Abstract
OBJECTIVE
Nephrin, an immunoglobulin-like protein essential for the function of the glomerular podocyte and regulated in diabetic nephropathy, is also expressed in pancreatic β-cells, where its function remains unknown. The aim of this study was to investigate whether diabetes modulates nephrin expression in human pancreatic islets and to explore the role of nephrin in β-cell function.
RESEARCH DESIGN AND METHODS
Nephrin expression in human pancreas and in MIN6 insulinoma cells was studied by Western blot, PCR, confocal microscopy, subcellular fractionation, and immunogold labeling. Islets from diabetic (n = 5) and nondiabetic (n = 7) patients were compared. Stable transfection and siRNA knockdown in MIN-6 cells/human islets were used to study nephrin function in vitro and in vivo after transplantation in diabetic immunodeficient mice. Live imaging of green fluorescent protein (GFP)-nephrin–transfected cells was used to study nephrin endocytosis.
RESULTS
Nephrin was found at the plasma membrane and on insulin vesicles. Nephrin expression was decreased in islets from diabetic patients when compared with nondiabetic control subjects. Nephrin transfection in MIN-6 cells/pseudoislets resulted in higher glucose-stimulated insulin release in vitro and in vivo after transplantation into immunodeficient diabetic mice. Nephrin gene silencing abolished stimulated insulin release. Confocal imaging of GFP-nephrin–transfected cells revealed nephrin endocytosis upon glucose stimulation. Actin stabilization prevented nephrin trafficking as well as nephrin-positive effect on insulin release.
CONCLUSIONS
Our data suggest that nephrin is an active component of insulin vesicle machinery that may affect vesicle-actin interaction and mobilization to the plasma membrane. Development of drugs targeting nephrin may represent a novel approach to treat diabetes.
In the U.S. alone, diabetes affects >20 million people. Although advances have been made in the clinical care of diabetes, one of the major limitations for finding a cure is that the mechanisms regulating the function of insulin-producing cells have not yet been fully characterized.
Nephrin is an immunoglobulin-like protein with important structural and signaling properties (1,2). It was initially described in podocytes, highly specialized cells in the kidney glomerulus (3,4). Nephrin is heavily downregulated in human diabetic nephropathy (5), and nephrin mutations are responsible for the congenital nephrotic syndrome of the Finnish type (6).
Nephrin expression has been reported in pancreatic β-cells (7), where its function remains unknown. In immortalized human podocytes, the COOH-terminal portion of nephrin appears to bind VAMP-2, a vesicle-associated membrane protein involved in exocytosis (8). The interaction of nephrin with VAMP-2, together with its well-known interaction with the actin cytoskeleton (9–13), suggests that nephrin may play an important role in vesicles trafficking, a recently described feature of podocyte biology (14).
In pancreatic β-cells, glucose stimulation affects actin reorganization, and redistribution of cortical actin is essential for proper β-cell function (15). However, the pathways responsible for the regulated targeting of vesicles to the plasma membrane have not yet been fully characterized. The aim of this study was to understand the regulation of pancreatic nephrin expression in patients with type 2 diabetes and the role of nephrin in the regulation of glucose-stimulated insulin release (GSIR).
RESEARCH DESIGN AND METHODS
Cell culture, islet culture, and RT-PCR.
MIN6 cells, and the B1 and C3 subclones (a gift from Dr. A. Thomas), were cultured in 25 mmol/l glucose DMEM (Invitrogen, Carlsbad, CA) (16), and pseudoislets were generated in culture on a shaker incubator (17). To study the effect of acute versus chronic glucose exposure, cells were cultured in 2 mmol/l glucose for 7 days and then assigned to receive either 2 or 20 mmol/l glucose culture media for 1 day (acute exposure) or 14 days (chronic exposure), a model we have used previously to mimic glucotoxicity in mesangial cells (18). Media supplemented with 5 mmol/l leucine and glutamine were used throughout the experiment to prevent apoptosis related to prolonged low glucose exposure (19), and cells were subcultured as necessary to prevent overconfluence. For selected experiments, cells were treated for 6 h with 0–200 nmol/l cytochalasin D (Sigma) (20), 0.5 μmol/l jasplakinolide (21), or 200 nmol/l phalloidin (Invitrogen), and the change in G/F actin ratio was assessed by Western blot (G-actin/F-actin Assay Kit; Cytoskeleton, Denver, CO). Human islets from cadaveric donors with research consent were obtained through the Islet Cell Resource Distribution system or were isolated at the local Human Cell Processing facility (22). Β-cell content was determined as previously described by laser scanning cytometry (23). Nephrin and 18S rRNA expression were evaluated using the 7500 Real-Time-PCR System (Applied Biosystems). For standard PCR and sequencing, primers were designed at the intron-exon junction (5′-ATCTCCAAGACCCCAGGTACACA and 3′-AGGGTCAGGACGGCTGAT for mouse, 5′-CCTGCTAGAGGTGAATTCCA and 3′-CCATTCTTCAGCCACTGCA for human). Additional human primers previously described were used to verify the presence of the two described splice variants of nephrin (24). Actin was used as the housekeeping gene (5′-TCATGAGGTAGTCCGTCAGG and 3′-TCTAGGCACCAAGGTGTG). A PCR product of 938 bp including part of the extracellular nephrin domain was amplified and sequenced from human pancreas, and the blast analysis matched completely the reported human nephrin mRNA sequence.
Western blotting and cell fractionation.
