Abstract
OBJECTIVE
The objectives of the study were to determine whether the cell cycle transcription factor, FoxM1, is required for glucose homeostasis and β-cell mass expansion in maternal islets during pregnancy and whether FoxM1 is essential for placental lactogen (PL)-induced β-cell proliferation.
RESEARCH DESIGN AND METHODS
β-Cell mass, β-cell proliferation, and glucose homeostasis were assessed in virgin, pregnant, and postpartum mice with a pancreas-wide Foxm1 deletion (FoxM1Δpanc). Wild-type islets were cultured with or without PL and examined for Foxm1 induction. Transgenic mice overexpressing PL in β-cells were bred with FoxM1Δpanc mice, and β-cell proliferation was examined.
RESULTS
Foxm1 was upregulated in maternal islets during pregnancy. In contrast to controls, β-cell proliferation did not increase in pregnant FoxM1Δpanc females. Mutant islets showed increased Menin and nuclear p27. FoxM1Δpanc females developed gestational diabetes mellitus as pregnancy progressed. After parturition, euglycemia was restored in FoxM1Δpanc females, but islet size was significantly reduced. Strikingly, β-cell mass was normal in postpartum FoxM1Δpanc pancreata due to a combination of increased β-cell size and islet neogenesis. Evidence for neogenesis included increased number of endocrine clusters, increased proportion of smaller islets, and increased neurogenin 3 or insulin expression in cells adjacent to ducts. PL induced Foxm1 expression in cultured islets, and FoxM1 was essential for PL-mediated increases in β-cell proliferation in vivo.
CONCLUSIONS
FoxM1 is essential for β-cell compensation during pregnancy. In the absence of increased β-cell proliferation, neogenesis is induced in postpartum FoxM1Δpanc pancreata. Our results suggest that FoxM1 functions downstream of PL to mediate its effects on β-cell proliferation.
Gestational diabetes mellitus (GDM) occurs in 3–7% of pregnancies (1,2). GDM onset typically occurs in the second trimester due to increased insulin resistance and inadequate β-cell compensation, similar to type 2 diabetes. GDM increases the risk of type 2 diabetes later in life, and increases risk for pregnancy complications such as preeclampsia and cesarean sections. Infants born to mothers with GDM are at higher risk for macrosomia. Postnatally, these infants are at risk of developing hypoglycemia, hypocalcemia, polycythemia, jaundice, and respiratory distress syndrome (2,3). Individuals born to mothers with GDM have higher risk of obesity and type 2 diabetes as adults (2). GDM has a strong genetic component, clustering in families and particular minority ethnic groups; common variants in several genes (KCNJ11, GK, and HNF4a) increase the risk of GDM (2,4,5). β-Cell mass adapts to physiological needs and increased functional demands (6–8). Changes in β-cell mass can be achieved by hyperplasia (increased cell number), hypertrophy (increased cell size), and neogenesis from progenitors. Pathways involved in maintaining or augmenting β-cell mass may be affected in diabetic individuals. This concept is reinforced by the recent finding that polymorphisms in genes that regulate proliferation, such as TCF7L2 and CDKN2, are linked with type 2 diabetes in humans (9,10). Although adult pancreatic ducts retain the ability to generate new endocrine cells in response to certain stimuli (11,12), most new adult β-cells arise from preexisting β-cells (13,14).
During pregnancy, maternal insulin demands increase due to the insulin resistance related to weight gain and placental hormone production, increased fetal burden, and increased food intake (7,15). Maternal islets adapt to this increased demand mainly through enhanced insulin secretion per β-cell and increased β-cell proliferation (15). β-Cell mass expands by 50% during pregnancy. The main stimuli of β-cell proliferation during pregnancy are placental lactogens (PLs), although prolactin (Prl) and growth hormone (GH) have similar effects on β-cells and are also elevated during pregnancy (6,15,16). Transgenic mice expressing PL within β-cells (RIP-mPL) exhibit increased β-cell proliferation and a doubling of β-cell mass (17).
Very little is known about the molecular effectors that act downstream of lactogenic hormones to regulate β-cell proliferation. Lactogens repress expression of the transcriptional coactivator, Menin (18,19), and transgenic overexpression of Menin in β-cells resulted in GDM due to decreased maternal β-cell proliferation. Menin target genes include p18 and p27, both cell cycle inhibitors (18,19). These studies provide a link between a known inducer of β-cell proliferation and inhibition of a cell cycle “brake.” Whether PL or other pregnancy hormones activate cell cycle “accelerators” in β-cells is currently unknown. A thorough understanding of molecular regulators of β-cell mass may lead to strategies for enhancing β-cell proliferation in individuals prone to GDM and type 2 diabetes.
The FoxM1 transcription factor is highly expressed in proliferating cells (20). FoxM1 directly activates genes involved in DNA synthesis, karyokinesis, and cytokinesis, including Cdc25A, Cdc25B, cyclin B1 (21–23), CENP-F (24), polo-like kinase 1 (Plk-1), and Aurora B kinase (25). FoxM1 target genes (Skp2, Cks1, KIS) also prevent nuclear localization of the Cdk inhibitor and Menin target gene, p27kip1 (26,27). FoxM1 is highly expressed in embryonic and neonatal pancreatic endocrine cells, but expression decreases as animals age (28). We previously showed that FoxM1 regulates postnatal β-cell proliferation and β-cell mass. Male mice lacking FoxM1 in their entire pancreas (FoxM1Δpanc) displayed a 75% reduction in β-cell mass at 9 weeks and were overtly diabetic (28). FoxM1Δpanc female mice maintained glucose homeostasis, despite a similar decrease in β-cell mass (12,28). We predicted that decreased FoxM1 activity could result in an inability to expand β-cell mass during times of increased metabolic demand. Consistent with this idea, we recently reported that FoxM1Δpanc females have decreased β-cell regeneration and impaired β-cell replication after partial pancreatectomy (PPx) (12).
