Abstract
OBJECTIVE
An emerging model of metabolic syndrome and type 2 diabetes is of adipose dysfunction with leukocyte recruitment into adipose leading to chronic inflammation and insulin resistance (IR). This study sought to explore potential mechanisms of inflammatory-induced IR in humans with a focus on adipose tissue.
RESEARCH DESIGN AND METHODS
We performed a 60-h endotoxemia protocol (3 ng/kg intravenous bolus) in healthy adults (n = 20, 50% male, 80% Caucasian, aged 27.3 ± 4.8 years). Before and after endotoxin, whole-blood sampling, subcutaneous adipose biopsies, and frequently sampled intravenous glucose tolerance (FSIGT) testing were performed. The primary outcome was the FSIGT insulin sensitivity index (Si). Secondary measures included inflammatory and metabolic markers and whole-blood and adipose mRNA and protein expression.
RESULTS
Endotoxemia induced systemic IR as demonstrated by a 35% decrease in Si (3.17 ± 1.66 to 2.06 ± 0.73 × 10−4 [μU · ml−1 · min−1], P < 0.005), while there was no effect on pancreatic β-cell function. In adipose, endotoxemia suppressed insulin receptor substrate-1 and markedly induced suppressor of cytokine signaling proteins (1 and 3) coincident with local activation of innate (interleukin-6, tumor necrosis factor) and adaptive (monocyte chemoattractant protein-1 and CXCL10 chemokines) inflammation. These changes are known to attenuate insulin receptor signaling in model systems.
CONCLUSIONS
We demonstrate, for the first time in humans, that acute inflammation induces systemic IR following modulation of specific adipose inflammatory and insulin signaling pathways. It also provides a rationale for focused mechanistic studies and a model for human proof-of-concept trials of novel therapeutics targeting adipose inflammation in IR and related consequences in humans.
Adipose dysfunction, insulin resistance (IR), and type 2 diabetes are proinflammatory states. Indeed, gene manipulation of Toll-like receptors (1,2), chemokines (3,4), and cytokines in experimental models has defined a role of innate and adaptive immunity in diet-induced adipose dysfunction and IR. An emerging model is one of early adipose recruitment of T-cells and macrophages with adipocyte inflammation and resistance to insulin-promoting metabolic syndrome, type 2 diabetes, and atherosclerosis.
Experimental data suggest that inflammation may attenuate adipocyte insulin signaling. Dietary and inflammatory activation of the inhibitor of nuclear factor κB kinase β-subunit (IKKβ), c-Jun NH2-terminal kinase (JNK) (5), protein kinase C (PKC) (6), and janus tyrosine kinases (JAK)/signal transducers and activators of transcription (STAT) (7) have been implicated in IR in adipocytes and rodent experimental models (7). These inflammatory kinases may promote IR by downregulating components of the insulin signaling cascade, including the insulin receptor and insulin receptor substrates (IRSs), and by inducing suppressors of cytokine signaling (SOCS), inhibitors of insulin receptor signaling. Despite substantial data in animal models, however, little is known of the specific role and mechanisms of inflammatory IR in humans. Defining whether adipose inflammation occurs in human IR and which specific pathways are involved will provide greater insight into human pathophysiology and inform preventive and therapeutic strategies for type 2 diabetes and its complications.
We and others utilize low-dose experimental endotoxemia to activate Toll-like receptor (TLR)-4 signaling in vivo as a model of inflammation-induced metabolic disturbances in humans (8–10). Here, we define the effects of endotoxemia on insulin sensitivity and focus on adipose inflammation because of its emerging relevance in dietary excess and adipose dysfunction in human IR. We demonstrate, for the first time in humans, that activation of innate immunity in vivo induces IR following modulation of specific adipose inflammatory and insulin signaling pathways.
RESEARCH DESIGN AND METHODS
Healthy volunteers were recruited from the general population of the Delaware Valley (10). The protocol was approved by the institutional review board of the University of Pennsylvania, and subjects gave written informed consent. Full details are described in the online appendix (available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0367/DC1). Briefly, criteria included healthy men or nonpregnant/lactating women, aged 18–40 years, with BMI of 18–30 kg/m2. Twenty subjects were recruited, equally divided by sex, to the University of Pennsylvania's Clinical Translational Research Center for three visits: visit 1 for screening; visit 2, 2 weeks later, for frequently sampled intravenous glucose tolerance (FSIGT) testing and dietary counseling; and visit 3, consisting of an overnight acclimatization phase, a 24-h saline control phase, and a 24-h post-lipopolysaccharide (LPS) study phase (60-h total). LPS (U.S. standard reference endotoxin, lot no. CC-RE-LOT-1 + 2; Clinical Center, Pharmacy Department, National Institutes of Health) was given intravenously as a 3 ng/kg bolus at 0600 h on day 2. Blood samples (nine before and nine after LPS) and subcutaneous gluteal adipose aspiration-biopsy samples (before and 4, 12, and 24 h after LPS) were collected (in n = 17 participants; 621.4 ± 253.1 mg average weight per sample).
Laboratory measures.
