary epithelial cells overexpressing PKCδKN were resistant to the loss of mitochondrial potential and apoptosis induced by etoposide, ultraviolet irradiation, bredfeldinA, and paclitaxel (19). Furthermore, it has been shown that PKCδ interacts with the tyrosine kinase cAbl. This complex transfers the endoplasmic reticulum (ER) stress to mitochondria, which further leads to apoptotic cell death (20,21). Although we cannot exclude direct effects of PKCδ in mitochondria and in ER, our data favor the idea that PKCδ leads to nuclear accumulation of FOXO1 with palmitate treatment. In fact, it has been previously demonstrated that FFA-induced ER stress and apoptosis are inhibited in insulin-secreting cells expressing the dominant-negative form of FOXO1 (14). Activation of FOXO1 leads to multiple cellular changes and plays a major role in stress resistance and survival of β-cells (22–27). In contrast, inhibition of FOXO1 is necessary for adaptive β-cell proliferation during insulin resistance (28,29). Our study therefore provides strong evidence that FFAs stimulate nuclear sequestration of FOXO1 through the activation of PKCδ because the nuclear accumulation of FOXO1 is inhibited in cells overexpressing PKCδKN. Moreover, phosphorylation of FOXO1 at the PKB/AKT phosphorylation site Ser256 is reduced in cells overexpressing PKCδWT but increased in cells expressing PKCδKN. The signaling pathway through PKCδ that leads to reduced Ser256 phosphorylation of FOXO1 may involve activation of inhibitory pathways such as JNK and protein phosphatase 2A (30,31). However, JNK inhibition did not affect nuclear FOXO1 accumulation induced by palmitate, suggesting a PKCδ-dependent but JNK-independent mechanism.
Our study delivers in vivo evidence that activation of PKCδ by FFAs mediates β-cell failure. Because PKCδKN inhibited FFA-induced nuclear accumulation of FOXO1, mitochondrial dysfunction, and apoptosis, it is anticipated that the underlying mechanism involves PKCδ-dependent chronic activation of FOXO1. In fact, a gene variation of FOXO1 associates with impaired glucose tolerance and type 2 diabetes in humans (32).
Supplementary Material
Acknowledgments
The study was supported by Deutsche Forschungsgemeinschaft grants UL140/7-1, GRK1302/1, and SFB685 and grants from the European Community (FP6: EUGENE2, LSHM-CT-2004-512013) and the Instituto Salud Carlos III (CIBER de Diabetes y Enfermedades Metabólicas Asociadas), Spain. D.M. was a PhD student of the international graduate school (GRK1302/1).
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
We thank Sieglinde Haug, Elisabeth Metzinger, and Dorothee Neuscheler as well as students Christian Klingler and Nico Trautwein for excellent technical help. We acknowledge the fruitful discussion with Dr. Martin Röcken.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 1.
REFERENCES
- 1.El Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M: Saturated fatty acids synergize with elevated glucose to cause pancreatic β-cell death. Endocrinol 2003;144:4154–4163 [DOI] [PubMed] [Google Scholar]
- 2.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL: Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinol 2004;145:5087–5096 [DOI] [PubMed] [Google Scholar]
- 3.Eitel K, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, Häring HU, Kellerer M: Different role of saturated and unsaturated fatty acids in β-cell apoptosis. Biochem Biophys Res Commun 2002;299:853–856 [DOI] [PubMed] [Google Scholar]
- 4.Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY: Distinct effects of saturated and monounsaturated fatty acids on β-cell turnover and function. Diabetes 2001;50:69–76 [DOI] [PubMed] [Google Scholar]
- 5.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ: Fatty acid and phorbol ester-mediated interference of mitogenic signaling via novel protein kinase C isoforms in pancreatic β-cells (INS-1). J Mol Endocrinol 2003;30:271–286 [DOI] [PubMed] [Google Scholar]
- 6.Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, Brendel MD, Bretzel RG, Häring HU, Kellerer M: Protein kinase Cδ activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes 2003;52:991–997 [DOI] [PubMed] [Google Scholar]
- 7.Carpenter L, Cordery D, Biden TJ: Inhibition of protein kinase C δ protects rat INS-1 cells against interleukin-1β and streptozotocin-induced apoptosis. Diabetes 2002;51:317–324 [DOI] [PubMed] [Google Scholar]
- 8.Welters HJ, Smith SA, Tadayyon M, Scarpello JH, Morgan NG: Evidence that protein kinase Cδ is not required for palmitate-induced cytotoxicity in BRIN-BD11 β-cells. J Mol Endocrinol 2004;32:227–235 [DOI] [PubMed] [Google Scholar]