FoxM1 activity is also required for liver regeneration in response to GH (29). We therefore hypothesized that FoxM1 would be required for the hormone-induced increase in β-cell replication during pregnancy. In the current study, FoxM1Δpanc females were used to examine the role of FoxM1 in β-cell mass expansion and maintenance of glucose homeostasis during pregnancy. Foxm1 expression was upregulated in maternal islets during pregnancy, and pregnant FoxM1Δpanc females showed decreased β-cell mass compared with controls. β-Cell replication failed to increase in mutant mice during pregnancy, resulting in overt GDM. Thus, FoxM1 plays a critical role in β-cell adaptation to pregnancy. Interestingly, islets from FoxM1Δpanc females showed sustained defects after parturition including decreased average islet size. However, β-cell mass was restored to normal in postpartum FoxM1Δpanc females, likely due to stimulation of islet neogenesis. In isolated islets, PL treatment induced Foxm1 expression, suggesting that FoxM1 acts downstream of PL and may mediate its effects on β-cell mass regulation. Strongly supporting this hypothesis, we show that inactivation of Foxm1 in pancreata of RIP-mPL transgenic mice completely prevents PL-mediated induction of β-cell proliferation.
RESEARCH DESIGN AND METHODS
Mice.
Foxm1fl/fl and FoxM1Δpanc mice and genotyping are described elsewhere (23,25,28). RIP-mPL transgenic mice were as previously described (17). All studies were performed with the approval of and in compliance with the Vanderbilt Institutional Animal Care and Use Committee.
Tissue preparation and histology.
Pancreata were fixed in 4% paraformaldehyde at 4°C for 4 h. Tissues were dehydrated, embedded in paraffin, and sectioned at 5 μm. Primary antibodies used are as follows: guinea pig anti-bovine insulin (Linco), 1:1,000; rat anti-bromodeoxyuridine (BrdU; Accurate Chemical & Scientific), 1:400; rabbit anti-Glut2 (Alpha Diagnostic), 1:500; mouse anti-Kip1/p27 (BD Biosciences), 1:100; mouse anti-neurogenin 3 (NGN3; Developmental Studies Hybridoma Bank), 1:100; and rabbit anti-cytokeratin (Dako), 1:1,000. Incubations were overnight in a humid chamber at 4°C. For BrdU detection, slides were treated with 1.5 N HCl for 20 min at 37°C, neutralized in sodium borate buffer for 1 min at room temperature, and treated with 0.005 mg/ml trypsin (Sigma-Aldrich) and 0.005 mg/ml CaCl2 (in Tris buffer; pH 7.5) for 3 min at 37°C. Vectastain ABC kit (Vector Labs) was used for immunohistochemical labeling of insulin for β-cell mass measurement. The Histomouse-SP kit (Invitrogen) was used for detection of p27. NGN3 and cytokeratin immunolabeling was performed as previously described (12). Donkey anti-guinea pig cyanin 2 (Cy2) and donkey anti-rat Cy3 were used as secondary antibodies at a 1:500 dilution. Mounting medium contained 1.5 μg/ml nuclear fluorogen DAPI (Molecular Probes).
Samples were viewed under bright-field illumination or appropriate optical filters (immunofluorescence) using an Olympus BX41 microscope (Tokyo, Japan) and digital camera with the Magnafire program (Optronics). Tagged image file format (TIFF) images from each experiment were processed equivalently in Adobe Photoshop.
β-Cell mass.
Entire pancreata were removed, weighed, and fixed as above. Five-micron longitudinal sections were prepared for insulin immunoperoxidase labeling and eosin counterstaining. Every 30th section (an average of 8–13 sections per pancreas) was used. Images of anti-insulin–labeled sections were scanned using a Nikon Super CoolScan 9000. Using Metamorph 6.1 software (Molecular Devices), β-cell mass was measured by obtaining the fraction of cross-sectional area of pancreatic tissue positive for insulin and multiplying this by the pancreatic weight (n = 3 animals of each genotype at each stage).
β-Cell replication.
BrdU (100 mg/kg; Sigma-Aldrich) was injected intraperitoneally every 2 h three times prior to pancreas tissue processing. BrdU-labeled β-cells were detected by double immunolabeling with insulin antibodies and DAPI to visualize nuclei. Using Metamorph, BrdU-positive and -negative nuclei in insulin-positive cells were counted at ×400 magnification. At least 3,000 β-cells were counted for each of three animals of each genotype at each stage. The proportion of BrdU-positive β-cell nuclei to total β-cell nuclei was calculated and represents the percentage of β-cells that have recently gone through S-phase.
β-Cell size.
Pancreas sections were colabeled for Glut2 and insulin as described above. Every islet from one section on each slide was photographed. Using Metamorph, the area of more than 1,000 individual β-cells was determined per mouse.
Total pancreatic and plasma insulin content.