For Western blotting, a polyclonal guinea pig anti-nephrin antibody (1:2,000, COOH-terminal; Fitzgerald Laboratories, Concord, MA) and a monoclonal antibody against the IgG8-like domain of human nephrin (1:500; 50A9, a gift of Dr. Tryggvason) were used. Both antibodies revealed a unique band of 180 kDa. Loading efficiency was verified by actin (1:5,000; Abcam, Cambridge, MA). Plasma membranes were separated after biotinylation of cultured cells (Thermo Scientific, Rockford, IL). Unbiotinylated cells were used as control, and separation efficiency was evaluated by Western blotting for Na/K-ATPase (1:1,000, rabbit polyclonal, Abcam). For sucrose gradient centrifugation, cells were glucose-starved for 1 h and then exposed to either 0.5 or 25 mmol/l glucose for 30 min and then homogenized in 250 μl HEPES buffer. Pooled supernatants were loaded onto a 4.4-ml linear sucrose density gradient, centrifuged in a SW50 rotor (110,000g) for 18 h, and 15–16 fractions were collected from the top of the gradient. A total of 20 μg protein from each fraction was analyzed by immunoblotting; E-Cadherin was used as a plasma membrane marker (1:1,000, mouse monoclonal; BD Bioscience, San Jose, CA); VAMP-2 (1:1,000, rabbit polyclonal; Assay Designs, Ann Arbor, MI) and insulin (1:3,000, chicken polyclonal; Abcam) were used for vesicles fractions.
Cell transfection and siRNA.
A full-length human nephrin-pCDNA3 vector was used to generate stable transfected MIN6 cells (Fugene-6; Millipore, Millerica, MA). The empty pCDNA3 vector was used as a negative control (CTRL). Four separate clones were used to repeat experiments. An enriched population of MIN6 cells transfected with a green fluorescent protein (GFP)-nephrin construct was also obtained with a combination of antibiotic selection and fluorescence-activated cell sorting (FACS; Aria cells sorter with Diva 6.0 software). For nephrin siRNA in MIN6 cells, a pool of four siRNAs was transfected (On Target Plus SMART pool; Dharmachon, Lafayette, CO). siRNAs were tested separately for their efficiency to downregulate nephrin without affecting four other randomly chosen genes. Nephrin expression after siRNA was verified by PCR and Western blotting. A pool of nontargeting siRNA was used as control. To perform gene silencing in human islets, islet cells were dissociated by incubation at 37°C for 5 min in Accutase (Innovative Cell Technologies, San Diego, CA). The 0.4 × 106 single cells in each well of a six-well plate were cultured overnight in CMRL-1066 (Invitrogen) and then transfected with 3.5 μg of a pool of four nontargeting siRNA and human nephrin siRNA (Dharmacon) using 6 μl of DharmaFECT1 in 2 ml final volume. After 3.5 days, 0.2 × 106 single islet cells were used for PCR and perifusion experiments.
Confocal microscopy, electron microscopy, and immunogold labeling.
The 50A9 anti-nephrin antibody was used for immunohistochemistry (Histostain-Plus; Zymed Laboratories, San Francisco, CA). For colocalization studies, immunofluorescence on frozen sections was performed with tyramide signal amplification (TSA kit; Invitrogen). Alexa-647–conjugated antibodies were used as secondary antibodies for other targets (1:200; Invitrogen). An irrelevant IgG2b antibody (R&D System, Minneapolis, MN) was used as the negative control. Antibodies for insulin (1:1,000; Mouse monoclonal, NeoMarkers), glucagon (rabbit polyclonal 1:500; Sigma), somatostatin (rabbit polyclonal 1:500; Sigma), CD31 (goat polyclonal; Abcam), and VAMP2 (1:500, mouse monoclonal; Synaptic System, Goettingen, Germany) were used. For immunocytochemistry, GFP-nephrin–transfected cells were fixed with 4% paraformaldehyde and counterstained with rhodhamine-phalloidin and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Invitrogen). When the same cells were used to study plasma membrane internalization upon glucose stimulation, cells were incubated with 2 or 20 mmol/l glucose medium for 20 min at 37°C, followed by a pulse incubation for 30 min with 5 μmol/l FM1–43 (Invitrogen) (25). Image acquisition was performed with a Leica SP5-confocal-DMI6000 microscope. For the quantification of vesicles number, cells were fixed in 2% glutaraldehyde/100 mmol/l sucrose/0.05 mol/l PO4. Slides were postfixed with 2% osmium, 0.1 mol/l PO4, dehydrated, and embedded in Epon-812. Image acquisition was performed with an electron microscope Phillips CM-10 (3,900× magnification). Double-immunogold labeling was performed as previously described (26) on human pancreatic sections. Stainings for insulin (5 nm gold) and nephrin (10 nm gold) were obtained with a rabbit polyclonal anti-insulin antibody (Dako, Glostrup, Denmark) and the mouse monoclonal anti-nephrin antibody 50A9.
Metabolic studies.