Two weeks prior to LPS and 24 h following LPS, the insulin sensitivity index (Si) was derived from an FSIGT test. We chose the 24-h post-LPS because we expected IR to be established by this time point based on human and animal experimental and observational data (8,10–12) and because of practical considerations given the experimental design. We chose FSIGT as the method for determining insulin sensitivity because the test also provides a measure of pancreatic β-cell function, the acute insulin response to glucose (AIRg), and so allows assessment of endotoxemia effects on the β-cell. The FSIGT test was conducted using the insulin-modified approach as previously described (13). Si was derived from Bergman's minimal model (14) using MINMOD Millennium software (15). AIRg was calculated as the incremental area under the curve for insulin from t = 0 to 10 min (12). Complementary estimates of IR and β-cell function, the homeostasis model assessment for IR (HOMA-IR) index [glucose (mmol/l) × insulin (μU/ml)/22.5], and the HOMA for β-cell function (HOMA-B) index [insulin (μU/ml) × 20/glucose (mmol/l) − 3.5] were calculated using fasting glucose and insulin values at 24 h and 5 min before and 24 h after LPS. Plasma biomarkers and lipoprotein measurement was described previously (10) and is outlined in the online appendix.
RNA isolation, real-time PCR quantification of mRNAs, and adipose protein isolation for Western blotting are described in detail in the online appendix. RNA extraction was performed for measurement of mRNA levels of interleukin (IL)-6, tumor necrosis factor (TNF)-α, TNF-induced protein 3 (A20), resistin, SOCS-1, SOCS-2, SOCS-3, SOCS-6, insulin receptor, IRS-1, IRS-2, IRS-3, IRS-4, GLUT4, and chemokines monocyte chemoattractant protein-1 (MCP-1) and C-X-C motif ligand 10 (CXCL10) (online appendix Table 1). Macrophage marker human epidermal growth factor module–containing mucin-like receptor 1 (EMR1-F4/80) mRNA also was assayed in adipose.
TABLE 1.
Baseline characteristics of study participants
| Age (years) | 25.7 ± 3.90 |
| Blood pressure | |
| Systolic | 116 ± 15 |
| Diastolic | 67 ± 8 |
| Total cholesterol (mmol/l) | 4.53 ± 0.52 |
| HDL cholesterol (mmol/l) | |
| Male | 1.33 ± 0.26 |
| Female | 1.69 ± 0.34 |
| Triglycerides (mmol/l) | 0.86 ± 0.39 |
| Fasting glucose (mmol/l) | 4.68 ± 0.28 |
| BMI (kg/m2) | |
| Male | 24.27 ± 1.62 |
| Female | 23.37 ± 0.78 |
| Waist circumference (cm) | |
| Male | 83.9 ± 5.81 |
| Female | 82.8 ± 5.1 |
| Total body fat dual-energy X-ray absorptiometry (%) | |
| Male | 15.3 ± 3.4 |
| Female | 29.7 ± 3.3 |
| FSIGT-Si (×10−4 μU · ml−1 · min−1) | 3.17 ± 1.66 |
| HOMA-IR insulin resistance index | 1.56 ± 0.63 |
| FSIGT-AIRg (μU · ml−1 · min−1) | 701 ± 120 |
| HOMA-B β-cell function index | 104 ± 36 |
Data are means ± SD.
Statistical analysis.
Data are reported as means ± SE for continuous variables and as proportions for categorical variables. In general, the effect of endotoxemia on plasma biomarkers, metabolic measures, blood and adipose mRNAs, and adipose protein levels were tested by mixed-effects modeling or repeated-measures ANOVA. For plasma data, we considered the time-matched difference (after minus before for matched time points prior to and following LPS) in biomarker responses and used a mixed-effects model to consider the effect due to LPS. A variety of models including linear, quadratic, and cubic polynomial were fit, and the best fit for each variable was used for final analyses (e.g., quadratic models for TNF, IL-6, and resistin). A similar time-matched mixed-effect modeling approach was applied to whole-blood mRNA data. Repeated-measures ANOVA was applied to FSIGT data (Si and AIRg), HOMA data (HOMA-IR and HOMA-B), and adipose mRNA data. When significant global differences were found in ANOVA, post hoc paired t tests were used to compare time points. Analyses were performed using the freeware statistical package R (version 2.4.1; The R Foundation for Statistical Computing). Statistical significance was defined as a P value <0.05.
RESULTS
Baseline characteristics of participants and clinical responses to endotoxemia.
Participants were healthy adults volunteers (n = 20, 50% male, 80% Caucasian, aged 25.7 ± 3.9 years). They had normal blood pressure, plasma lipoproteins, BMI, and body fat distribution (Table 1), and baseline Si and HOMA-IR were consistent with data from healthy subjects published by our group (13). Endotoxemia produced a transient febrile illness in all subjects with increases in temperature, heart rate, and white blood cell count largely resolving by 8–12 h following LPS (Table 2).
TABLE 2.
Peak clinical and systemic inflammatory responses during endotoxemia
| Pre-LPS | Post-LPS | Time of peak (h) | |
|---|---|---|---|
| Plasma IL-6 (ng/ml) | 3.2 ± 17.1 | 1,607 ± 650* | 2 |
| Plasma TNF (ng/ml) | 3.0 ± 1.1 | 507 ± 251* | 2 |
| Heart rate (bpm) | 83 ± 11 | 103 ± 14 | 4 |
| Temperature (°C) | 36.5 ± 0.5 | 37.7 ± 1.0† | 4 |
| White blood cell count (mg/μl) | 6.5 ± 0.9 | 12.9 ± 4.1† | 12 |
| Plasma hsCRP | 0.49 ± 0.21 | 42.4 ± 8.4† | 24 |
Clinical parameters and plasma levels of cytokines are presented as means ± SD.