Dissected pancreata were rinsed in PBS, blotted with filter paper, weighed, and homogenized (Polytron PT 10/35; Brinkmann Instruments) in 1 ml of acid alcohol. The homogenate was extracted with an additional 5 ml of acid alcohol for 48 h at 4°C and centrifuged at 2,500 rpm for 30 min. Supernatants were assayed for insulin by radioimmunoassay (30). Insulin concentrations were normalized to pancreas wet weight.
For plasma insulin content, blood samples were collected at 0 and 30 min after glucose injection during an intraperitoneal glucose tolerance test (IPGTT) from the saphenous vein. The plasma supernatant was analyzed using the Ultrasensitive ELISA kit (Alpco).
IPGTT.
IPGTTs were performed on Foxm1fl/fl and FoxM1Δpanc virgin females and at gestational day (GD) 12.5 and GD15.5 as described (28). Controls for these studies were Foxm1fl/fl mice.
Islet RNA isolation and quantitative real-time RT-PCR.
Islets were isolated from virgin, GD14.5, and postpartum day 8 (P8) females by intraductal collagenase perfusion. At least three mice were used per group. Total islet RNA (75–125 islets/mouse) was extracted using the RNAqueous kit (Ambion). RNA concentration and integrity were assessed using the ND-1000 Spectrophotometer (NanoDrop) and the 2100 Electrophoresis Bioanalyzer (Agilent).
cDNA was synthesized using the Superscript III First-Strand synthesis system (Invitrogen). Reactions were carried out in duplicate with iQ SYBR Green supermix (Bio-Rad) at an annealing temperature of 58°C. Data were collected using an iCycler iQ Real-Time PCR Detection System (Bio-Rad) and software (Bio-Rad). Primers optimized by melting curve analysis were generated as follows: Foxm1 (forward, cacttggattgagaccactt; reverse, gtcgtttctgctgtgattcc), Bcl-xl (forward, ccttggatccaggagaacg; reverse, caggaaccagcggttgaa), and hypoxanthine-guanine phosphoribosyltransferase (Hprt) (forward, agtcaacgggggacataaaa; reverse, tgcattgttttaccagtgtcaa). Expression levels were normalized against the levels of Hprt, a ubiquitously expressed gene that shows little change during cellular growth or differentiation. Results were analyzed using the 2−ΔΔCt method (31).
Western blotting.
Islets from 8- to 12-week-old virgin and GD15.5 Foxm1fl/fl and FoxM1Δpanc females were lysed and sonicated with a Virsonic 100 sonicator (Virtis Company). The supernatant was quantified using the Bio-Rad DC protein assay (Bio-Rad). Western blotting was done as previously described using 10 μg islet protein/lane from one animal (32). Membranes were probed with primary antibodies diluted in 3% nonfat milk in Tris-buffered saline (TBS) overnight at 4°C: goat anti-Menin (Bethyl Laboratories) 1:1,000; rabbit anti-Pdx1 (a gift from Dr. Chris Wright, Vanderbilt University), 1:10,000; mouse anti-p27 (BD Biosciences), 1:2000; and mouse anti–β-actin (Santa Cruz Biotechnology), 1:10,000. Peroxidase-conjugated species-specific secondary antibodies (Jackson ImmunoResearch Laboratories) were diluted to 1:5,000 in 3% nonfat milk in TBS and incubated for 1 h at room temperature. Protein levels in individual lanes were quantified using ImageJ 1.42 (National Institutes of Health [NIH]) and normalized to the level of β-actin signal in corresponding lanes. Three to five animals per group were analyzed.
In vitro islet culture.
Islets from 8- to 12-week-old Foxm1fl/fl virgin females were cultured for 4 days in 1× RPMI 1640 supplemented with 10% horse serum, 11 mmol/l glucose, 100 units/ml penicillin, and 100 μg/ml streptomycin with or without 500 ng/ml human PL (National Hormone and Peptide Program). An average of 50–60 islets were plated in one 60 × 15-mm non–tissue culture–treated Petri dish at 37°C in 5% CO2. Cultures were replenished with fresh medium with or without PL after 48-h incubation. After 4 days, RNA was extracted and subjected to quantitative (q)RT-PCR analysis.
β-Cell apoptosis.
Transferase-mediated dUTP nick-end labeling was performed on paraffin sections of adult pancreata using the In Situ Cell Death Detection kit (Roche) and followed by immunofluorescent staining of insulin. Cleaved caspase-3 was detected using an antibody raised in rabbit (Cell Signaling Technology) at a dilution of 1:1,500. Immunoreactivity was detected using the Vectastain ABC and DAB kits (Vector Labs).
Statistical analysis.
Data were analyzed by Student t test or two-way ANOVA with Bonferroni post-tests, using GraphPad Prism V5.01. A value of P ≤ 0.05 was considered significant.
RESULTS
Foxm1 expression is increased in islets during pregnancy.
In pregnant mice, maternal β-cell replication peaks at GD14.5 (18) (supplementary Fig. 1, which is available in the online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0050/DC1). β-Cell mass expansion during pregnancy is due mainly to stimulation of proliferation by elevated circulating pregnancy hormones, including, GH, Prl, and PL (6,15,16), as well as via cell hypertrophy (8,33). We previously showed that FoxM1 is required for postnatal β-cell mass expansion and proliferation and is also necessary for β-cell mass regeneration after PPx (12,28). In the liver, FoxM1 activity is required for GH-mediated stimulation of liver regeneration (29). Thus, we hypothesized that FoxM1 would be required for the hormone-induced increases in β-cell proliferation and β-cell mass during pregnancy.