In vitro and in vivo analysis of insulin production at baseline and after glucose stimulation were performed as previously described (27). Briefly, control, nephrin siRNA treated, and nephrin-transfected MIN6 cells were used for the in vitro analysis of the glucose stimulation index (expressed as the ratio of insulin secreted at 20 mmol/l glucose to 2 mmol/l glucose) after a 30-min stimulation with 20 mmol/l glucose in static incubation experiments. Insulin content (cell lysates plus supernatants) and insulin secretion (cell supernatants) were studied by enzyme-linked immunosorbent assay (ELISA) (Mercodia, SW) and normalized per DNA content (Quanti-iT PicoGreen dsDNA assay kit, Invitrogen). For perifusion studies in human islets, 200,000 dissociated cells were loaded on microcolumns connected to an inflow and an outflow port of a customized perifusion system (Biorep, Miami, FL). Cells were perifused with media of defined composition (3 mmol/l glucose, 11 mmol/l glucose, and 25 mmol/l KCl), and samples were collected every 2 min for insulin and DNA determination. For in vivo experiments, pseudoislets were transplanted under the kidney capsule of diabetic immunodeficient athymic nu/nu (nude) mice (Harlan Labs) under protocols approved by the University of Miami Institutional Animal Care and Use Committee (27). Sampling for glycemia was consistently performed in the morning during the first hour of light exposure. Intraperitoneal glucose tolerance test (IPGTT) after 5-h fasting and after administration of a glucose bolus (2 g/kg) was performed to assess the role of nephrin expression on insulin secretion in vivo. Fasting serum before IPGTT was tested for the presence of insulin by ultrasensitive insulin ELISA (Mercodia, SW).
Data analysis.
Results represent the mean and SD of four to eight independent experiments. When one-way ANOVA showed statistical significance, results were compared using t test after Tukey's correction for multiple comparisons (Graph Pad Prism software). Statistical significance was set at P < 0.05.
RESULTS
Nephrin expression is decreased in islets from patients with type 2 diabetes and is regulated by glucose.
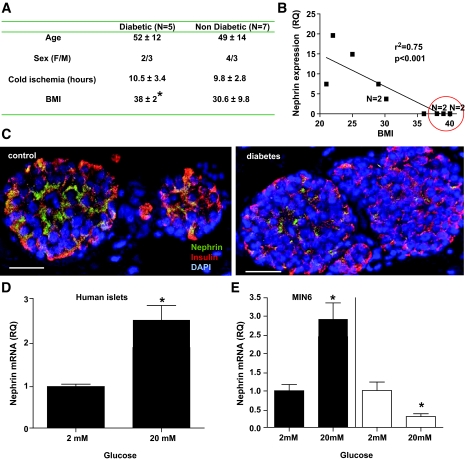
We have studied nephrin mRNA expression in human islets isolated from five patients with type 2 diabetes and seven nondiabetic patients matched for age, sex, and cold ischemia time (Fig. 1A). None of the diabetic patients had reached insulin dependence. BMI was significantly higher in diabetic than in nondiabetic donors (P < 0.05), and nephrin expression was inversely related to BMI (Fig. 1B). β-Cell content was not significantly different (42 ± 11% in diabetic vs. 49 ± 14% in nondiabetic patients). Confocal assessment of nephrin expression in human islets from the same patients was consistent with the downregulation of nephrin expression observed by PCR (Fig. 1C). To understand if glucose had a role in the regulation of nephrin expression observed in diabetes, we studied nephrin expression after acute and chronic exposure to glucose. We found that an acute exposure of islet cells (both human islets and MIN-6 cells) to 20 mmol/l glucose causes upregulation of nephrin expression (Fig. 1D and E), whereas the opposite effect was observed with chronic exposure of MIN-6 cells to 20 mmol/l glucose (Fig. 1E). Nephrin expression was absent in human islets after 14 days in culture (data not shown).
FIG. 1.
Modulation of nephrin expression by diabetes and glucose. A: Characteristics of pancreas donors with or without type 2 diabetes. BMI was significantly different among groups. B: Correlation between the nephrin mRNA expression relative quantification (RQ) in isolated islets and BMI among the 12 patients studied. An r2 of 0.75 with P < 0.001 revealed that higher BMIs were associated with lower nephrin expression. Circled in red are the five diabetic donors. n = 2 denotes overlapping points. C: A representative immunofluorescence confocal image for nephrin, insulin, and DAPI in human islets from a diabetic and nondiabetic donor is shown (20× magnification). A white scale bar of 50 μm is provided. D–E: Quantitative analysis of nephrin mRNA expression in human islets (D) or MIN6 cells (E, ■) cultured at either 2 or 20 mmol/l glucose for 24 h. Chronic exposure to high glucose concentration was tested in MIN6 cells only. Cells “starved” in 2 mmol/l glucose for 7 days were assigned to either 2 or 20 mmol/l glucose culture for an additional 14 days. A downregulation of nephrin mRNA expression was observed (E, □). Mean and SD of four independent experiments are shown. *P < 0.05. (A high-quality color digital representation of this figure is available in the online issue.)
Nephrin expression in islets is predominantly in β-cells.
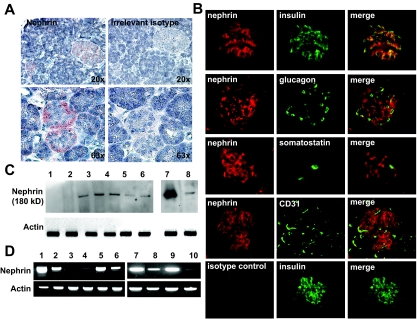
Nephrin immunostaining in normal human pancreas was present in islet cells regardless of islet size (Fig. 2A). Immunofluorescence colocalization studies with nephrin, insulin, glucagon, somatostatin, and CD31 showed nephrin expression primarily in β-cells (Fig. 2B). Nephrin expression in adult and fetal human pancreas, human islets, and murine insulinoma cells was confirmed by Western blot (Fig. 2C) and standard RT-PCR (Fig. 2D). A 938-bp product of PCR amplification was sequenced to confirm specific nephrin amplification. Both transcript splice variants described for podocytes (24) were detected in pancreatic islets.
FIG. 2.
