Abstract
OBJECTIVE
Carbohydrate-responsive element-binding protein (ChREBP) is a transcription factor that has been shown to regulate carbohydrate metabolism in the liver and pancreatic β-cells in response to elevated glucose concentrations. Because few genes have been identified so far as bona fide ChREBP-target genes, we have performed a genome-wide analysis of the ChREBP transcriptome in pancreatic β-cells.
RESEARCH DESIGN AND METHODS
Chromatin immunoprecipitation and high-density oligonucleotide tiling arrays (ChIP-chip; Agilent Technologies) using MIN6 pancreatic β-cell extracts were performed together with transcriptional and other analysis using standard techniques.
RESULTS
One of the genes identified by ChIP-chip and linked to glucose sensing and insulin secretion was aryl hydrocarbon receptor nuclear translocator (ARNT)/hypoxia-inducible factor-1β (HIF-1β), a transcription factor implicated in altered gene expression and pancreatic-islet dysfunction in type 2 diabetes. We first confirmed that elevated glucose concentrations decreased ARNT/HIF-1β levels in INS-1 (832/13) cells and primary mouse islets. Demonstrating a role for ChREBP in ARNT gene regulation, ChREBP silencing increased ARNT mRNA levels in INS-1 (832/13) cells, and ChREBP overexpression decreased ARNT mRNA in INS-1 (832/13) cells and primary mouse islets. We demonstrated that ChREBP and Max-like protein X (MLX) bind on the ARNT/HIF-1β promoter on the proximal region that also confers the negative glucose responsiveness.
CONCLUSIONS
These results demonstrate that ChREBP acts as a novel repressor of the ARNT/HIF-1β gene and might contribute to β-cell dysfunction induced by glucotoxicity.
Pancreatic β-cell dysfunction or loss is the hallmark of all forms of diabetes (1,2). Transcription factors have proven to be critical for the maintenance of normal β-cell function, and mutations in many of these, including pancreatic duodenum homeobox-1 (PDX1) (3) and the hepatocyte nuclear factors HNF1α (4) and HNF4α (5) lead to monogenic forms of inherited type 2 diabetes (6), whereas polymorphisms in others, notably T-cell factor 7-like 2 (TCF7L2), are associated with more common forms of the disease (7).
Recently, a transcription factor termed aryl hydrocarbon receptor nuclear translocator (ARNT), or hypoxia-inducible factor-1β (HIF-1β), has emerged as a potentially important player in the pathogenesis of pancreatic β-cell dysfunction and type 2 diabetes in humans (8). ARNT/HIF-1β is a member of the basic helix-loop-helix (HLH) Per/AhR/ARNT/Sim (PAS) family of transcription factors and binds DNA as an obligate heterodimer with the oxygen-sensitive HIF-1α, HIF-2α, or aryl hydrocarbon receptor (AhR). When mRNA levels were compared in human pancreatic islets of Langerhans isolated from five type 2 diabetic donors and seven nondiabetic donors by oligonucleotide microarrays and real-time PCR, an 82% reduction in the expression of the ARNT/HIF-1β gene was observed in islets from type 2 diabetic donors. Confirming a role for ARNT/HIF-1β in β-cell function, β-cell–specific ARNT/HIF-1β gene knockout in mice, or ARNT/HIF-1β silencing in MIN6 cells, led to defects in glucose-stimulated insulin secretion (GSIS) and alterations in islet gene expression comparable to those observed in human type 2 islets (8).
Carbohydrate-responsive element-binding protein (ChREBP) (9) (also termed MondoB or Williams-Beuren syndrome critical region gene 14 [WBSCR14]) (10) is a transcription factor that regulates de novo lipogenesis in the liver in response to elevated glucose concentrations (11). It is a member of the basic HLH (bHLH) family and transactivates glucose-responsive genes by binding DNA on carbohydrate response element (ChoRE), as a heterodimer with Max-like protein X (MLX) (12). In addition to its lipogenic role in the liver, we have recently shown (13) that, in clonal pancreatic β-cells, 1) ChREBP mRNA levels are increased in response to elevated glucose concentrations; 2) ChREBP translocates to the nucleus in response to high glucose; 3) ChREBP directly binds to the promoters of lipogenic genes fatty acid synthase (FAS) and l-type pyruvate kinase (l-PK) in a glucose- and calcium-dependent, but insulin-independent, manner in intact cells; and 4) ChREBP silencing by small-interfering RNA (siRNA) improves GSIS (13).
To identify other genes directly regulated by ChREBP in the β-cell, we performed a genome-wide analysis of the ChREBP transcriptome using chromatin immunoprecipitation and high-density oligonucleotide tiling arrays (ChIP-on-chip; Agilent Technologies) in MIN6 cells. ARNT/HIF-1β fell within the statistical cutoff and was considered positive for ChREBP binding. Consequently, we demonstrate that ChREBP directly binds to ARNT/HIF-1β promoter in a glucose-dependent manner in clonal MIN6 and INS-1 (832/13) cells and negatively regulates ARNT/HIF-1β gene expression in pancreatic islet β-cells.
These results suggest that ChREBP, in addition to being a critical regulator of lipogenic genes in the liver and pancreatic β-cell, may also play a role in the development of β-cell failure and type 2 diabetes through alteration of ARNT/HIF-1β gene expression.
RESEARCH DESIGN AND METHODS
Reagents.