Antibodies that specifically recognize FoxM1 in mouse tissue are not available. Thus, we examined whether Foxm1 mRNA expression in maternal islets correlated with β-cell proliferation during pregnancy. Based on the maternal β-cell proliferation profile during pregnancy, qRT-PCR analysis was performed on isolated control islets at virgin, GD14.5, and P8 stages. Maternal Foxm1 expression in control islets was elevated more than 50% at GD14.5 and returned back to prepregnancy levels at P8 (Fig. 1). Thus, Foxm1 expression correlated with the dynamics of β-cell proliferation during pregnancy.
FIG. 1.
Foxm1 expression is increased during pregnancy. Quantitative real-time RT-PCR was performed on isolated islets from 8- to 12-week-old virgin (■), GD14.5 (□), and P8 Foxm1fl/fl females (▧). The relative expression units at GD14.5 and P8 were normalized to that of virgin mice. A 1.7-fold increase in Foxm1 mRNA was observed in islets from GD14.5 females compared with islets from virgin females. Unpaired t tests were used to measure significance. n = 3–6 per group. Error bars represent SD. **P < 0.01.
Impaired β-cell mass expansion in pregnant FoxM1Δpanc mice.
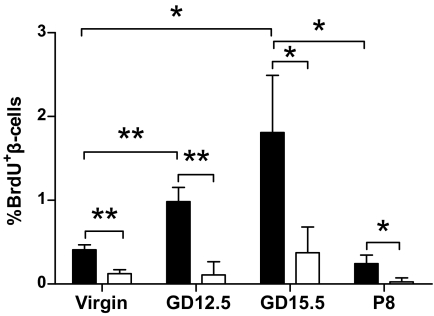
We predicted that lack of FoxM1 would lead to decreased β-cell proliferation and mass during pregnancy. Significantly fewer proliferating β-cells were observed in FoxM1Δpanc females compared with controls (Fig. 2) at all stages. Controls displayed an approximately threefold increase in β-cell proliferation at GD15.5, with no corresponding increase in FoxM1Δpanc mice, clearly indicating that FoxM1 is important for increased maternal β-cell proliferation during pregnancy.
FIG. 2.
Decreased β-cell proliferation in FoxM1Δpanc female mice. Foxm1fl/fl mice (■) showed a significant increase in β-cell proliferation at midgestation compared with before pregnancy. FoxM1Δpanc females (□) showed much lower β-cell proliferation than control mice at every time point, with no increase in β-cell proliferation during pregnancy in mutant mice. Two-way ANOVA with Bonferroni post-tests and two-tailed Student t test were used to measure significance. n = 3–4 per group. Error bars represent SD. *P < 0.05, **P < 0.01.
Our previous studies revealed an increase in nuclear p27 in neonatal islets in the absence of FoxM1 (28). Examination of islets from virgin and pregnant females revealed a decrease in intensity of nuclear p27 expression in control islets at GD15.5 (Fig. 3A and B). In contrast, islets from virgin and GD15.5 FoxM1Δpanc females showed increased nuclear p27 compared with control females (Fig. 3C and D). There was no change in total p27 protein as detected by Western blotting (data not shown). In addition, we examined expression of Menin in control and FoxM1Δpanc islets at virgin and GD15.5 stages. Islets from pregnant control animals showed a decrease in Menin expression compared with virgin mice, consistent with published results (18). However, in FoxM1Δpanc islets from pregnant females, Menin protein levels remained elevated (Fig. 3E).
FIG. 3.
Increased nuclear p27 in islets of GD15.5 FoxM1Δpanc females. p27 (brown) was detected in some islet nuclei in virgin control and FoxM1Δpanc pancreata at 8–12 weeks of age (A and C). The number of nuclei strongly positive for p27 decreased in control pregnant females at GD15.5 (B) compared with virgin controls (A). In both virgin and GD15.5 FoxM1Δpanc islets (C and D), nuclear localization of p27 was increased compared with control islets at GD15.5 (B). The larger nuclei in FoxM1Δpanc pancreata are due to the endoreduplication known to occur in the absence of Foxm1 (21). Representative images are shown (n = 3 animals per group). Magnification ×400. E: Quantification of Western blotted Menin protein from isolated islets. Menin protein decreases in control islets during pregnancy, but remains elevated in FoxM1Δpanc islets. n = 3–4 animals per group. *P < 0.05. (A high-quality color digital representation of this figure is available in the online issue.)
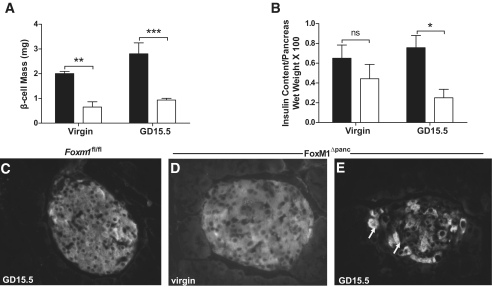
The decrease in β-cell proliferation observed in FoxM1Δpanc females translated into decreased β-cell mass. Virgin FoxM1Δpanc females had a significant decrease in β-cell mass compared with controls (Fig. 4A), although we did not detect a significant difference in total pancreatic insulin content at baseline (Fig. 4B). A dramatic decrease in β-cell mass in FoxM1Δpanc females was also observed at GD15.5 (Fig. 4A). Pregnancy neither stimulated β-cell mass expansion nor increased total pancreatic insulin content in FoxM1Δpanc females compared with virgin females (Fig. 4A and B).