Nephrin is expressed in human islets and β-cells. A: Immunostaining for nephrin on normal human pancreas revealed a predominant localization of nephrin to both small and large islets. B: Immunofluorescence colocalization studies revealed a predominant localization of nephrin (red) to insulin-producing cells (green, first row) but not to glucagon, somatostatin, and CD31-positive cells (green, subsequent rows). An irrelevant IgG2b was used as control and is shown in the last row. C: Western blotting analysis for nephrin. 1: COS7 (negative control), 2: human podocytes, 3: fetal human pancreas, 4–6: human islets from three different donors, 7: human kidney, 8: MIN6 cells. D: Standard RT-PCR for nephrin and actin. Samples 1–6 are of human origin, whereas 7–10 are of mouse origin. 1: kidney, 2: fetal pancreas, 3 and 5: whole pancreas from two different donors, 4 and 6: islets from same donors, 7: kidney, 8: islets, 9: fetal pancreas, and 10: MIN6 cells. (A high-quality color digital representation of this figure is available in the online issue.)
Nephrin affects GSIR in MIN6 cells.
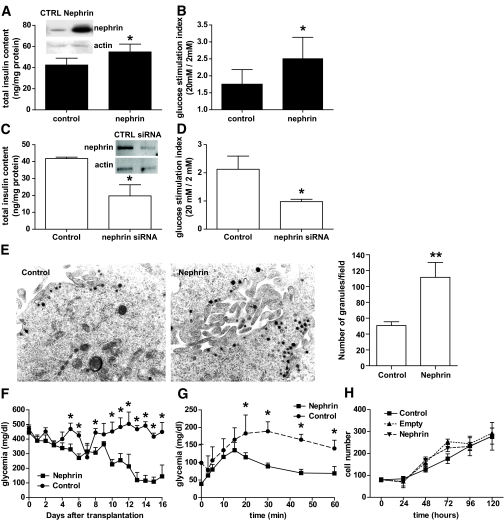
Nephrin overexpression was associated with significantly higher insulin content (Fig. 3A) and improved GSIR in static incubation experiments when compared with control cells (Fig. 3B). To further characterize the role of nephrin in β-cell function, insulinoma cells were treated with a selected pool of four nephrin siRNAs with 85% efficiency. The depletion of nephrin expression was paralleled by a decrease in insulin content (Fig. 3C) and in GSIR in vitro (Fig. 3D). Nephrin expression was also compared between the glucose responsive and the glucose unresponsive B1 and C3 MIN6 subclones. MIN6 B1 cells had threefold higher nephrin expression than MIN6 C3 cells (3.1 ± 0.5 vs. one relative quantification in B1 vs. C3 respectively, P = 0.023). However, nephrin transfection of C3 cells was not sufficient to make them glucose responsive. Monolayers of nephrin-transfected and empty vector–transfected (control) cells were analyzed by electron microscopy for vesicle quantification. Nephrin-transfected cells had a significantly higher content of secretory granules and clusters of secretory vesicles than control cells (Fig. 3E). The effect of nephrin overexpression on insulin production and glucose responsiveness was confirmed in vivo upon transplantation of nephrin and control pseudoislets in immunodeficient diabetic mice. Mice receiving nephrin-positive pseudoislets had significantly lower glycemia starting at day 5 after transplantation (Fig. 3F), and glucose tolerance tests performed at day 7 after transplantation showed a faster reversal to normoglycemia in mice that had received nephrin-positive pseudoislets when compared with mice with pseudoislets transfected with the empty vector control (Fig. 3G). All animals reverted to a glycemia of <100 mg/dl after 80 min of glucose administration. Serum insulin was detectable in nephrin-transplanted mice only (data not shown). All mice reverted to hyperglycemia after nephrectomy of the graft-bearing kidney, suggesting that the transplanted pseudoislets were responsible for the correction of diabetes. No difference in the growth rate of nephrin-transfected cells and cells transfected with an empty vector or untransfected cells was observed (Fig. 3H).
FIG. 3.
Nephrin affects insulin content and glucose responsiveness in MIN6 cells. Nephrin overexpression resulted in increased total insulin content (A) and glucose response in static incubation experiments (B, graphic representation with mean and SD of eight independent experiments). The effect of nephrin siRNA on total insulin content (C) and glucose response in static incubation experiments (D) are also shown. Representative blots showing nephrin protein level in transfected and siRNA-treated cells are shown (A and C). Nephrin overexpression resulted in increased number of secretory vesicles (as evaluated by vesicles count in 20 separate electromicrographs) when compared with empty vector–transfected cells (control) (E). F: Graphic representation of nonfasting glycemia evaluated daily in diabetic nude mice recipients of nephrin-overexpressing pseudoislets (n = 6) or control pseudoislets (n = 5). G: IPGTT profiles of recipients of nephrin-overexpressing or control pseudoislets performed 1 week after transplantation. H: Growth curves of monolayer of untransfected cells (control) when compared with empty vector or nephrin-transfected cells. *P < 0.05, **P < 0.01.
Nephrin affects GSIR in human islets.