*P < 0.0001;
†P < 0.005.
Systemic inflammatory and metabolic responses.
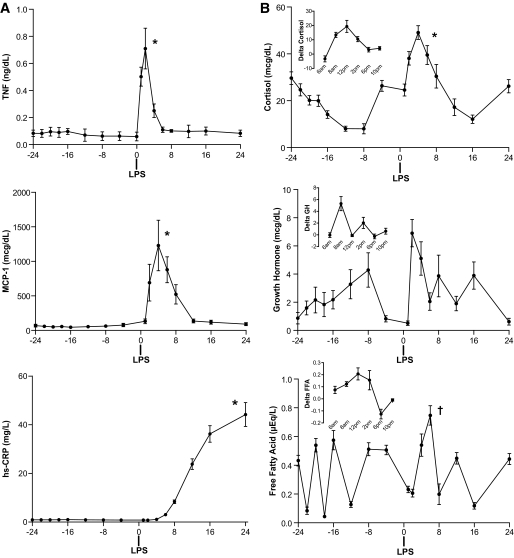
We have previously described plasma adipokine responses in this study sample (10). Briefly, endotoxemia induced a marked, rapid, and transient induction of plasma TNF and IL-6 (Table 2), followed by a robust increase in circulating resistin and a delayed but significant increase in leptin and leptin-soluble leptin ratio in plasma. We now report significant increases in additional circulating inflammatory markers including MCP-1 and high-sensitivity C-reactive protein (hsCRP) (Fig. 1A) as well as plasma cortisol and free fatty acids, with a trend toward increased growth hormone (GH) (P = 0.08) levels (Fig. 1B). Overall, these findings confirm a transient inflammatory response during human endotoxemia with modulation of several inflammatory, adipokine, and hormonal cascades that are known to impact insulin sensitivity.
FIG. 1.
Plasma levels of inflammatory and hormonal markers. A: Endotoxemia increased plasma TNF-α, MCP-1, and hsCRP. B: Plasma cortisol and free fatty acids increased significantly with a trend for increase in GH (P = 0.08). Inset graphs show the difference for 24 h after versus 24 h before LPS. *P < 0.0001 and †P < 0.005 in mixed-effects model.
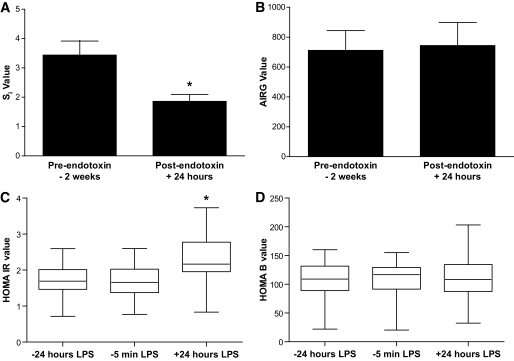
Experimental endotoxemia induces IR in humans.
We hypothesized that these inflammatory and metabolic perturbations would induce IR without altering pancreatic β-cell function. Insulin sensitivity, measured as Si, declined by over 30% from 2 weeks prior to 24 h after LPS (3.17 ± 1.66 to 2.06 × 10−4 [μU · ml−1 · min−1], P < 0.005) (Fig. 2A). In contrast, AIRg, a measure of pancreatic β-cell function, showed no change following LPS (711 ± 418 to 744 ± 488 μU · ml−1 · min−1, P = 0.7) (Fig. 2B). Consistent with the FSIGT data, HOMA-IR was 39 and 46% higher at 24 h after LPS (2.17 ± 0.83) than 24 h (1.56 ± 0.62; P < 0.001) and 5 min (1.49 ± 0.63; P < 0.001) prior to LPS (Fig. 2C), while HOMA-B, an estimate of pancreatic β-cell function, was not influenced by the endotoxemia state (P = 0.81) (Fig. 2D). We observed a modest negative correlation (Spearman R = −0.51) between HOMA-IR and Si consistent with prior work highlighting the differences in fasting versus evoked physiology captured by these indexes (16).
FIG. 2.
Endotoxemia induced IR without change in pancreatic β-cell function. Endotoxemia suppressed the insulin sensitivity index (Si) at FSIGT testing (A), whereas there was no change in the FSIGT test-derived acute insulin response to glucose (AIRG) (B), a measure of pancreatic β-cell function. Consistent with this, the HOMA of IR fell following LPS (C), while the HOMA-B estimate of β-cell function was unchanged (D). *ANOVA P < 0.001.
We explored whether peak levels of inflammatory and metabolic biomarkers (TNF, IL-6, MCP-1, hsCRP, resistin, cortisol, GH, or free fatty acids) correlated with the degree of induced IR. We found that peak hsCRP (Spearman R = 0.57, P < 0.03) and resistin (r = 0.47, P = 0.08) tended to correlate with change in HOMA-IR, a measure of hepatic IR, while the peak FFA levels were inversely correlated (r = −0.81, P = 0.004) with Si, a measure of peripheral insulin sensitivity.