FIG. 4.
Decreased β-cell mass in virgin and pregnant FoxM1Δpanc mice. A: Compared with controls (■), β-cell mass was significantly decreased in virgin and GD15.5 FoxM1Δpanc females (□; n = 3–4 per group). B: Virgin Foxm1Δpanc females have total pancreatic insulin content similar to virgin controls. Insulin content in FoxM1Δpanc females decreased slightly during pregnancy. Two-way ANOVA with Bonferroni post-tests was used to measure significance (n = 4–7 per group). C: Islets from control female mice at GD15.5 showed uniform insulin immunoreactivity as did islets from virgin FoxM1Δpanc females (D). E: Islets from female FoxM1Δpanc mice showed patchy insulin reactivity at GD15.5 with some insulin-producing cells labeling more strongly than others (arrows). *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent SEM. ns, not significant.
To determine whether loss of FoxM1 might also affect β-cell differentiation, we examined Pdx1 expression by immunolabeling and Western blotting. Pdx1 expression was unchanged in FoxM1Δpanc islets from either virgin or pregnant females compared with controls (data not shown). Sections from virgin and pregnant mice control and FoxM1Δpanc pancreata were also examined for insulin expression. Islets from control animals showed uniform insulin reactivity (Fig. 4C and data not shown). In contrast, although insulin expression in virgin FoxM1Δpanc islets resembled controls, many cells labeled weakly in islets from female FoxM1Δpanc at GD15.5 (Fig. 4D and E). These low-level insulin-expressing cells were not undergoing apoptosis, as there was no increase in apoptotic β-cells in pregnant FoxM1Δpanc females (data not shown). Thus, loss of FoxM1 results in both a decrease in the number of proliferating maternal β-cells during pregnancy as well as a decrease in insulin expression in individual β-cells. Overall, these data suggest that FoxM1 is a key factor promoting maternal β-cell compensation during pregnancy.
Development of GDM in FoxM1Δpanc pregnant females.
Virgin FoxM1Δpanc female mice remain euglycemic (12,28) despite defects in β-cell mass. Thus, IPGTT was performed on 8- to 12-week-old virgin and pregnant females at GD12.5 and GD15.5 to determine whether pregnant female FoxM1Δpanc mice could maintain glucose homeostasis despite defects in β-cell proliferation and β-cell mass expansion. FoxM1Δpanc pregnant females showed no dramatic differences in fasting blood glucose levels compared with control females at either time point examined. However, mutant females displayed impaired glucose tolerance at GD12.5 (Fig. 5A) and overt GDM at GD15.5 (Fig. 5B). In humans, GDM is diagnosed if patients fail two or more values on the 100-g glucose tolerance test. The normal cutoff values are below 95 mg/dl at fasting, 180 mg/dl at 1 h, 155 mg/dl at 2 h, and 140 mg/dl at 3 h (2). An elevated fasting plasma glucose concentration is not required for a diagnosis of GDM (2). One hour after glucose challenge, blood glucose levels in FoxM1Δpanc females were greater than 300 mg/dl. In addition, the blood glucose concentration 2 h after glucose challenge was ∼200 mg/dl (Fig. 5B). The mean plasma insulin level in control mice at GD15.5 was 231.7 pg/ml at fasting and 977.2 pg/ml at 30 min (n = 2). In contrast, mean plasma insulin level in FoxM1Δpanc females was 304.6 pg/ml at fasting and 361.3 pg/ml at 30 min (n = 4). These results demonstrate that FoxM1 is absolutely essential for the maintenance of glucose homeostasis during pregnancy and that loss of FoxM1 results in a reduced capacity for β-cell compensation in the face of increasing insulin resistance as pregnancy proceeds.
FIG. 5.
FoxM1Δpanc mice developed glucose intolerance at GD12.5 and GDM at GD15.5. IPGTTs were performed on Foxm1fl/fl (●) and FoxM1Δpanc (○) females at GD12.5 (A) and GD15.5 (B) after a 16-h fast. At GD12.5, FoxM1Δpanc females displayed impaired glucose tolerance at 90 and 120 min (A). FoxM1Δpanc females progressed to GDM by GD15.5 (B). Two-way ANOVA with Bonferroni post-test was used to measure significance of difference. n = 5–10 per group. Error bars represent SEM. *P < 0.05.
Loss of Foxm1 results in sustained islet defects after parturition.
Because prior GDM elevates the risk for type 2 diabetes later in life, we examined whether there were any deleterious lasting consequences of pregnancy in FoxM1Δpanc females. Eight days after giving birth, FoxM1Δpanc females tended to have higher blood glucose levels during an IPGTT than postpartum control females; however, these did not reach statistical significance (supplementary Fig. 2). The impaired β-cell proliferation observed in FoxM1Δpanc virgin females was still observed at P8 (Figs. 2 and 6). In addition, the islets in P8 FoxM1Δpanc pancreata were smaller than those in controls (Figs. 6A and B and 7A). Sections from P8 control islets contained an average of 21 β-cells per islet, whereas sections from P8 FoxM1Δpanc islets contained an average of only 9 β-cells per islet. After parturition, FoxM1Δpanc females exhibited a significant increase in the proportion of small endocrine cell clusters (1–8 cells) versus definitive islets (>8 cells) in comparison with control mice (Fig. 6C), likely contributing to the differences observed in average islet size (Fig. 7A). Although it is possible that small endocrine clusters represent sections through the “top” of an islet of normal size, we observe this increase in small islet clusters only in postpartum FoxM1Δpanc females; no differences were observed in the proportion of small endocrine cell clusters between control and FoxM1Δpanc virgin females (12) (data not shown).