The Silencer siRNA construction kit was from Ambion (Huntingdon, U.K.). Primers for siRNA construction and PCR were from MWG Biotech (Milton Keynes, U.K.). TransIT-TKO transfection reagent was from Mirrus (Madison, WI). The rat insulin radioimmunoassay kit was from Linco (St. Charles, MO). Tissue culture reagents were from Sigma-Aldrich (Dorset, U.K.) or Invitrogen (Paisley, U.K.). Lipofectamine 2000 and SYBR Green platinum were from Invitrogen (Paisley, U.K.). Collagenase from C. histolyticum was obtained from Serva Electrophoresis (Heidelberg, Germany). Anti-ChREBP antibody was described by da Silva Xavier et al. (13). Mouse monoclonal anti-hemaglutinin (HA) antibody was provided by Anindiya Roy (Cancer Research U.K.). Other reagents were from Sigma or Fisher.
Plasmids, adenoviruses, and SiRNA generation.
Plasmid pChREBP was described (13). Plasmid bearing HA-tagged MLX cDNA was provided by Dr H. Towle (University of Minnesota). Plasmid pARNT-2369.LucFF was generated by PCR using MIN6 genomic DNA, AccuPrime TaqDNA Polymerase High Fidelity (Invitrogen), and the following primers: forward, 5′-cgcg GAGCTC CAC AAG CTA AGA TCA TCT GAG AGG (SacI site underlined) and reverse, 5′-cgcg CTCGAG GTT ACT TAC TAG GCA TCA GGG GAA (XhoI site underlined). The resulting 2,662-bp fragment was subcloned into pCR2.1 by TA cloning, digested by SacI, and subcloned into pGL3basic (Promega) at the SacI site. Plasmids pARNT-1028.LucFF, pARNT-630.LucFF, and pARNT-1028-769.LucFF were generated by digesting pARNT-2369.LucFF with BglII, KpnI, and SpeI, respectively, gel purification, and self-ligation. Plasmids pARNT-1028[-443T>A;-442_-402del].LucFF and pARNT-630[-440_-402del].LucFF were generated by PCR using plasmids pARNT-1028.LucFF and pARNT-630.LucFF as templates, respectively; Phusion Hot Start High-Fidelity DNA Polymerase (Finnzymes); and the following primers: forward, 5′- TTCTCTTTGCATTTCACTGACG and reverse, 5′- TCATCAGCATATGCTTCCTACAGAC. The resulting PCR products were gel purified, phosphorylated using T4 kinase and forward buffer (Invitrogen), and self-ligated. The differences in the sequences of the resulting deletion mutants is probably because the primers used were not PAGE purified. The E-boxes point mutations in pARNT-1028.LucFF and pARNT-630.LucFF plasmids were introduced using a QuikChange Site-Directed Mutagenesis Kit (Stratagene). Primer sequences for each mutant are available on demand. pLPK-183.LucFF has been described previously (14). pRL-CMV was from Promega. Adenovirus-encoding ChREBP was generated by subcloning ChREBP cDNA into pAdTrackCMV using NotI-XbaI sites and then amplified according to He et al. (15) and Ainscow and Rutter (16). Adenovirus encoding GFP was described by Leclerc et al. (17). All constructs were verified by DNA sequencing. SiRNAs were generated as described by da Silva Xavier et al. (13) using The Silencer siRNA construction kit from Ambion and the following primers (T7 underlined): rat ChREBP, 508 AAACCTGAGGCTGTTGTCTTCCTGTCTC 527; scrambled, AAGGCTGTTTCACCTGAGTT CCTGTCTC.
Islet isolation and culture.
Male CD1 mice (12–13 weeks old) were killed by cervical dislocation, and collagenase (1.0 mg/ml in Hanks' balanced salt solution) was injected into the pancreatic duct (5.0 ml/mouse). The distended pancreata were then incubated in a shaking water bath at 37°C in 1.0 mg/ml collagenase in Hanks' balanced salt solution for 10 min, and the islets were recovered using Histopaque gradient solutions (3 ml of 1.119 g/l; 3 ml of 1.083 g/l; 3 ml of 1.077 g/l). After centrifugation for 20 min at 2,500g, islets were removed from the top layer and washed once with RPMI 1640. Mouse islets were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FCS plus antibiotics and incubated in a humidified atmosphere at 37°C with 5% CO2. Infection of islets with adenoviruses was performed at a multiplicity of infection of 100, and islets of no greater than 150 μm diameter were used to ensure infection of 30–50% of cells within each islet (18). Measurements of insulin secretion were performed 48 h after infection.
Cell culture and transfection.
MIN6 β-cells (19) were used between passages 19 and 30 and grown in Dulbecco's modified Eagle's medium containing 25 mmol/l glucose and supplemented with 15% (vol/vol) heat-inactivated FCS, 4 mmol/l l-glutamine, 25 μmol/l β-mercaptoethanol, 100 IU/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere at 37°C with 5% CO2 unless specified otherwise. INS-1 (832/13) β-cells (20) were cultured in RPMI 1640 containing 11 mmol/l glucose, 10% (vol/vol) FCS, 4 mmol/l l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, 10 mmol/l HEPES, 1 mmol/l sodium pyruvate, 0.05 mmol/l β-mercaptoethanol, or as indicated. HEK293 cells were cultured in Dulbecco's modified Eagle's medium containing 10% (vol/vol) heat-inactivated FCS, 25 mmol/l glucose, 2 mmol/l glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. INS1 (832/13) cells were transfected using Lipofectamine 2000 and Opti-Mem, according to the manufacturer's instructions, whereas HEK293 cells were transfected using the calcium phosphate method, pH 7.12 (21).
Chromatin immunoprecipitation assay and ChIP-on-chip.