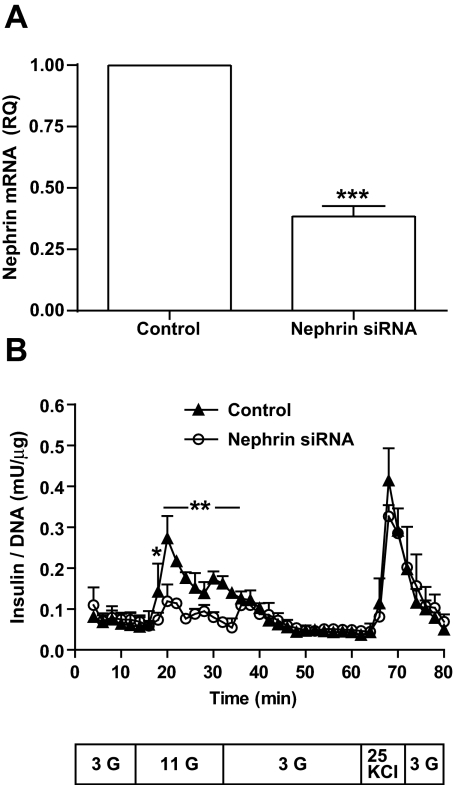
To overcome the limitation of using insulinoma cells to study the role of nephrin in insulin secretion, we applied nephrin siRNA gene downregulation to dissociated human islet cells (from four different donors). A mean silencing efficiency of 62% was achieved (Fig. 4A). Cell viability evaluated in siRNA-treated dissociated cells by Trypan blue exclusion was also conserved (data not shown). Nephrin siRNA affected both the first and the second phase of glucose response in perifused islet cells when compared with nontargeting siRNA (Fig. 4B). Response to KCl was conserved (Fig. 4B), suggesting that cell depolarization–dependent insulin exocytosis is probably not under the direct influence of nephrin.
FIG. 4.
Nephrin affects glucose responsiveness in human islets. A: Dispersed dissociated human islets exposed to either nephrin siRNA or nontargeting siRNA (control). PCR analysis of nephrin siRNA efficiency from four independent experiments reached a mean value of 62%. ***P < 0.001. B: Nephrin siRNA (○) or nontargeting siRNA (control, ▲) were perifused with 3 mmol/l glucose (G), 11 mmol/l glucose, 3 mmol/l glucose, 25 mmol/l KCl, and 3 mmol/l glucose in the time and sequence described in the text box below the graph. A graphic representation of four independent experiments with mean and SD for each time point is provided. *P < 0.05, **P < 0.001.
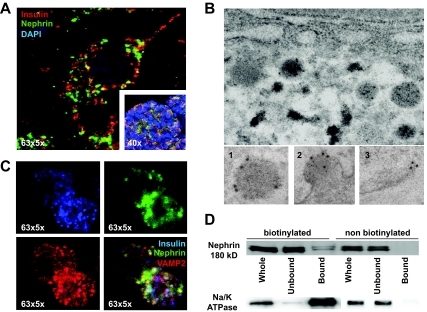
Nephrin localizes to both plasma membrane and insulin vesicles of pancreatic β-cells.
Confocal microscopy of β-cells from human pancreatic sections revealed a prevalent granular cytoplasmic localization with partial insulin colocalization (Fig. 5A). Double-immunogold labeling for nephrin and insulin of normal human pancreas revealed a prevalent localization of nephrin at the periphery of insulin vesicles (Fig. 5B1). When analyzing vesicles in the process of fusing to the plasma membrane, nephrin remains predominantly localized to the fused membrane (Fig. 5B2). Nephrin localization to the plasma membrane independent of insulin vesicles could also be observed (Fig. 5B3). Nephrin staining was also detectable in few empty vesicles (<5%). Immunofluorescence analysis of nephrin localization in cultured MIN6 cells revealed a partial colocalization of nephrin with insulin and VAMP2 (Fig. 5C). Biotinylation experiments revealed a predominant cytosolic localization (unbound) and a limited plasma membrane localization (bound, Fig. 5D). Proper separation was confirmed by reblotting the membranes for Na/K ATPase (Fig. 5D).
FIG. 5.
Nephrin colocalizes with vesicles in β-cells. A: 63×5× and 40× magnified confocal images of human pancreatic β-cells and of human islets reveals a granular cytoplasmic nephrin localization (green) with partial overlap with insulin (red). B: Double-immunogold labeling for nephrin (10 nm gold) and insulin (5 nm gold) in human pancreatic sections. Enlarged are a single insulin vesicle (1), an insulin vesicle fused to the plasma membrane (2), and a plasma membrane without insulin vesicle (3), showing the localization of nephrin in the different phases of insulin secretion. C: Confocal microscopy at 63× magnification of MIN6 cells showing a more intense nephrin staining (green) in insulin-positive cells (blue) but not necessarily in VAMP2-positive cells (red). D: Biotinylation of MIN6 cells and separation of plasma membrane (bound) and cytoplasmic (unbound) fractions revealed that only a modest fraction of nephrin was localized to the plasma membrane and that the molecular weight of plasma membrane and cytoplasmic nephrin are identical. Blotting for Na/K ATPase confirmed a proper membrane and cytoplasm separation. All experiments were repeated four times. (A high-quality color digital representation of this figure is available in the online issue.)
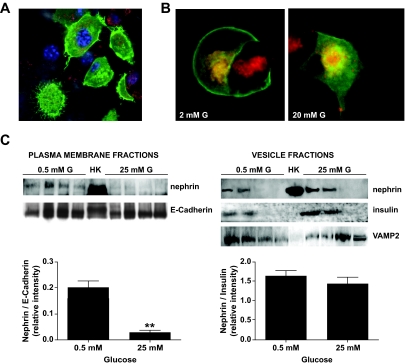
Glucose affects nephrin endocytosis.