- 9.Uchida T, Iwashita N, Ohara-Imaizumi M, Ogihara T, Nagai S, Choi JB, Tamura Y, Tada N, Kawamori R, Nakayama KI, Nagamatsu S, Watada H: Protein kinase Cδ plays a non-redundant role in insulin secretion in pancreatic β cells. J Biol Chem 2007;282:2707–2716 [DOI] [PubMed] [Google Scholar]
- 10.Li W, Yu JC, Shin DY, Pierce JH: Characterization of a protein kinase C-δ (PKC-δ) ATP binding mutant. An inactive enzyme that competitively inhibits wild type PKC-δ enzymatic activity. J Biol Chem 1995;270:8311–8318 [DOI] [PubMed] [Google Scholar]
- 11.George M, Ayuso E, Casellas A, Costa C, Devedjian JC, Bosch F: β-cell expression of IGF-I leads to recovery from type 1 diabetes. J Clin Invest 2002;109:1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullrich S, Berchtold S, Ranta F, Seebohm G, Henke G, Lupescu A, Mack AF, Chao CM, Su J, Nitschke R, Alexander D, Friedrich B, Wulff P, Kuhl D, Lang F: Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes 2005;54:1090–1099 [DOI] [PubMed] [Google Scholar]
- 13.Ranta F, Avram D, Berchtold S, Düfer M, Drews G, Lang F, Ullrich S: Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes 2006;55:1380–1390 [DOI] [PubMed] [Google Scholar]
- 14.Martinez SC, Tanabe K, Cras-Méneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA: Inhibition of Foxo1 protects pancreatic islet β-cells against fatty acid and endoplasmic reticulum stress–induced apoptosis. Diabetes 2008;57:846–859 [DOI] [PubMed] [Google Scholar]
- 15.Jackson DN, Foster DA: The enigmatic protein kinase Cδ: complex roles in cell proliferation and survival. FASEB J 2004;18:627–636 [DOI] [PubMed] [Google Scholar]
- 16.Waraich RS, Weigert C, Kalbacher H, Hennige AM, Lutz S, Haring HU, Schleicher ED, Voelter W, Lehmann R: Phosphorylation of Ser357 of rat insulin receptor substrate-1 mediates adverse effects of protein kinase C-δ on insulin action in skeletal muscle cells. J Biol Chem 2008;283:11226–11233 [DOI] [PubMed] [Google Scholar]
- 17.Orrenius S: Mitochondrial regulation of apoptotic cell death. Toxicol Lett 2004;149:19–23 [DOI] [PubMed] [Google Scholar]
- 18.Wiederkehr A, Wollheim CB: Minireview: implication of mitochondria in insulin secretion and action. Endocrinol 2006;147:2643–2649 [DOI] [PubMed] [Google Scholar]
- 19.Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME: PKCδ is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem 2001;276:29719–29728 [DOI] [PubMed] [Google Scholar]
- 20.Qi X, Mochly-Rosen D: The PKCδ-Abl complex communicates ER stress to the mitochondria: an essential step in subsequent apoptosis. J Cell Sci 2008;121:804–813 [DOI] [PubMed] [Google Scholar]
- 21.Ito Y, Pandey P, Mishra N, Kumar S, Narula N, Kharbanda S, Saxena S, Kufe D: Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis. Mol Cell Biol 2001;21:6233–6242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glauser DA, Schlegel W: The emerging role of FOXO transcription factors in pancreatic β cells. J Endocrinol 2007;193:195–207 [DOI] [PubMed] [Google Scholar]
- 23.Buteau J, Accili D: Regulation of pancreatic β-cell function by the forkhead protein FoxO1. Diabetes Obes Metab 2007;9(Suppl. 2):140–146 [DOI] [PubMed] [Google Scholar]
- 24.Kitamura T, Ido Kitamura Y: Role of FoxO proteins in pancreatic β cells. Endocr J 2007;54:507–515 [DOI] [PubMed] [Google Scholar]
- 25.Taguchi A, White MF: Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol 2008;70:191–212 [DOI] [PubMed] [Google Scholar]
- 26.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ: Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic β-cells (INS-1). J Biol Chem 2002;277:49676–49684 [DOI] [PubMed] [Google Scholar]
- 27.Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D: FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab 2005;2:153–163 [DOI] [PubMed] [Google Scholar]
- 28.Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D: Role of the forkhead protein FoxO1 in β cell compensation to insulin resistance. J Clin Invest 2006;116:775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakae J, Biggs WH, 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D: Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 2002;32:245–253 [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Kanthasamy A, Yang Y, Anantharam V, Kanthasamy A: Protein kinase C δ negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci 2007;27:5349–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y: The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem 2006;281:1091–1098 [DOI] [PubMed] [Google Scholar]
- 32.Müssig K, Staiger H, Machicao F, Stancáková A, Kuusisto J, Laakso M, Thamer C, Machann J, Schick F, Claussen CD, Stefan N, Fritsche A, Häring HU: Association of common genetic variation in the FOXO1 gene with β-cell dysfunction, impaired glucose tolerance, and type 2 diabetes. J Clin Endocrinol Metab 2009;94:1353–1360 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.