Chromatin immunoprecipitation was performed as described by da Silva Xavier et al. (13). Briefly, MIN6 β or INS-1(832/13) cells were cultured in medium containing 3 mmol/l glucose for 16 h before stimulation with medium containing 3 or 30 mmol/l [20 mmol/l for INS-1(832/13)] glucose for 20 h before cross-linking with 1% formaldehyde and sonication to obtain fragments of chromatin <1 kb. DNA-protein complexes were immunoprecipitated with antibodies against ChREBP or HA, followed by digestion with proteinase K (Boehringer Mannheim). The resulting DNA was extracted using phenol-chloroform and precipitated with ethanol. The mouse ARNT/HIF-1β promoter from −530 to −249 bp was amplified using the following primers: 5′-TGCTGATGAGCCCACTAAGA-3′ and 5′-ATAGAGGCCGGGCTAGTGAC-3′. The mouse ARNT/HIF-1β promoter from −1,059 bp to −759 bp and −392 bp to 94 bp was amplified using the following primer pairs: 5′-GTCTTCAAACACACCAGACGA-3′ and 5′-GTGAGGTGTCCTATGTCAC-3′; 5′-CAGAAAGGACTGACAAGTGC-3′ and 5′-GCTGGCAGAGGGTTAAAAG-3′, respectively. The sequence of the mouse l-PK primers are given in the study by da Silva Xavier et al. (13). The rat ARNT/HIF-1β promoter from −197 to −609 bp was amplified using the following primers: 5′-TTGCCTAGGAAGCACAAGG-3′ and 5′-AGCCTCCC TGGGAAAACTGACC-3′ and the rat ARNT/HIF-1β promoter −1,001 to −1,300 bp was amplified using the following primers: 5′-GTCAGTCTAGGCTACTTGAG-3′ and 5′-GGGCTAGTGGTGCATACCTTTAATC-3′. The rat l-PK promoter from 129 to −392 bp was amplified using the following primers: 5′-AAAGTCTGCCTTAAGTGGGGC-3′ and 5′-TCCACCTTCCCTCCACCCA-3′. ChIP-on-chip was carried out using Agilent high-density oligonucleotide tiling arrays (Agilent Technologies, Cheshire, U.K.) according to the manufacturer's protocol. The antibody-enriched DNA was blunt-ended and ligated to specific linker and amplified using a two-stage PCR protocol. Fluorescence dyes Cy3 (3 mmol/l glucose) and Cy5 (30 mmol/l glucose) were used to label the antibody-enriched DNA. The DNA was hybridized onto microarray slides for up to 40 h at 40°C. The slides were washed, and the data were analyzed by high-resolution scanning and Agilent ChIP Analytics software. To detect significant binding events, a minimum cutoff P value of <0.001 was used.
RNA isolation and quantitative real-time PCR.
Primary mouse pancreatic islets were treated for 24 h with ChREBP or null adenovirus in culture medium containing 11 mmol/l glucose and then incubated for 16 h at 3 mmol/l glucose and finally for 20 h at either 3 or 17 mmol/l glucose as indicated. Levels of mRNA encoding the indicated genes were determined by quantitative real-time RT-PCR and were normalized compared with cyclophillin mRNA as described by da Silva Xavier et al. (22). Results are expressed as the fold change over control (null, 3 mmol/l glucose) and presented as the means ± SE.
Insulin secretion and assay.
After adenoviral infection (48 h), islets were incubated for 60 min in a shaking water bath at 37°C in 1 ml Krebs-Ringer bicarbonate HEPES buffer: 125 mmol/l NaCl, 3.5 mmol/l KCl, 1.5 mmol/l CaCl2, 0.5 mmol/l MgSO4, 0.5 mmol/l NaH2PO4, 2.0 mmol/l NaHCO3, 10 mmol/l HEPES) supplemented with 3 mmol/l glucose/0.1% (wt/vol) BSA, equilibrated with a mixture of 95% O2/5% CO2, pH 7.4. The islets were then separated into four groups of six islets per condition and incubated for 30 min in 1.0 ml Krebs-Ringer bicarbonate HEPES buffer as above, containing either 3 or 17 mmol/l glucose. Total insulin extracted from the islets in acidified ethanol was carried out as described previously (17). Insulin was measured by radioimmunoassay (Linco Research, St. Charles, MO).
Luciferase assays.
Dual-Luciferase Reporter Assay System (Promega) was used according to the manufacturer's instructions.
DNA-protein interactions studies.
HEK293 cells were cotransfected with Myc-tagged ChREBP and HA-tagged MLX for 48 h using calcium phosphate as above before cell lysis and nuclear extracts preparation using NucBuster Protein Extraction Kit (Novagen) according to the manufacturer's instructions. DNA-protein interactions were analyzed using the NoShift Transcription Factor Assay Kit (Novagen). Briefly, 5 μg nuclear extracts were incubated with a 3′ biotinylated dsDNA probe and salmon sperm DNA plus Poly(dI-dC) for 30 min on ice before transfer to a streptavidin-coated plate. Mouse monoclonal anti-HA antibody (1/1,000) was added for 1 h at 37°C followed by anti-mouse horseradish peroxidase–conjugated secondary antibody (1/1,000) for 30 min at 37°C. 3,3′,5,5′-Tetramethylbenzidine substrate was added for 30 min to develop colorimetric signal before quenching with 1 N HCl. Absorbance was read at 450 nm.
Statistical analysis.
Data are given as means ± SE of three to five individual experiments. Comparisons between means were performed using two-tailed Student's t test for unpaired data, with Bonferroni's correction for multiple tests where appropriate.