FIG. 6.
Lasting postpartum changes in FoxM1Δpanc female pancreata. BrdU incorporation (red) was used to assess active β-cell proliferation (insulin [Ins]: green) in maternal islets at P8. Control islets (A) were larger and showed increased BrdU incorporation compared with FoxM1Δpanc islets (B; see also Fig. 2). C: The proportion of small islet clusters (1–8 cells) was significantly increased in FoxM1Δpanc pancreata at P8 compared with controls (n = 3; P < 0.05). Rare NGN3-positive cells (green) were observed adjacent to ducts (cytokeratin: red) at P2 (not shown) and P4 (D). Juxtaductal insulin-positive cells were observed in FoxM1Δpanc pancreata at P8 (arrow in E). Magnification ×400. (A high-quality color digital representation of this figure is available in the online issue.)
FIG. 7.
Postpartum FoxM1Δpanc pancreata show normal β-cell mass due to increased number of islets. A: Average islet size was decreased in FoxM1Δpanc pancreata, although β-cell mass was normal as assessed by two different calculations (B, C). This was likely due to an increase in the number of insulin+ clusters (D) and increased average β-cell size (E) in FoxM1Δpanc pancreata compared with controls. n = 3–4 per group. *P < 0.05.
Despite the decrease in islet size, total β-cell mass was normal in FoxM1Δpanc females after parturition (Fig. 7B and C). This is likely due to a combination of increased individual β-cell size (Fig. 7E) and an increase in the actual number of islet clusters in FoxM1Δpanc pancreata (Fig. 7D), possibly due to neogenesis. Postpartum FoxM1Δpanc pancreata contained rare NGN3-expressing cells adjacent to ducts at P2 and P4 and juxtaductal insulin-positive cells at P8 (Fig. 6D and E). Thus, NGN3 expression preceded insulin expression, suggestive of neogenesis. NGN3-positive cells were not observed in control pancreata at any time point examined (virgin, GD15.5, P2, P4, and P8) or in FoxM1Δpanc pancreata from virgin, GD15.5, or P8 females.
FoxM1 is essential for PL-mediated increases in β-cell proliferation and β-cell mass.
PL is the primary lactogenic hormone that stimulates β-cell proliferation during pregnancy (34). To examine whether FoxM1 might act downstream of PL to promote β-cell proliferation during pregnancy, Foxm1 expression was examined in isolated Foxm1fl/fl female islets exposed to PL in culture. As shown in Fig. 8A, PL treatment increased expression of Bcl-xl, a known target of PL signaling. In addition, Foxm1 expression was increased approximately threefold. As a more direct test of whether FoxM1 functions downstream of PL, RIP-mPL transgenic mice were interbred with FoxM1Δpanc mice. RIP-mPL mice express PL under the control of the rat insulin promoter and have a twofold increase in β-cell proliferation (17). FoxM1 was absolutely essential for the PL-mediated increase in β-cell proliferation in RIP-mPL transgenic mice (Fig. 8B).
FIG. 8.
FoxM1 acts downstream of PL to mediate increases in β-cell proliferation and β-cell mass. A: Expression of Foxm1 and Bcl-xl was elevated in isolated islets in response to four days of PL treatment (n = 5 per group). B: Foxm1 inactivation completely inhibited PL-mediated induction of β-cell proliferation in RIP-mPL transgenic mice (n = 3–4 per group). Paired, two-tailed Student t test was used to measure significance. ■, 0 ng/ml PL; □, 500 ng/ml PL. *P < 0.05, **P < 0.01.
DISCUSSION
During pregnancy, β-cell mass increases by ∼50% due to stimulation of proliferation by GH, Prl, and most importantly, PL (6,15,16). Foxm1 expression strongly correlated with the dynamics of maternal β-cell proliferation during pregnancy and was induced in response to PL in cultured islets. In contrast to the partial requirement we recently described for FoxM1 in β-cell regeneration and proliferation after PPx (12), we found that FoxM1 is absolutely essential for β-cell mass expansion during pregnancy. These data, combined with the fact that FoxM1 is necessary for PL-induced β-cell proliferation in RIP-mPL transgenic mice, point to FoxM1 as a downstream target of PL signaling and an essential mediator of PL activity in islets. Because pregnancy is a physiological stimulus of β-cell proliferation, understanding the molecular pathways involved in pregnancy-induced β-cell mass expansion could facilitate identification of therapeutic targets for enhancing β-cell mass in vitro or in vivo.
Our data show that FoxM1 negatively regulates protein levels of the cell cycle inhibitor, Menin. We do not yet know whether this is at the level of transcription. However, the effect of FoxM1 on p27 in our model is mainly at the level of protein localization because total p27 levels were unchanged in FoxM1Δpanc pancreata. FoxM1 promotes p27 nuclear export in other systems (27). Interestingly, Menin expression is negatively regulated by lactogens (18). In the future, it will be interesting to examine whether Stat5 directly regulates the Foxm1 gene in β-cells in response to PL and to determine the mechanism whereby FoxM1 regulates Menin in conjunction with pregnancy hormones.