Endotoxemia induces adipose inflammation in humans.
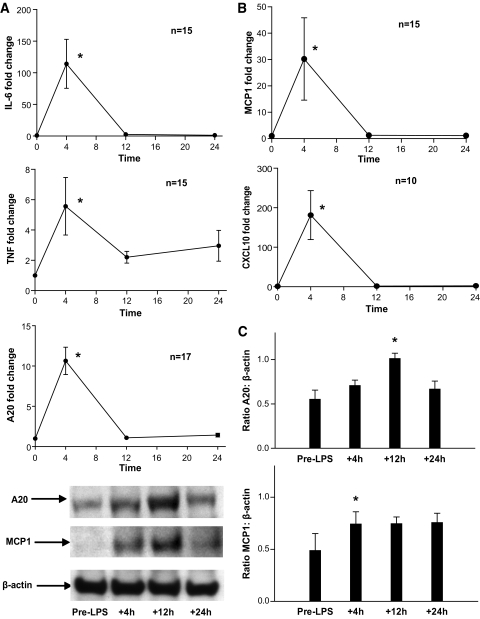
Endotoxemia induced a robust innate inflammatory response in adipose with increases in mRNA levels of IL-6 (peak 110-fold, ANOVA F = 64.5, P < 0.001), TNF (peak 6-fold, F = 4.9, P < 0.001), and A20 (peak 11-fold, F = 79.7, P < 0.001) (Fig. 3A). In parallel, adipose mRNA levels of MCP-1 (peak 30-fold, F = 3.49, P = 0.03), a chemokine implicated in adipose macrophage recruitment (3,4), and CXCL10 (peak 15-fold, F = 8.5, P < 0.005), a T-cell chemokine, were markedly induced during endotoxemia (Fig. 3B). Supporting these mRNA changes, protein levels of both A20 and MCP-1 were significantly increased in adipose at Western blotting (Fig. 3C). Remarkably, adipose mRNA levels of resistin (fourfold, F = 6.9, P < 0.001), a leukocyte-derived cytokine in humans, and EMR1-F4/80 (peak 13-fold, F = 13.8, P < 0.001), a macrophage marker, were increased, suggesting recruitment of leukocytes to adipose during endotoxemia.
FIG. 3.
Endotoxemia induced adipose inflammation in humans. A: Endotoxemia increased adipose IL-6, TNF-α, and TNF-induced protein 3 (A20) mRNA levels. B: In parallel, mRNA levels of MCP-1 and CXCL10, monocyte, and T-cell chemokines were induced. C: Western blotting confirmed increases in adipose A20 and MCP-1 proteins (representative blot shown; densitometry n = 3; *ANOVA P < 0.05).
Endotoxemia modulates the insulin signaling pathway in adipose and blood.
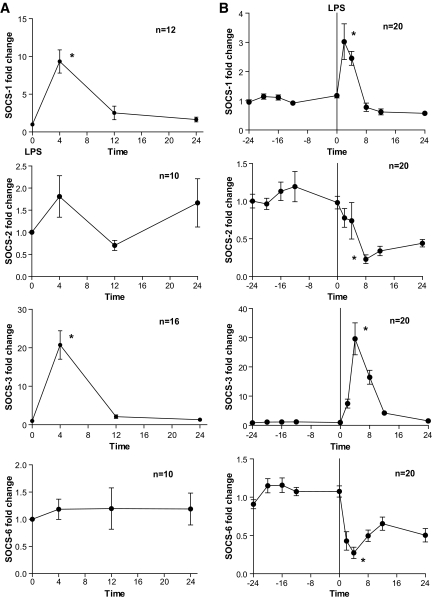
We sought evidence that adipose inflammation modulated insulin signaling pathways in adipose. SOCS family proteins inhibit tyrosine kinase receptor signaling including the insulin receptor. At baseline prior to LPS, SOCS-3, SOCS-6, and SOCS-2 mRNAs were the most abundant in human adipose with evidence of differential expression compared with whole blood (supplementary Table 2). Following endotoxin, adipose SOCS-1 (10-fold) and SOCS-3 (20-fold), but not SOCS-2 or SOCS-6, mRNAs increased markedly (Fig. 4A). There were similar striking changes in whole-blood SOCS-1 and SOCS-3 (3-fold and 30-fold, respectively), but endotoxin actually reduced SOCS-2 and SOCS-6 mRNA in blood (Fig. 4B).
FIG. 4.
Differential modulation of adipose and whole-blood SOCS proteins. Endotoxemia induced (A) adipose and (B) whole-blood mRNA levels of SOCS-1 and SOCS-3, but not SOCS-2 and SOCS-6. *P < 0.0001 in ANOVA (adipose) or mixed-effects model (blood).