RESULTS
ChREBP binds directly and glucose dependently to the ARNT/HIF-1β proximal promoter in living clonal β-cells to regulate its transcription.
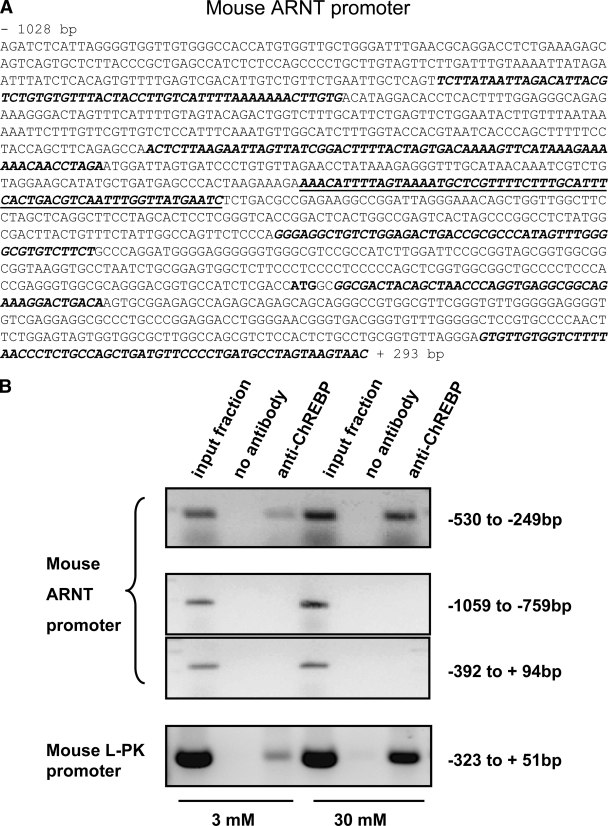
To identify genomic targets of ChREBP, we used an unbiased “ChIP-on-chip” approach using high-density oligonucleotide arrays (see research design and methods). The immediate 5′ flanking region of the ARNT/HIF-1β gene was one of the promoter elements identified on the array as a site at which binding of ChREBP achieved statistical confidence at P < 0.1%. Given previous reports that ARNT/HIF-1β expression in islets is downregulated in type 2 diabetes in humans and that this factor is required for the normal regulation of insulin secretion (8), we explored the nature and role of the ChREBP-ARNT/HIF-1β promoter interaction in more detail. First, we confirmed, by specific ChIP assay, the binding of ChREBP to a region of the mouse ARNT/HIF-1β promoter that gave the strongest signal on the ChIP-on-chip (Fig. 1A, underlined), which was located between −249 and −530 bp upstream of the ATG (Fig. 1B, upper panel). Our anti-ChREBP antibody also immunoprecipitated a region on the mouse l-PK promoter containing the ChoRE (23,24) and known to bind ChREBP (13,25) (Fig. 1B, lower panel), whereas regions on the ARNT/HIF-1β promoter located either upstream or downstream of −530 to −249 bp area were not pulled down (Fig. 1B, middle panels). Whereas immunoprecipitation of ChREBP from cells incubated at 3 mmol/l glucose, and PCR amplification of this fragment, generated a relatively minor product, a band of more than threefold greater intensity was generated from cells incubated at elevated glucose concentrations (30 mmol/l; Fig. 1B, upper panel). These findings thus demonstrated that ChREBP was bound 1) directly and 2) in a glucose-regulated manner to the ARNT/HIF-1β promoter in living MIN6 β-cells.
FIG. 1.
ChREBP binds to ARNT/HIF-1β promoter in vivo in a glucose-dependent manner. A: Sequence of the mouse ARNT/HIF-1β promoter highlighting (bold-italic) those sequences present on the Agilent chip. Underlined is the region that gave the strongest hybridization signal in the ChIP-on-chip assay. The position of the ATG is shown in bold. B: MIN6 cells were incubated in 3 versus 30 mmol/l glucose for 16 h, as indicated before cross-linking and chromatin immunoprecipitation using our polyclonal anti-ChREBP antibody. The primer sequences used to amplify mouse ARNT/HIF-1β and l-PK promoters and the PCR conditions are given in research design and methods. Data are representative of three independent experiments.
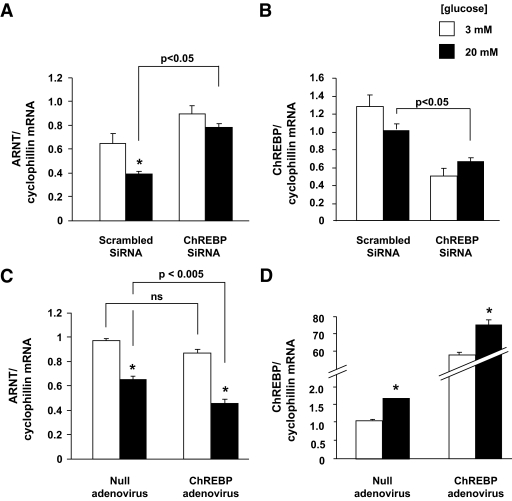
To further explore the roles of glucose and ChREBP in controlling ARNT/HIF-1β gene expression, we measured ARNT/HIF-1β mRNA levels in the highly glucose-responsive β-cell line, INS-1 (832/12) (20). Confirming the glucose sensitivity of ARNT/HIF-1β expression, as recently described (26), incubation of INS-1 (832/13) cells at 20 versus 3 mmol/l glucose led to a substantial (40–50%) decrease in the corresponding ARNT/HIF-1β mRNA levels (Fig. 2A). These findings are consistent with the view that ChREBP induction (27) and nuclear accumulation (13) at high glucose concentrations may be involved in the effects of glucose on ARNT/HIF-1β expression. Accordingly, transfection with a siRNA selective for rat ChREBP, which decreased the corresponding mRNA levels by ∼50% (Fig. 2B), resulted in a twofold increased in ARNT/HIF-1β mRNA levels at high glucose (Fig. 2A). Conversely, adenoviral ChREBP overexpression (supplementary Fig. 1 and Fig. 2C in the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db08-0868/DC1) resulted in significant reduction of ARNT/HIF-1β mRNA levels.