Because nephrin was localized in both plasma membrane and insulin vesicles, and nephrin endocytosis was reported in podocytes (28), we investigated if the glucose concentration affected the subcellular localization of nephrin. For this purpose, GFP-nephrin–transfected cells were used for confocal imaging (Fig. 6A). When cells were co-stained with the live membrane dye FM4–64 used to visualize vesicles, nephrin localization was primarily at the plasma membrane in cells cultured at 2 mmol/l glucose and partially underwent endocytosis after stimulation with 20 mmol/l glucose (Fig. 6B). Subcellular fractionation experiments in untransfected MIN6 cells were used to confirm findings by immunoflorescence. Nephrin was clearly detectable in E-cadherin–positive plasma membrane fractions from MIN6 cells cultured in 0.5 mmol/l glucose, but the nephrin/E-cadherin relative intensity was markedly decreased in 25 mmol/l glucose (Fig. 6C). On the contrary, when analyzing vesicle fractions (VAMP2-positive), nephrin- and insulin-positive vesicle fractions were present in both 0.5 and 25 mmol/l glucose.
FIG. 6.
Nephrin endocytosis occurs upon glucose stimulation. A: GFP-nephrin–transfected into MIN6 cells localizes both at the plasma membrane and in the cytoplasm. B: MIN6 cells starved in 2 mmol/l glucose revealed that nephrin is predominantly localized to the plasma membrane and only partially to FM6-64–stained vesicles (red). Upon stimulation with 20 mmol/l glucose, nephrin disappears from the plasma membrane and localizes solely to the endocytosed vesicles compartment. C: Plasma membrane and vesicle fractions were collected from MIN6 cells cultured in either 0.5 or 25 mmol/l glucose for 30 min. Human kidney (HK) was used as positive control for nephrin staining. While in 0.5 mmol/l glucose, nephrin was present in both plasma membrane (E-Cadherin positive) fractions and insulin vesicle fractions (insulin positive and VAMP2 positive); in 25 mmol/l glucose, nephrin almost disappeared from the plasma membrane. Shown are a representative blot analysis and the bar graph representation of the nephrin–to–E-cadherin ratios as well as the nephrin-to-insulin ratios (mean and SD of three independent experiments). **P < 0.01. (A high-quality color digital representation of this figure is available in the online issue.)
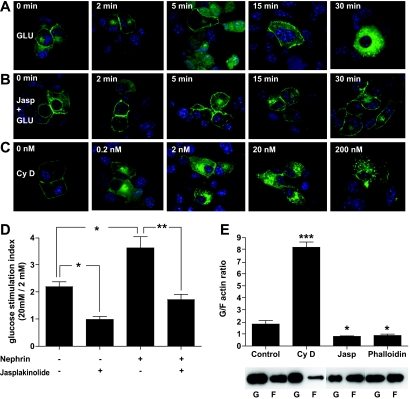
Nephrin augmentation of insulin secretion is partially dependent on actin cytoskeleton remodeling.
Actin depolymerizing agents have been shown to affect insulin secretion by pancreatic β-cells (15,29), while more complex may be the role of actin-stabilizing/nucleating drugs such as jasplakinolide, which has been clearly shown to increase glucose responsiveness in MIN6 cells when used at high concentrations (30). Nephrin increases insulin secretion (Fig. 3), and nephrin modulates actin cytoskeleton remodeling (9,13). Therefore, we tested if nephrin trafficking from the plasma membrane to the cytoplasm can be prevented by actin stabilization with low-dose jasplakinolide and if the positive nephrin effect on insulin secretion could be abolished. Both glucose and cytochalasin D induced a redistribution of nephrin from the plasma membrane to the cytoplasm (Fig. 7A and C), and jasplakinolide completely prevented this effect (Fig. 7B), similar to what we observed with 200 nmol/l phalloidin treatment (data not shown). The augmentation of GSIR in nephrin-transfected cells was also prevented by jasplakinolide (Fig. 7D), suggesting that a nephrin-mediated increase in insulin secretion is partially dependent on the polymerization status of actin. The actin remodeling function of cytochalasin D, jasplakinolide, and phalloidin was studied by Western blotting for G- and F-actin subcellular fractions. While untreated MIN6 cells are characterized by a content of F-actin of ∼40% of total actin, cytochalasin D treatment resulted in a decrease F-actin content to 10%, and jasplakinolide and phalloidin both increased F-actin content to 60 and 65%, respectively (Fig. 7E).
FIG. 7.
Nephrin trafficking depends on actin and is essential for GSIR. A: GFP-nephrin–transfected into MIN6 cells is mainly localized at the sites of cell-to-cell contact in 2 mmol/l glucose. Upon glucose stimulation, nephrin undergoes a time-dependent endocytosis. B: Actin stabilization with 0.5 μmol/l jasplakinolide prevented glucose-induced relocation of GFP-nephrin from the plasma membrane to the cytoplasm. C: Actin depolymerization with cytochalasin D led instead to nephrin redistribution from plasma membrane to the cytoplasm in a dose-dependent manner. D: The positive effect of nephrin overexpression on GSIR was also totally prevented by pretreatment with 0.5 μmol/l jasplakinolide. *P < 0.05, **P < 0.01. All experiments were repeated four times. E: MIN6 cells exposed to cytochalasin D (Cy D), jasplakinolide (Jasp), and phalloidin were analyzed for the change in F-actin content compared to control untreated cells. While an increased G-to-F actin ratio was observed with CyD (***P < 0.001), both jasplakinolide and phalloidin treatment resulted in a 50% reduction of G-to-F actin ratios (*P < 0.05). (A high-quality color digital representation of this figure is available in the online issue.)