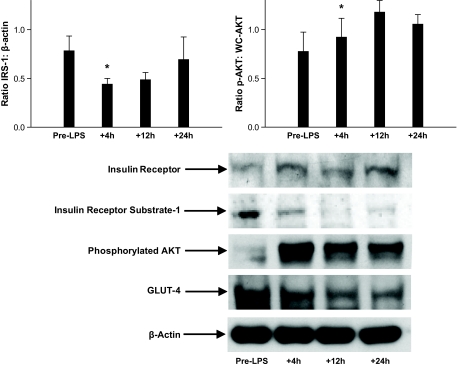
IRS family members are key mediators of cellular insulin signaling. Prior to LPS, we detected mRNA for IRS-1 and IRS-2 but not IRS-3 or IRS-4 in adipose with differential expression compared with whole blood (supplementary Table 2). Endotoxin reduced IRS-1 (by 47%) mRNA in adipose. In whole blood, IRS-1 mRNA levels also fell (by 53%), but IRS-2 mRNA increased (2.3-fold increase) (Table 3). In parallel, insulin receptor mRNA levels decreased significantly in blood but not in adipose, and there was no significant change in GLUT4 mRNA in adipose or whole blood (Table 3). Western blotting of adipose proteins generally supported mRNA data with reduction in IRS-1 but no change in insulin receptor; however, GLUT4 protein levels tended to fall (Fig. 5). Furthermore, enhanced serine phosphoryation of AKT, with no change in total AKT (Fig. 5), was observed consistent with previous findings of TNF-induced serine phosphorylation of AKT in adipocytes (17). Overall, endotoxemia induced specific SOCS proteins and modulated several components of the insulin signaling pathway in adipose.
TABLE 3.
Insulin signaling proteins are modulated in adipose and whole blood during endotoxemia
| Gene | Adipose mRNA expression |
Blood mRNA expression |
||||
|---|---|---|---|---|---|---|
| Maximal percent change | Time (h) | ANOVA P value | Maximal percent change | Time (h) | Mixed-effects model P value | |
| Insulin receptor | −25 | 12 | 0.26 | −20 | 2 | <0.001 |
| IRS-1 | −47 | 4 | 0.03 | −53 | 4 | 0.03 |
| IRS-2 | −25 | 4 | 0.36 | +230 | 12 | <0.001 |
| IRS-3 | ND | — | — | −50 | 4 | <0.001 |
| GLUT4 | −24 | 12 | 0.33 | −25 | 12 | 0.02 |
ND, not detected.
FIG. 5.
Western blot of insulin signaling proteins in adipose tissue. Relative to β-actin, endotoxin reduced protein levels of IRS-1 and GLUT4, increased phosphorylated-AKT (p-AKT), and had no effect on insulin receptor expression (representative blot shown; densitometry for IRS-1 and p-AKT n = 3; *ANOVA P < 0.01).
DISCUSSION
Inflammation, particularly in adipose tissue, has been implicated in diet- and obesity-related IR in experimental models. Resistance to insulin also occurs acutely in human states of infection and sepsis. However, the specific mechanisms and the potential for therapeutic targeting in humans are poorly understood. In this work, we found that endotoxemia induced systemic IR but not pancreatic β-cell dysfunction in humans. Further, IR measured at 24 h post-LPS was preceded by specific modulation of adipose inflammatory and insulin signaling pathways. This work defines specific targets for inflammatory modulation of insulin signaling in humans and also provides a human model for proof-of-concept studies of novel therapeutics in IR and its complications.
Epidemiological studies (18,19) suggest causal links between chronic inflammation, IR, and incident type 2 diabetes, while observational data demonstrate that IR and overt type 2 diabetes may emerge during human infections and sepsis (11). Agwunobi et al. (8) were the first to show impaired insulin sensitivity 6–7 h following LPS administration utilizing euglycemic clamp studies. Our study goes beyond the findings of Agwunobi et al. by demonstrating persistence of IR at 24 h after endotoxin in the absence of any effect on pancreatic β-cell function while also identifying adipose tissue inflammatory responses and modulation of specific adipose insulin signaling proteins that precede systemic IR. Interestingly, Agwunobi et al. also noted enhanced insulin sensitivity 2 h after LPS as determined by a significant increase in the glucose infusion rate required during the clamp. A recent elegant study (20) using isotope tracers with a euglycemic clamp showed that this acute and transient increase in insulin sensitivity at 1–2 h after LPS was due to increases in both hepatic and peripheral insulin sensitivity.
Experimental models support an important role for innate and adaptive immunity in diet- and obesity-induced IR (1,3–5,21). Deficiency of TLR-4, the innate antigen/LPS receptor, protects against diet-induced obesity and IR in rodents (2). TNF impairs insulin-mediated glucose disposal, and functional TNF deficiency in mice protects from obesity-induced IR (21). However, the relevance to human pathophysiology of individual signaling pathways implicated in rodent models remains unknown. In fact, species heterogeneity in inflammatory modulation (22) and of insulin signaling has been documented (9,23). Thus, use of human models of inflammation can provide unique insight into clinically relevant mechanisms and therapeutic targets for IR and type 2 diabetes.
Our study is the first to demonstrate loss of insulin sensitivity without any apparent effect on pancreatic β-cell function during acute human inflammation. Because fasting-based HOMA-IR estimates have been shown to correlate best with measures of hepatic insulin sensitivity and FSIGT Si with measures of peripheral insulin sensitivity (24), endotoxemia appears to trigger both hepatic and peripheral IR. Indeed, we note that Agwunobi et al. (8) published data with euglycemic clamps that demonstrate hepatic IR following LPS. Further, we describe several inflammatory perturbations that may impact tissue and systemic insulin sensitivity, induction of inflammatory cytokines and chemokines, modulation of adipokine signaling (10), activation of the hypothalamic-pituitary-adrenal axis (25), and altered flux of plasma free fatty acids (26). Indeed, the degree of evoked change in several inflammatory and metabolic markers, including free fatty acids, hsCRP, resistin, and GH tended to precede and correlate with the degree of IR. Taken together, therefore, these data support a model of both hepatic and peripheral IR during endotoxemia, with peripheral IR likely to be occurring at the level of skeletal muscle as well as adipose tissue.