FIG. 2.
Glucose and ChREBP inhibits ARNT/HIF-1β gene expression in INS-1 (832/13) cells. A and B: INS-1 (832/13) cells were transfected for 16 h with either ChREBP-specific or -scrambled SiRNAs using TransIT-TKO and Opti-mem or (C and D) infected with ChREBP expressing or null adenovirus at an multiplicity of infection of 100 and then incubated in 3 mmol/l glucose RPMI for 16 h followed by either 3 or 20 mmol/l glucose RPMI as indicated for 20 h, before total RNA extraction. ARNT/HIF-1β (A and C) and ChREBP (B and D) mRNA levels were estimated by quantitative real-time RT-PCR. The results are expressed as mean ± SE of three independent experiments. *P < 0.05 for the effect of glucose.
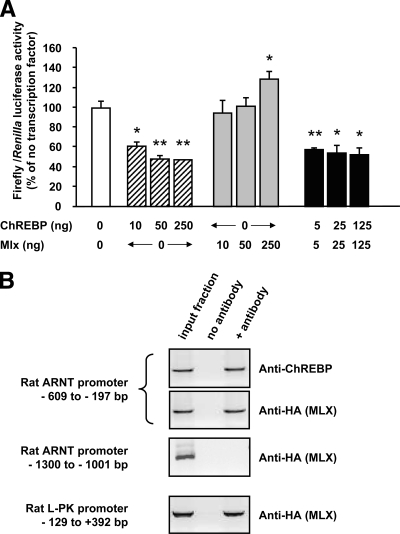
To further analyze the effect of ChREBP binding on ARNT/HIF-1β gene transcription, we next generated promoter-reporter luciferase constructs using fragments of the mouse ARNT/HIF-1β promoter from −2,369 to 293 base pairs with respect to the ATG, a region of the promoter highly conserved between species. Because ChREBP has been shown to regulate lipogenic gene expression as a heterodimer with MLX (28), we next examined the effects of ChREBP and/or MLX overexpression on ARNT/HIF-1β reporter activity in HEK293 cells. As shown in Fig. 3A, overexpression of ChREBP resulted in substantial inhibition of ARNT/HIF-1β promoter activity, even without cotransfection with exogenous MLX, whereas MLX overexpression alone activated the ARNT/HIF-1β promoter. However, as verified by qRT-PCR, MLX was expressed in HEK293 cells [although at lower levels than in INS-1 (832/13) cells], whereas ChREBP mRNA was practically undetectable (not shown). To find out whether ChREBP binding to the ARNT/HIF-1β promoter occurs after heterodimerization with MLX in pancreatic β-cells, we performed a ChIP assay in INS-1 (832/13) cells transfected with MLX-HA, using an anti-HA antibody. As shown in Fig. 3B, both l-PK and ARNT/HIF-1β segments of promoter previously pulled down using anti-ChREBP antibody were also immunoprecipitated using anti-HA antibody, whereas the region of ARNT/HIF-1β promoter located between −1,300 and −1,001 bp was not detected. This strongly suggests that ChREBP and MLX form a heterodimeric complex on each promoter.
FIG. 3.
ChREBP represses ARNT/HIF-1β promoter activity. A: HEK293 cells were seeded in six-well plates and transfected using calcium phosphate with 2 μg pARNT-2369.LucFF, 5 ng pRL-CMV to correct for transfection efficiency, and the indicated amount of plasmid encoding transcription factors ChREBP and/or MLX. Luciferase assays were performed 48 h later as described in research design and methods. Results are expressed as a percentage of the ratio of Firefly/Renilla luciferase in cells with no added transcription factor, as a mean ± SE of four separate experiments. *P < 0.05 and **P < 0.005 for the effect of added transcription factor. B: INS-1 (832/13) cells seeded in T75 flasks were transfected using lipofectamine 2000 with 8 μg HA-MLX before cross-linking and chromatin immunoprecipitation using either anti-ChREBP or anti-HA antibody as indicated. The primer sequences to amplify the rat l-PK and ARNT/HIF-1β promoters are given under research design and methods. Data are representative of three separate experiments.