In addition to enhancing β-cell mass and β-cell proliferation, PL and other pregnancy hormones enhance β-cell function. Functional changes in maternal β-cells include a lowered threshold for glucose-stimulated insulin secretion, increased glucokinase activity, enhanced insulin biosynthesis, increased gap junctional communication, and increased islet blood flow (6,34–37). Islets from FoxM1Δpanc pregnant females showed reduced insulin labeling per β-cell and reduced total pancreatic insulin content. Thus, β-cell defects in the absence of FoxM1 may not be limited to a dramatic decrease in β-cell proliferation. Preliminary data from our laboratory suggest that loss of FoxM1 impairs β-cell function when animals are exposed to a high-fat diet (A. Ackermann Misfeldt, U.G. Kopsombut, and M.G., unpublished observations).
After parturition, a combination of decreased proliferation, increased apoptosis, and decreased cell size normally restores β-cell mass to prepregnancy levels within 10 days (38). It was striking to us that 8 days after giving birth, islets in FoxM1Δpanc pancreata were significantly smaller than those in controls, although glucose homeostasis and β-cell mass were restored. Our data strongly suggest that in the absence of β-cell proliferation, postpartum FoxM1Δpanc pancreata generate new β-cells via neogenesis, a process we previously showed does not require FoxM1 in embryos or adults (12).
GDM results from an interaction between genetic and environmental risk factors. Common variants in several candidate genes increase the risk of GDM in humans (e.g., KCNJ11, GK, and HNF4a) (4); however, very few mouse models of GDM currently exist. Heterozygous leptin receptor–deficient mice (db/+) spontaneously develop GDM due to increased peripheral insulin resistance (39). In addition, β-cell–specific inactivation of HNF4α resulted in impaired glucose tolerance in virgin females that worsens during pregnancy, although these animals do not manifest definitive GDM (40). HNF4α is required for increased β-cell proliferation and β-cell mass during pregnancy (40). Similarly, transgenic induction of Menin expression in islets of pregnant females resulted in moderate hyperglycemia by GD9.0 that worsened as pregnancy progressed (18); however, even the control females in this study showed elevated blood glucose. The model presented here is more similar to human GDM in that a phenotype is manifested specifically at midgestation; virgin and P8 FoxM1Δpanc females show no overt phenotype. Human GDM patients likely have an underlying undetected β-cell defect that becomes apparent only under the physiological stress of pregnancy. Future studies will examine whether prior GDM predisposes FoxM1Δpanc females to type 2 diabetes later in life, a phenotype that never manifests in FoxM1Δpanc virgin females.
Supplementary Material
Acknowledgments
R.C.V. is supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (RO1 DK072264). M.G. is supported by NIH/NIDDK (R01 DK071052).
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
We thank Dr. Amanda Ackermann Misfeldt and members of the Gannon laboratory for critical reading of the article and helpful suggestions. We also thank Young Ah Oh, Nikul Patel, Shidrokh Ardestani, Obi Umunakwe, Paige Cooper, David Lowe, and Usa G. Kopsombut for their technical assistance and Gregory Ayers in the Department of Biostatistics for assistance with data analysis. Islets were isolated with the help of Dr. Yanyun Gu and Dr. Zhongyi Chen and Anastasia Golovin of the Vanderbilt Islet Procurement and Analysis Core (DRTC P60 DK20593). RNA quality was assessed by the Vanderbilt Microarray Shared Resource, supported by the Vanderbilt Ingram Cancer Center (P30 CA68485), the Vanderbilt Digestive Disease Center (P30 DK58404), and the Vanderbilt Vision Center (P30 EY08126).
This article is dedicated to the memory of the late Dr. Robert H. Costa, who was instrumental in initiating our studies of FoxM1.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Brăniçsteanu DD, Mathieu C: Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol Metab 2003;14:54–56 [DOI] [PubMed] [Google Scholar]
- 2.Perkins JM, Dunn JP, Jagasia SM: Perspectives in gestational diabetes mellitus: a review of screening, diagnosis, and treatment. Clin Diabetes 2007;25:57–62 [Google Scholar]
- 3.Frías JL, Frías JP, Frías PA, Martínez-Frías ML: Infrequently studied congenital anomalies as clues to the diagnosis of maternal diabetes mellitus. Am J Med Genet A 2007;143A:2904–2909. [DOI] [PubMed] [Google Scholar]
- 4.Shaat N, Groop L: Genetics of gestational diabetes mellitus. Curr Med Chem 2007;14:569–583 [DOI] [PubMed] [Google Scholar]
- 5.Robitaille J, Grant AM: The genetics of gestational diabetes mellitus: evidence for relationship with type 2 diabetes mellitus. Genet Med 2008;10:240–250 [DOI] [PubMed] [Google Scholar]
- 6.Sorenson RL, Brelje TC: Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 1997:29:301–307 [DOI] [PubMed] [Google Scholar]
- 7.Kawai M, Kishi K: Adaptation of pancreatic islet B-cells during the last third of pregnancy: regulation of B-cell function and proliferation by lactogenic hormones in rats. Eur J Endocrinol 1999;141:419–425 [DOI] [PubMed] [Google Scholar]
- 8.Bonner-Weir S: Life and death of the pancreatic beta cells. Trends Endocrinol Metab 2000;11:375–378 [DOI] [PubMed] [Google Scholar]
- 9.Cauchi S, El Achhab Y, Choquet H, Dina C, Krempler F, Weitgasser R, Nejjari C, Patsch W, Chikri M, Meyre D, Froguel P: TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: a global meta-analysis. J Mol Med 2007;85:777–782 [DOI] [PubMed] [Google Scholar]