Recent experimental studies in rodents demonstrated that adipose recruitment of T-cell and macrophages in obesity promotes adipocyte inflammation leading to local and systemic IR (27). We hypothesized that adipose inflammation would be a consequence of human endotoxemia that might contribute to local and systemic IR. Endotoxemia induced a rapid and transient increase in adipose TNF and IL-6. There was also a marked induction of adipose MCP-1, which is known to recruit chemokine CC motif receptor (CCR)-2–expressing monocytes, increase inflammatory-M1 adipose tissue macrophage (ATM), and promote IR (27). Recent studies of diet-induced obesity suggest that upregulation of T-cell chemokines in adipose and recruitment of inflammatory TH1 cells precedes recruitment of monocytes and the development of systemic IR. Remarkably, we found that endotoxemia induced CXCL10, a potent T-cell chemokine. The emergence of resistin mRNA in adipose suggests leukocyte recruitment because expression of this adipokine is restricted to myeloid lineage in humans (23). In addition, we found increased mRNA levels of the macrophage marker EMR1-F4/80 (28) in adipose, further supporting that endotoxemia may promote adipose recruitment of macrophages. Overall, these data suggest that endotoxemia induces human adipose inflammatory responses similar to those observed in models of diet- and obesity-related IR (1–4,21,27).
Whether inflammation attenuates adipose insulin signaling in humans and which signaling pathways are involved has not been defined. Several inflammatory adipokines such as TNF, IL-6, and resistin, as well as endotoxin itself, induce SOCS proteins that inhibit insulin receptor signaling and target IRS proteins for ubiquitination and proteosomal degradation (29,30). The SOCS family, consisting of eight members, is recognized as a general negative feedback mechanism for receptor tyrosine kinase signaling including the insulin receptor. Using the yeast two-hybrid system, SOCS-1, SOCS-3, and SOCS-6 have been shown to bind to the insulin receptor (31), and cells from SOCS-1–deficient mice exhibit enhanced insulin sensitivity (30). Conversely, in obesity, SOCS-1 and SOCS-3 are increased in liver, muscle, and fat coincident with reduced tyrosine phosphorylation of IRS proteins. We report for the first time the pattern of SOCS family mRNA expression in human adipose and a marked and selective induction of adipose SOCS proteins during endotoxemia; SOCS-1 and SOCS-3 were increased with no effect of SOCS-2 and SOCS-6. Our findings suggest that induction of SOCS-3 in adipose may be an important molecular mechanism of IR in human inflammatory states (31).
The in vivo effect of inflammation on insulin receptor signaling in human adipose is unknown. The insulin receptor, a transmembrane dimeric protein with intrinsic kinase activity, recruits IRS proteins upon insulin binding. Tyrosine phosphorylation of IRS proteins activates phosphatidylinositol-3-kinase, leading to AKT phosphorylation and GLUT4 mobilization (32). Inflammatory kinases including IKKβ (5,33), JNK (5), PKCs (6), and JAK-STATs attenuate insulin signaling in adipocytes and in rodent models. These kinases induce serine phosphorylation of IRS-1, which inhibits IRS-1 tyrosine phosphorylation during insulin signaling (32). We found tissue-specific IRS expression and downregulation of adipose IRS-1 protein coincident with reduced IRS-1 mRNA. Our data also suggest species heterogeneity in the pattern of adipose IRS expression with more abundant IRS-2 in human adipose compared with that reported in rodents (32). The effect of endotoxemia on IRS-1 protein levels is one of several mechanisms by which endotoxemia may impair insulin signaling in human adipose. Remarkably, changes in several mRNAs in whole blood paralleled that in adipose (e.g., IL-6, MCP1, SOCS-1, and SOCS-3). However, inflammation appears to modulate specific insulin signaling–related proteins (insulin receptor, SOCS-2 and SOCS-6, and IRS-2) in a tissue-specific manner. This should prompt caution in extrapolating tissue-specific effects from global characterization of whole-blood mRNAs.