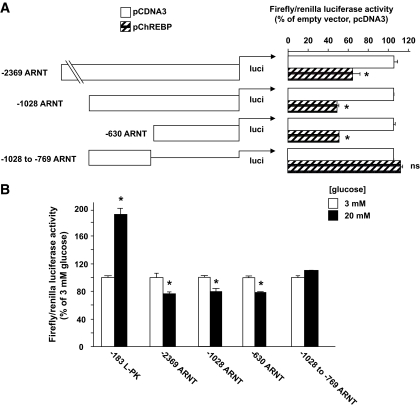
In an attempt to locate the ChREBP/MLX binding site, truncation and deletion mutants were generated in ARNT/HIF-1β luciferase reporter plasmid by restriction digests (see research design and methods for details) and cotransfected with either empty pcDNA3 vector or pChREBP into HEK293 cells. As expected, and in accordance with the ChIP-on-chip data and the ARNT/HIF-1β–specific ChIP experiment presented in Fig. 1, the deletion mutant lacking the whole proximal region pulled down by ChIP was no longer repressed by exogenous ChREBP (Fig. 4A). We next mutated the five consensus individual E-boxes present between −512 and −329 bp on the ARNT/HIF-1β promoter. None of these mutants, at least individually, displayed a loss of ChREBP repression (supplemental Fig. 3A in the online appendix). Subsequently, using the NoShift Transcription Factor Assay Kit (Novagen) as an alternative to electrophoretic mobility shift assay, and nuclear extracts from HEK293 cells cotransfected with c-Myc–tagged ChREBP and HA-tagged MLX, we scanned the ARNT/HIF-1β promoter between −519 and −220 bp, using overlapping double-stranded DNA probes (supplementary Fig. 2A). The l-PK ChoRE was used as an internal positive control. Because only the probe located between −441 to −411 bp gave a positive signal (supplementary Fig. 2B), we generated deletion mutants around this region, but none of these mutants lost ChREBP repression (supplementary Fig. 3B), suggesting that ChREBP repression of ARNT/HIF-1β promoter could demand a complex cis element configuration.
FIG. 4.
ChREBP and glucose repression of ARNT/HIF-1β promoter activity requires cis elements located in the proximal region of the promoter. A: HEK293 cells were seeded in six-well plates and transfected using calcium phosphate with 2 μg of the various pARNT.LucFF constructs, 5 ng pRL-CMV to correct for transfection efficiency, and 250 ng pcDNA3 empty vector or pChREBP as indicated. Luciferase assays were performed 48 h later as in Fig. 3. Results are expressed as a percentage of the ratio of Firefly/Renilla luciferase in cells with no added transcription factor, as a mean ± SE of three separate experiments. *P < 0.05 for the effect of ChREBP. B: INS-1 (832/13) cells were seeded into 12-well plates and transfected overnight with 450 ng of the various pARNT.LucFF constructs or pLPK-183.LucFF as indicated and 1 ng of pRL-CMV as internal control using Lipofectamine 2000 and Opti-mem. The medium was then changed for either 3 or 20 mmol/l glucose RPMI for 20–24 h before cell lysis and luciferase assay. *P < 0.05 for the effect of glucose (n = 3–6).
Glucose repression of ARNT/HIF-1β gene expression requires the proximal promoter.
To investigate the role of ChREBP in mediating the glucose repression of the ARNT/HIF-1β gene, we transfected the various ARNT promoter-reporter luciferase constructs in INS-1 (832/13) cells and incubated them in 3 or 20 mmol/l glucose for 24 h. Only the construct lacking the whole of the proximal promoter (−1,028 to −769 ARNT) was no longer repressed by glucose; all the other mutants still showed significant glucose repression (Fig. 4B and data not shown).
ChREBP overexpression inhibits ARNT/HIF-1β expression and regulates glucose-induced insulin secretion from primary mouse islets.
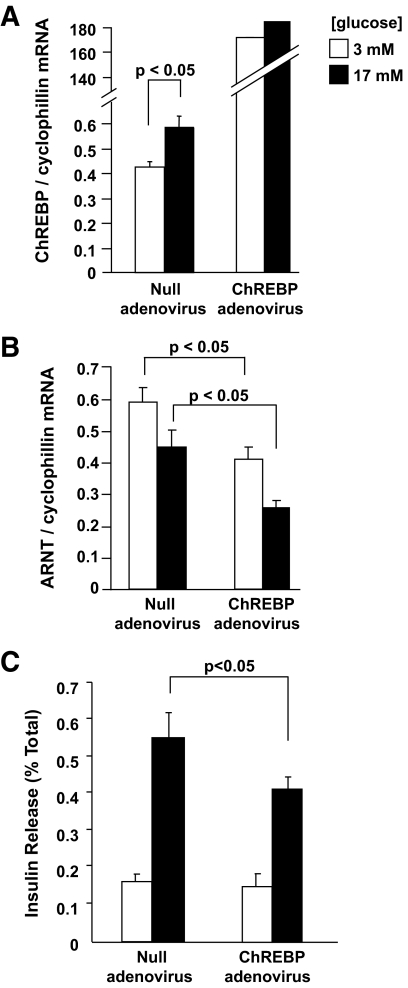
To determine whether the observations reported above in clonal β-cells were relevant to the primary β-cell, we infected isolated mouse islets of Langerhans with ChREBP-overexpressing adenovirus. Whereas endogenous ChREBP mRNA levels were augmented in islets cultured for 20 h at 17 versus 3 mmol/l glucose, as expected, transduction of islets with ChREBP adenovirus caused a >100-fold increase in ChREBP mRNA levels compared with those observed at either 3 or 17 mmol/l glucose in null adenovirus-transduced islets (Fig. 5A). This forced increase in ChREBP expression was associated with a decrease in ARNT/HIF-1β mRNA levels at both low and high (3 and 17 mmol/l) glucose (Fig. 5B).
FIG. 5.
Overexpression of ChREBP-encoding adenovirus decreases ARNT/HIF-1β gene expression and insulin secretion in primary mouse islets of Langerhans. A and B: Isolated mouse islets were cultured in 11 mmol/l glucose RPMI and infected with either null or ChREBP adenoviruses at an multiplicity of infection of 100 for 24 h before incubation for 16 h in 3 mmol/l glucose RPMI followed by 20 h in 3 or 17 mmol/l glucose RPMI before total RNA extraction. ARNT/HIF-1β and ChREBP mRNA levels were estimated by quantitative real-time RT-PCR. C: Isolated mouse islets were infected as above and maintained in 11 mmol/l RPMI for 48 h before insulin secretion assay. Four groups of six islets per condition were selected and preincubated for 1 h in Krebs-Ringer bicarbonate HEPES buffer containing 3 mmol/l glucose, followed by 30 min at either 3 or 17 mmol/l glucose. Released and total insulin were quantified by radioimmunoassay. The results are expressed as the means ± SE of three independent experiments.