DISCUSSION
The present study unveiled that nephrin affects the function of pancreatic β-cells and promotes insulin secretion in response to glucose. This function of nephrin is independent from its well-known and described role in kidney podocytes, where it regulates structure and function of the glomerular slit diaphragm (10,31,32). The regulation of insulin secretion by nephrin is also different from a recently described function in skeletal muscles, where nephrin is responsible for secondary fusion of myoblasts into nascent myotubules (33). Despite such apparent discrepant functions of nephrin in different organs, a common mechanisms of action remains the ability of nephrin to modulate actin cytoskeleton remodeling (9,13), a phenomenon that is equally important for the function of pancreatic β-cells, podocytes, and skeletal muscles. The existing controversial data on nephrin expression in pancreatic islets (7,34,35) has prompted us to confirm nephrin expression in human pancreas. Here we clearly showed that nephrin is expressed in human pancreas predominantly in pancreatic β-cells (Fig. 2). Of note, in β-cells, nephrin is localized primarily on the surface of insulin containing vesicles (Fig. 5B), suggesting a role for nephrin as a modulator of vesicle trafficking during insulin secretion. This vesicle associated intracellular localization was unexpected, because in podocytes, nephrin has been described as a plasma membrane protein involved in cell-cell adhesion via homophilic (36,37) and heterophilic interactions (38–40). As it stands, there are no reports of any endocrine dysfunctions in patients with gene mutations in the nephrin NPHS1 gene, and glucose tolerance tests performed in this subgroup of patients are normal (35). This, however, does not exclude that nephrin may be relevant to β-cell function, since truncated nephrin isoforms may still be functional in the pancreas. Interestingly, while nephrin in podocytes interacts with other proteins such as podocin and Neph1 to facilitate nephrin-mediated signaling, those proteins have not been detected in the pancreas (41). Together, these observations raise the intriguing possibility that nephrin serves a different function in the pancreas than in the kidney.
To investigate nephrin function, we manipulated nephrin expression in insulinoma MIN6 cells and dissociated human islets and established a role for nephrin in both GSIR and in total insulin production (Figs. 3 and 4). Although the increased total insulin content could result from a direct effect of nephrin on insulin transcription, it could also be the consequence of a well-characterized feedback mechanism to increase insulin production and vesicle formation upon insulin secretion (42), as suggested by the electron microscopy quantification of the number of secretory vesicles in nephrin-transfected cells (Fig. 3E). It is also possible that nephrin is involved in the machinery enabling glucose entry and metabolism, which may explain why nephrin downregulation did not affect significantly KCl response. Decreased nephrin expression in the MIN6-C3 subline compared with the MIN6-B1 subline was not responsible for decreased glucose responsiveness. In this context, it will be interesting to test whether this is due to the decreased MIN6-C3 expression of gelsolin, another actin regulator that may play an important role in glucose-stimulated actin remodeling (29).
The localization of nephrin on both the plasma membrane and insulin vesicles, together with the known ability of nephrin to modulate actin cytoskeleton remodeling and to undergo endocytosis in podocytes (28), has prompted us to explore if nephrin endocytosis would occur as a consequence of glucose stimulation and would affect insulin release. Our finding that nephrin undergoes endocytosis upon glucose stimulation is in keeping with the hypothesis that nephrin relocation from the plasma membrane to the cytoplasm facilitates insulin release, probably through disruption of the dense web of cortical actin that prevents insulin vesicle fusion to the plasma membrane (43,44). In fact, actin stabilization with phalloidin and jasplakinolide prevented nephrin endocytosis and abolished nephrin effect on increased insulin secretion. We have used a dose of jasplakinolide that specifically leads to actin stabilization, as the effects of jasplakinolide on the kinetics of actin polymerization are highly time and dose dependent (45). The opposite effect on insulin secretion and glucose responsiveness were reported in very elegant studies in whole islets and insulinoma cells where a 10-fold higher concentration of jasplakinolide was used (30), suggesting a multifaceted role of the actin cytoskeleton in the regulation of GSIR. Further studies will be needed to define the precise mechanism whereby nephrin augments insulin secretion.
Another interesting finding of the present study was the observation that nephrin expression is decreased in islets from diabetic patients when compared with age-matched nondiabetic control subjects, similar to what has been reported in the kidney (5). BMI and nephrin mRNA expression was inversely related in pancreatic islets, supporting a critical role of nephrin in GSIR. Consistent with these ideas, prolonged exposure to high glucose led to a downregulation of nephrin expression in MIN6 cells, while acute exposure to high glucose increased nephrin mRNA expression. Although the relevance of an in vitro model of acute and prolonged glucose exposure in insulinoma cells remains to be established, the opposite effect of acute and chronic glucose exposure on nephrin gene expression is consistent to what has been described in kidney cells (46), where chronic glucose exposure has been widely accepted as a model of glucotoxicity (18). One possible interpretation of our results is that nephrin allows a more dynamic vesicle mobilization and increased insulin secretion, which may be impaired in diabetes because of the downregulation of nephrin in pancreatic β-cells.
In conclusion, our results identified nephrin as an important regulator of glucose-stimulated insulin secretion in pancreatic β-cells. Our data suggest that nephrin is an active component of the vesicle machinery that guarantees the interaction of insulin vesicles with the actin cytoskeleton and thereby their mobilization toward the plasma membrane. Thus, interventions that lead to increased nephrin expression and/or function may delay the need for insulin therapy in type 2 diabetes. Whether an active role of nephrin in vesicle exocytosis in podocytes contributes to the integrity of the slit diaphragm remains to be explored.
Acknowledgments
The current study was supported by grants from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK-82636 [to A.F.] and DK-25802-21 [to C.R.]), from NIH/National Center for Research Resources (U42 RR016603 and M01RR16587 [to C.R.]), from the American Diabetes Association (7-09-JF-23 [to A.F.]), from the Diabetes Research Institute Foundation (diabetesresearch.org), and from the Karasik Foundation.
No potential conflicts of interest relevant to this article were reported.