Overall, endotoxemia induces IR in humans following modulation of adipose tissue inflammatory and insulin signal pathways in vivo. While adipose dysfunction in genetically and environmentally susceptible patients may increase inflammation, experimental endotoxemia may also induce adipose inflammation and subsequent adipocyte dysfunction, which then leads to IR. However, our study has several limitations. While we have not definitively proven that adipose inflammation is causal in systemic IR during endotoxemia, our work provides proof of principle that inflammation-induced systemic IR emerges after inflammatory modulation of adipose insulin signaling in humans. We emphasize the need for specific study of the chronic low-grade human inflammation observed in obesity, metabolic syndrome, and type 2 diabetes. Our approach to study insulin sensitivity using the FSIGT-derived Si at 24 h post-LPS is limited in that we cannot differentiate various contributions of changes in hepatic and peripheral insulin sensitivity versus the total body change and cannot define the kinetics of the development and resolution of IR. However, we utilized the FSIGT Si as a sensitive measure of peripheral IR and combined this with the HOMA-IR that correlates best with measures of hepatic insulin sensitivity (24). Furthermore, FSIGT enabled examination of whether pancreatic β-cell function changes during inflammation-induced IR, while the hyperinsulinemic-eugylemic clamp does not. We acknowledge that inflammatory effects are likely to occur in liver and in skeletal muscle during endotoxemia and that these could impact systemic IR. Indeed, our HOMA-IR data and our FSIGT data support both hepatic and peripheral IR consistent with published euglycemic clamp studies. Detailed examination of changes in skeletal muscle and greater study of adipose tissue inflammation and function in relationship to the kinetics of IR is warranted in future studies. Despite these limitations, our study provides the first tissue level data on evoked inflammatory pathways in human IR.
Finally, endotoxemia may not represent accurately the pathophysiology of chronic inflammatory, insulin-resistant disease states. Several lines of evidence, however, support its relevance to the pathophysiology of IR in humans. First, an inflammatory IR and metabolic dyslipidemia emerges clinically during acute sepsis (11) and chronic infections (34). Second, we and others have shown that cytokine/adipokine (10,23,35), acute-phase reactant responses, and lipoprotein changes (36,37) observed acutely during experimental endotoxemia resemble those chronically observed in the metabolic syndrome. Third, gene manipulation and drug targeting of the TLR-4 (2,38) and nuclear factor κB (5,39) have provided proof of concept that modulation of innate immune signaling attenuates IR and type 2 diabetes in dietary and obesity models. Last, and directly relevant to the effect on adipose, we recently demonstrated that endotoxemia induces gene expression responses in subcutaneous adipose (40) that are remarkably similar to the changes observed in visceral adipose in insulin-resistant states (41–43).
Conclusion.
Human endotoxemia induces systemic IR but not pancreatic β-cell dysfunction. Remarkably, evoked adipose inflammation and modulation of adipose insulin signal pathways, similar to some of those described in rodent models of diet-induced obesity and IR, precede the emergence of systemic IR in humans. Our findings suggest specific targets in humans that warrant further mechanistic focus. For example, induction of specific SOCS proteins and downregulation of IRS-1 are likely to play roles in the inflammatory induction of adipose and systemic IR in humans. This work also provides a human experimental model for studies of novel therapeutics targeting systemic and adipose inflammation in IR and its metabolic consequences.
Supplementary Material
Acknowledgments
This work was supported by a Clinical and Translational Science Award (UL1RR024134) from the National Center for Research Resources (NCRR) and a Diabetes and Endocrine Research Center (P20-DK019525) award, both to the University of Pennsylvania. M.P.R. is also supported by RO1 HL-073278 and P50 HL-083799-SCCOR from the National Institutes of Health. N.N.M. is a recipient of the ACC Young Investigator Award for the metabolic syndrome.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW: Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 2007;100:1589–1596 [DOI] [PubMed] [Google Scholar]
- 2.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ: Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007;56:1986–1998 [DOI] [PubMed] [Google Scholar]
- 3.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M: MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006;116:1494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoelson SE, Lee J, Goldfine AB: Inflammation and insulin resistance. J Clin Invest 2006;116:1793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, Littman DR, Shulman GI: PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 2004;114:823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo D, Dunbar JD, Yang CH, Pfeffer LM, Donner DB: Induction of Jak/STAT signaling by activation of the type 1 TNF receptor. J Immunol 1998;160:2742–2750 [PubMed] [Google Scholar]
- 8.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL: Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab 2000;85:3770–3778 [DOI] [PubMed] [Google Scholar]
- 9.Hudgins LC, Parker TS, Levine DM, Gordon BR, Saal SD, Jiang XC, Seidman CE, Tremaroli TD, Lai J, Rubin AL: A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J Lipid Res 2003;44:1489–1498 [DOI] [PubMed] [Google Scholar]
- 10.Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L, Comiskey LL, Tabita-Martinez J, Sellers KF, Rickels MR, Ahima RS, Reilly MP: Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab 2007;92:2036–2037 [DOI] [PubMed] [Google Scholar]
- 11.Van Cromphaut SJ, Vanhorebeek I, Van den Berghe G: Glucose metabolism and insulin resistance in sepsis. Curr Pharm Des 2008;14:1887–1899 [DOI] [PubMed] [Google Scholar]