We have previously demonstrated that ChREBP silencing enhanced basal and glucose-stimulated insulin secretion from MIN6 β-cells (13). Correspondingly, we observed here that adenovirus-mediated ChREBP overexpression in mouse islets inhibited GSIS by ∼30% (Fig. 5C).
DISCUSSION
The principal aim of the present study was to identify and characterize new target genes that may mediate the effects of ChREBP on β-cell function. To this end, and using an unbiased genome-wide approach, we have identified ARNT/HIF-1β as such a target. Previously shown to be downregulated in type 2 diabetic human islets, and to modulate insulin secretion in the mouse (8), repression of ARNT/HIF-1β by ChREBP represents an important potential mechanism through which the inhibitory effects of high glucose and of ChREBP may be exerted.
Regulation of ARNT/HIF-1β by ChREBP and glucose.
ARNT/HIF-1β expression has recently been shown (26) to be elevated at low glucose concentrations in pancreatic MIN6 β-cells. Both ARNT/HIF-1β and presenilin-1 expression were induced by low glucose, the latter being a component of the γ-secretase complex involved in the processing of the β-amyloid precursor processing in the brain, which may regulate β-cell survival via the Notch pathway (26). It was suggested in these earlier studies that ARNT/HIF-1β lies downstream of presenilin in a signaling pathway activated at low intracellular Ca2+ levels. The current data support and extend these findings by demonstrating that ARNT/HIF-1β is regulated by glucose in primary islets as well as in clonal β-cells. Moreover, the present study also supports the view that ChREBP may play a role that is complementary to that of presenilin-1 to control transcription of the ARNT/HIF-1β gene as glucose concentrations change. However, elevated glucose concentrations were still able to decrease ARNT/HIF-1β mRNA levels in the presence of increased, inhibitory, ChREBP levels (Fig. 3), suggesting that multiple signaling mechanisms may be involved in the regulation of ARNT/HIF-1β transcription by glucose. Interestingly, in a previous report (13), we demonstrated that ChREBP binding to the liver type (l-) pyruvate kinase gene was regulated in part by an increase in intracellular free Ca2+ concentrations. Recent studies by Scott and colleagues (29) suggested that activation by glucose of the l-PK gene in INS-1–derived 832/13 cells involves recruitment of c-Myc, Max, and ChREBP to the promoter and a glucose-stimulated increase in ChREBP transactivation. Whether the inhibitory effects of ChREBP binding at the ARNT/HIF-1β gene promoter are similarly mediated remains to be explored.
ChREBP has previously been shown to regulate transcription of target genes both positively or negatively, depending on the gene and context (10,12). Using ChIP, NoShift assay, and mutational analysis, we located the ChREBP/MLX binding site to the proximal region of ARNT/HIF-1β promoter, a region also mediating the negative glucose effect, but the precise cis element(s) have remained elusive. Although glucose repression of gene expression in yeast is now well understood (30), and involves the AMP-activated protein kinase analog Snf1 (31), our understanding of glucose inhibition of gene expression in mammalian cells is still rudimentary. In primary rat hepatocytes, Ma et al. (28) found 59 genes that were repressed by high glucose, and only eight of these were also increased by dominant-negative MLX. Whether or not ChREBP was the interacting partner of MLX in regulating the expression of these genes and whether ChREBP/MLX was mediating the glucose response needs further study.
Potential impact of ARNT/HIF-1β regulation on β-cell function.
What may be the physiological consequences for β-cell function or survival of ChREBP-mediated ARNT/HIF-1β repression at high glucose concentrations? The HIF-1 complex is believed to be an important regulator of the response to hypoxic stresses in other cell types (32). Inhibition of ARNT/HIF-1β expression by high glucose may thus contribute to the deleterious effects on β-cell mass or function during sustained hyperglycemia observed in type 2 diabetes. This may further exacerbate the effects of elevated nuclear levels of ChREBP to drive lipogenic gene expression and hence, potentially, contribute to “gluco-lipotoxicity” (1,33) under these conditions. Correspondingly, we show here for the first time that ChREBP overexpression inhibits glucose-stimulated insulin secretion from primary mouse islets by ∼50%. Therapeutic approaches that may suppress ChREBP function in islets may thus be useful for the treatment of insulin secretory deficiencies in some forms of type 2 diabetes.
Supplementary Material
Acknowledgments
This study was supported by a grant from Diabetes U.K. (BDA:RD04/0002895) to I.L. and support to G.A.R. from MRC (G0401641), the Wellcome Trust (Programme Grants 081958/Z/07/Z and 067081/Z/02/Z, Project Grant WT082366MA), the European Union (STREP6 “Save Beta”), and the National Institute of Health (DK071962-01).