- 10.Grarup N, Rose CS, Andersson EA, Andersen G, Nielsen AL, Albrechtsen A, Clausen JO, Rasmussen SS, Jørgensen T, Sandbaek A, Lauritzen T, Schmitz O, Hansen T, Pedersen O: Studies of association of variants near the HHEX, CDKN2A/B, and IGF2BP2 genes with type 2 diabetes and impaired insulin release in 10,705 Danish subjects: validation and extension of genome-wide association studies. Diabetes 2007;56:3105–3111 [DOI] [PubMed] [Google Scholar]
- 11.Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H: Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008;132:197–207 [DOI] [PubMed] [Google Scholar]
- 12.Ackermann Misfeldt A, Costa RH, Gannon M: β-Cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes 2008;57:3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 14.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA: Growth and regeneration of adult beta cells does not involve specialized progenitors. Developmental Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 15.Parsons JA, Brelje TC, Sorenson RL: Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 1992;130:1459–1466 [DOI] [PubMed] [Google Scholar]
- 16.Nielsen JH, Galsgaard ED, Moldrup A, Friedrichsen BN, Billestrup N, Hansen JA, Lee YC, Carlsson C: Regulation of beta-cell mass by hormones and growth factors. Diabetes 2001;50:S25–S29 [DOI] [PubMed] [Google Scholar]
- 17.Vasavada RC, Garcia-Ocaña A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF: Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 2000;275:15399–15406 [DOI] [PubMed] [Google Scholar]
- 18.Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK: Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007;318:806–809 [DOI] [PubMed] [Google Scholar]
- 19.Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK: Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A 2005;102:14659–14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye H, Kelly TF, Samadani U, Lim L, Rubio S, Overdier DG, Roebuck KA, Costa RH: Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues. Mol Cell Biol 1997;17:1626–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korver W, Roose J, Heinen K, Weghuis DO, de Bruijn D, van Kessel AG, Clevers H: The human TRIDENT/HFH-11/FKHL16 gene: structure, localization, and promoter characterization. Genomics 1997;46:435–442 [DOI] [PubMed] [Google Scholar]
- 22.Leung TW, Lin SS, Tsang AC, Tong CS, Ching JC, Leung WY, Gimlich R, Wong GG, Yao KM: Over-expression of FoxM1 stimulates cyclin B1 expression. FEBS Lett 2001;507:59–66 [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Kiyokawa H, Dennewitz MB, Costa RH: The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci U S A 2002;99:16881–16886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laoukili J, Kooistra MR, Brás A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH: FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 2005;7:126–136 [DOI] [PubMed] [Google Scholar]
- 25.Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, Yoder HM, Kiyokawa H, Kaestner KH, Costa RH: The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Dev Biol 2004;276:74–88 [DOI] [PubMed] [Google Scholar]
- 26.Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, Tan Y, Ackerson T, Costa RH: Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol 2005;25:10875–10894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrovic V, Costa RH, Lau LF, Raychaudhuri P, Tyner AL: FoxM1 regulates growth factor-induced expression of kinase-interacting stathmin (KIS) to promote cell cycle progression. J Biol Chem 2008;283:453–460 [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, Costa RH, Gannon M: The Foxm1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol 2006;20:1853–1866 [DOI] [PubMed] [Google Scholar]
- 29.Krupczak-Hollis K, Wang X, Dennewitz MB, Costa RH: Growth hormone stimulates proliferation of old-aged regenerating liver through forkhead box m1b. Hepatology 2003;38:1552–1562 [DOI] [PubMed] [Google Scholar]
- 30.Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC: Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem 2002;277:11225–11232 [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 32.Crawford LA, Guney MA, Oh YA, Deyoung RA, Valenzuela DM, Murphy AJ, Yancopoulos GD, Lyons KM, Brigstock DR, Economides A, Gannon M: Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and β-cell proliferation during embryogenesis. Mol Endocrinol 2009;23:324–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonner-Weir S: Islet growth and development in the adult. J Mol Endocrinol 2000;24:297–302 [DOI] [PubMed] [Google Scholar]
- 34.Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL: Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 1993;132:879–887 [DOI] [PubMed] [Google Scholar]
- 35.Kawai M, Kishi K: In vitro studies of the stimulation of insulin secretion and B-cell proliferation by rat placental lactogen-II during pregnancy in rats. J Reprod Fertil 1997;109:145–152 [DOI] [PubMed] [Google Scholar]
- 36.Svensson AM, Bodin B, Andersson A, Jansson L: Pancreatic islet blood flow during pregnancy in the rat: an increased islet mass is associated with decreased islet blood flow. J Endocrinol 2004;180:409–415 [DOI] [PubMed] [Google Scholar]
- 37.Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorenson RL: Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy. J Endocrinol 2007;193:367–381 [DOI] [PubMed] [Google Scholar]
- 38.Scaglia L, Smith FE, Bonner-Weir S: Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology 1995;136:5461–5468 [DOI] [PubMed] [Google Scholar]
- 39.Yamashita H, Shao J, Qiao L, Pagliassotti M, Friedman JE: Effect of spontaneous gestational diabetes on fetal and postnatal hepatic insulin resistance in Lepr(db/+) mice. Pediatr Res 2003;53:411–418 [DOI] [PubMed] [Google Scholar]
- 40.Gupta RK, Gao N, Gorski RK, White P, Hardy OT, Rafiq K, Brestelli JE, Chen G, Stoeckert CJ, Jr, Kaestner KH: Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev 2007;21:756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.