- 12.McCowen KC, Ling PR, Ciccarone A, Mao Y, Chow JC, Bistrian BR, Smith RJ: Sustained endotoxemia leads to marked down-regulation of early steps in the insulin-signaling cascade. Crit Care Med 2001;29:839–846 [DOI] [PubMed] [Google Scholar]
- 13.Rickels MR, Naji A, Teff KL: Insulin sensitivity, glucose effectiveness, and free fatty acid dynamics after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab 2006;91:2138–2144 [DOI] [PubMed] [Google Scholar]
- 14.Bergman RN, Prager R, Volund A, Olefsky JM: Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 1987;79:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN: MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 2003;5:1003–1015 [DOI] [PubMed] [Google Scholar]
- 16.Anderson RL, Hamman RF, Savage PJ, Saad MF, Laws A, Kades WW, Sands RE, Cefalu W: Exploration of simple insulin sensitivity measures derived from frequently sampled intravenous glucose tolerance (FSIGT) tests: the Insulin Resistance Atherosclerosis Study. Am J Epidemiol 1995;142:724–732 [DOI] [PubMed] [Google Scholar]
- 17.Guo D, Donner DB: Tumor necrosis factor promotes phosphorylation and binding of insulin receptor substrate 1 to phosphatidylinositol 3-kinase in 3T3–L1 adipocytes. J Biol Chem 1996;271:615–618 [DOI] [PubMed] [Google Scholar]
- 18.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM: C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM: Insulin resistance, inflammation, and the prediabetic state. Am J Cardiol 2003;92:18J–26J [DOI] [PubMed] [Google Scholar]
- 20.van der Crabben SN, Blumer RM, Stegenga ME, Ackermans MT, Endert E, Tanck MW, Serlie MJ, van der Poll T, Sauerwein HP: Early endotoxemia increases peripheral and hepatic insulin sensitivity in healthy humans. J Clin Endocrinol Metab 2009;94:463–468 [DOI] [PubMed] [Google Scholar]
- 21.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS: Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997;389:610–614 [DOI] [PubMed] [Google Scholar]
- 22.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D: Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol 2005;12:60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaev I, Bokarewa M, Tarkowski A, Smith U: Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes. PLoS ONE 2006;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdul-Ghani MA, Sabbah M, Kher J, Minuchin O, Vardi P, Raz I: Different contributions of insulin resistance and beta-cell dysfunction in overweight Israeli Arabs with IFG and IGT. Diabetes Metab Res Rev 2006;22:126–130 [DOI] [PubMed] [Google Scholar]
- 25.Gerich JE: Role of growth hormone in diabetes mellitus. N Engl J Med 1984;310:848–850 [DOI] [PubMed] [Google Scholar]
- 26.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI: Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 1999;103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW, Jr: CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006;116:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khazen W, M'Bika P, Tomkiewicz C, Benelli C, Chany C, Achour A, Forest C: Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett 2005;579:5631–5634 [DOI] [PubMed] [Google Scholar]
- 29.Ueki K, Kondo T, Kahn CR: Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 2004;24:5434–5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawazoe Y, Naka T, Fujimoto M, Kohzaki H, Morita Y, Narazaki M, Okumura K, Saitoh H, Nakagawa R, Uchiyama Y, Akira S, Kishimoto T: Signal transducer and activator of transcription (STAT)-induced STAT inhibitor 1 (SSI-1)/suppressor of cytokine signaling 1 (SOCS1) inhibits insulin signal transduction pathway through modulating insulin receptor substrate 1 (IRS-1) phosphorylation. J Exp Med 2001;193:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mooney RA, Senn J, Cameron S, Inamdar N, Boivin LM, Shang Y, Furlanetto RW: Suppressors of cytokine signaling-1 and -6 associate with and inhibit the insulin receptor: a potential mechanism for cytokine-mediated insulin resistance. J Biol Chem 2001;276:25889–25893 [DOI] [PubMed] [Google Scholar]
- 32.White MF: IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 2002;283:E413–E422 [DOI] [PubMed] [Google Scholar]
- 33.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M: IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191–198 [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Real JM, Lopez-Bermejo A, Vendrell J, Ferri MJ, Recasens M, Ricart W: Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care 2006;29:1058–1064 [DOI] [PubMed] [Google Scholar]
- 35.Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA: An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med 2004;1:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP: Inflammation impairs reverse cholesterol transport in vivo. Circulation 2009;119:1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O'Grady NP: New insights into the biology of the acute phase response. J Clin Immunol 1999;19:203–214 [DOI] [PubMed] [Google Scholar]
- 38.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME: Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16:1248–1255 [DOI] [PubMed] [Google Scholar]
- 39.Shoelson SE, Lee J, Yuan M: Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord 2003;27(Suppl. 3):S49–S52 [DOI] [PubMed] [Google Scholar]
- 40.Shah R, Lu Y, Hinkle CC, McGillicuddy FC, Kim R, Hannenhalli S, Cappola T, Heffron S, Wang X, Mehta NN, Putt M, Reilly MP: Gene profiling of human adipose tissue during evoked inflammation in vivo. Diabetes 2009;58:2211–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolinkova M, Dostalova I, Lacinova Z, Michalsky D, Haluzikova D, Mraz M, Kasalicky M, Haluzik M: The endocrine profile of subcutaneous and visceral adipose tissue of obese patients. Mol Cell Endocrinol 2008;291:63–70 [DOI] [PubMed] [Google Scholar]
- 42.Alvarez-Llamas G, Szalowska E, de Vries MP, Weening D, Landman K, Hoek A, Wolffenbuttel BH, Roelofsen H, Vonk RJ: Characterization of the human visceral adipose tissue secretome. Mol Cell Proteomics 2007;6:589–600 [DOI] [PubMed] [Google Scholar]
- 43.Gomez-Ambrosi J, Catalan V, Diez-Caballero A, Martinez-Cruz LA, Gil MJ, Garcia-Foncillas J, Cienfuegos JA, Salvador J, Mato JM, Fruhbeck G: Gene expression profile of omental adipose tissue in human obesity. FASEB J 2004;18:215–217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.