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Christopher Newgard (Duke University) for providing INS-1(832/13) cells, Prof. Howard Towle (University of Minnesota) for providing MLX-HA plasmid, and Dr. Anindiya Roy (Cancer Research U.K.) for providing ChIP grade anti-HA antibody.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Rutter GA, Parton LE: The beta-cell in type 2 diabetes and in obesity. Front Horm Res 2008;36:118–134 [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM: Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 3.Stoffers DA, Ferrer J, Clarke WL, Habener JF: Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet 1997;17:138–139 [DOI] [PubMed] [Google Scholar]
- 4.Vaxillaire M, Boccio V, Philippi A, Vigouroux C, Terwilliger J, Passa P, Beckmann JS, Velho G, Lathrop GM, Froguel P: A gene for maturity onset diabetes of the young (MODY) maps to chromosome 12q. Nat Genet 1995;9:418–423 [DOI] [PubMed] [Google Scholar]
- 5.Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M, Bell GI: Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1). Nature 1996;384:458–460 [DOI] [PubMed] [Google Scholar]
- 6.Mitchell SM, Frayling TM: The role of transcription factors in maturity-onset diabetes of the young. Mol Genet Metab 2002;77:35–43 [DOI] [PubMed] [Google Scholar]
- 7.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P: A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007;445:881–885 [DOI] [PubMed] [Google Scholar]
- 8.Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O'Connell PJ, Gonzalez FJ, Kahn CR: Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 2005;122:337–349 [DOI] [PubMed] [Google Scholar]
- 9.Johansson LE, Lindblad U, Larsson CA, Råstam L, Ridderstråle M: Polymorphisms in the adiponutrin gene are associated with increased insulin secretion and obesity. Eur J Endocrinol 2008;159:577–583 [DOI] [PubMed] [Google Scholar]
- 10.Cairo S, Merla G, Urbinati F, Ballabio A, Reymond A: WBSCR14, a gene mapping to the Williams–Beuren syndrome deleted region, is a new member of the Mlx transcription factor network. Hum Mol Genet 2001;10:617–627 [DOI] [PubMed] [Google Scholar]
- 11.Uyeda K, Repa JJ: Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab 2006;4:107–110 [DOI] [PubMed] [Google Scholar]
- 12.Stoeckman AK, Ma L, Towle HC: Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem 2004;279:15662–15669 [DOI] [PubMed] [Google Scholar]
- 13.da Silva Xavier G, Rutter GA, Diraison F, Andreolas C, Leclerc I: ChREBP binding to fatty acid synthase and L-type pyruvate kinase genes is stimulated by glucose in pancreatic beta-cells. J Lipid Res 2006;47:2482–2491 [DOI] [PubMed] [Google Scholar]
- 14.Kennedy HJ, Viollet B, Rafiq I, Kahn A, Rutter GA: Upstream stimulatory factor-2 (USF2) activity is required for glucose stimulation of L-pyruvate kinase promoter activity in single living islet beta-cells. J Biol Chem 1997;272:20636–20640 [DOI] [PubMed] [Google Scholar]
- 15.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B: A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 1998;95:2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ainscow EK, Rutter GA: Mitochondrial priming modifies Ca2+ oscillations and insulin secretion in pancreatic islets. Biochem J 2001;353:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leclerc I, Woltersdorf WW, da Silva Xavier G, Rowe RL, Cross SE, Korbutt GS, Rajotte RV, Smith R, Rutter GA: Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 2004;286:E1023–E1031 [DOI] [PubMed] [Google Scholar]
- 18.Diraison F, Parton L, Ferré P, Foufelle F, Briscoe CP, Leclerc I, Rutter GA: Over-expression of sterol-regulatory-element-binding protein-1c (SREBP1c) in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release: modulation by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR). Biochem J 2004;378:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K: Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990;127:126–132 [DOI] [PubMed] [Google Scholar]
- 20.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB: Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000;49:424–430 [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual Cold Spring Harbor, Cold Spring Harbor Laboratory Press, 1989 [Google Scholar]
- 22.da Silva Xavier G, Qian Q, Cullen PJ, Rutter GA: Distinct roles for insulin and insulin-like growth factor-1 receptors in pancreatic beta-cell glucose sensing revealed by RNA silencing. Biochem J 2004;377:149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih HM, Towle HC: Definition of the carbohydrate response element of the rat S14 gene: evidence for a common factor required for carbohydrate regulation of hepatic genes. J Biol Chem 1992;267:13222–13228 [PubMed] [Google Scholar]
- 24.Bergot MO, Diaz-Guerra MJ, Puzenat N, Raymondjean M, Kahn A: Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acid Res 1992;20:1871–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K: Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem 2002;277:3829–3835 [DOI] [PubMed] [Google Scholar]
- 26.Dror V, Kalynyak TB, Bychkivska Y, Frey MH, Tee M, Jeffrey KD, Nguyen V, Luciani DS, Johnson JD: Glucose and endoplasmic reticulum calcium channels regulate HIF-1beta via presenilin in pancreatic beta-cells. J Biol Chem 2008;283:9909–9916 [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Wollheim CB: ChREBP rather than USF2 regulates glucose stimulation of endogenous L-pyruvate kinase expression in insulin-secreting cells. J Biol Chem 2002;277:32746–32752 [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Robinson LN, Towle HC: ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem 2006;281:28721–28730 [DOI] [PubMed] [Google Scholar]
- 29.Collier JJ, Zhang P, Pedersen KB, Burke SJ, Haycock JW, Scott DK: c-Myc and ChREBP regulate glucose-mediated expression of the L-type pyruvate kinase gene in INS-1-derived 832/13 cells. Am J Physiol Endocrinol Metab 2007;293:E48–E56 [DOI] [PubMed] [Google Scholar]
- 30.Carlson M: Glucose repression in yeast. Curr Opin Microbiol 1999;2:202–207 [DOI] [PubMed] [Google Scholar]
- 31.Hedbacker K, Carlson M: SNF1/AMPK pathways in yeast. Front Biosci 2008;13:2408–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS: Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol 2003;23:359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poitout V, Robertson RP: Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.