We thank Maria O. Saenz, Jorge Gonzalez-Quintana, and Nahir Y. Sanabria for excellent technical assistance. We also extend personal thanks to Dr. Daniel H. Mintz for continuous guidance and encouragement.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Huber TB, Benzing T: The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 2005;14:211–216 [DOI] [PubMed] [Google Scholar]
- 2.Patrakka J, Tryggvason K: Nephrin: a unique structural and signaling protein of the kidney filter. Trends Mol Med 2007;13:396–403 [DOI] [PubMed] [Google Scholar]
- 3.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1998;1:575–582 [DOI] [PubMed] [Google Scholar]
- 4.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A 1999;96:7962–7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G: Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes 2003;52:1023–1030 [DOI] [PubMed] [Google Scholar]
- 6.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 2006;354:1387–1401 [DOI] [PubMed] [Google Scholar]
- 7.Palmén T, Ahola H, Palgi J, Aaltonen P, Luimula P, Wang S, Jaakkola I, Knip M, Otonkoski T, Holthöfer H: Nephrin is expressed in the pancreatic beta cells. Diabetologia 2001;44:1274–1280 [DOI] [PubMed] [Google Scholar]
- 8.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavaré JM, Mathieson PW, Saleem MA: Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 2007;56:1127–1135 [DOI] [PubMed] [Google Scholar]
- 9.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 2006;116:1346–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 2007;17:428–437 [DOI] [PubMed] [Google Scholar]
- 11.Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW: Co-localization of nephrin, podocin, and the actin cytoskeleton: evidence for a role in podocyte foot process formation. Am J Pathol 2002;161:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB: Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol 2007;27:8698–8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 2006;440:818–823 [DOI] [PubMed] [Google Scholar]
- 14.Rastaldi MP, Armelloni S, Berra S, Calvaresi N, Corbelli A, Giardino LA, Li M, Wang GQ, Fornasieri A, Villa A, Heikkila E, Soliymani R, Boucherot A, Cohen CD, Kretzler M, Nitsche A, Ripamonti M, Malgaroli A, Pesaresi M, Forloni GL, Schlöndorff D, Holthofer H, D'Amico G: Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J 2006;20:976–978 [DOI] [PubMed] [Google Scholar]
- 15.Thurmond DC, Gonelle-Gispert C, Furukawa M, Halban PA, Pessin JE: Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Mol Endocrinol 2003;17:732–742 [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K: Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990;127:126–132 [DOI] [PubMed] [Google Scholar]
- 17.Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM: Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 1999;48:1402–1408 [DOI] [PubMed] [Google Scholar]
- 18.Fornoni A, Striker LJ, Zheng F, Striker GE: Reversibility of glucose-induced changes in mesangial cell extracellular matrix depends on the genetic background. Diabetes 2002;51:499–505 [DOI] [PubMed] [Google Scholar]
- 19.Van de Casteele M, Kefas BA, Cai Y, Heimberg H, Scott DK, Henquin JC, Pipeleers D, Jonas JC: Prolonged culture in low glucose induces apoptosis of rat pancreatic beta-cells through induction of c-myc. Biochem Biophys Res Commun 2003;312:937–944 [DOI] [PubMed] [Google Scholar]
- 20.Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P: Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest 2005;115:1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JR, Ludowyke RI, Biden TJ: A redistribution of actin and myosin IIA accompanies Ca(2+)-dependent insulin secretion. FEBS Lett 2001;492:101–106 [DOI] [PubMed] [Google Scholar]
- 22.Ricordi C, Strom TB: Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol 2004;4:259–268 [DOI] [PubMed] [Google Scholar]
- 23.Ichii H, Inverardi L, Pileggi A, Molano RD, Cabrera O, Caicedo A, Messinger S, Kuroda Y, Berggren PO, Ricordi C: A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant 2005;5:1635–1645 [DOI] [PubMed] [Google Scholar]
- 24.Holthöfer H, Ahola H, Solin ML, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D: Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 1999;155:1681–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang SN, Wenna ND, Yu J, Yang G, Qiu H, Yu L, Juntti-Berggren L, Köhler M, Berggren PO: Glucose recruits K(ATP) channels via non-insulin-containing dense-core granules. Cell Metab 2007;6:217–228 [DOI] [PubMed] [Google Scholar]
- 26.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D: Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999;154:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornoni A, Pileggi A, Molano RD, Sanabria NY, Tejada T, Gonzalez-Quintana J, Ichii H, Inverardi L, Ricordi C, Pastori RL: Inhibition of c-jun N terminal kinase (JNK) improves functional beta cell mass in human islets and leads to AKT and glycogen synthase kinase-3 (GSK-3) phosphorylation. Diabetologia 2008;51:298–308 [DOI] [PubMed] [Google Scholar]
- 28.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L: Beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 2006;103:14110–14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomas A, Yermen B, Min L, Pessin JE, Halban PA: Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. J Cell Sci 2006;119:2156–2167 [DOI] [PubMed] [Google Scholar]
- 30.Nevins AK, Thurmond DC: Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol 2003;285:C698–C710 [DOI] [PubMed] [Google Scholar]
- 31.Tryggvason K: Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 1999;10:2440–2445 [DOI] [PubMed] [Google Scholar]
- 32.Benzing T: Signaling at the slit diaphragm. J Am Soc Nephrol 2004;15:1382–1391 [DOI] [PubMed] [Google Scholar]
- 33.Sohn RL, Huang P, Kawahara G, Mitchell M, Guyon J, Kalluri R, Kunkel LM, Gussoni E: A role for nephrin, a renal protein, in vertebrate skeletal muscle cell fusion. Proc Natl Acad Sci U S A 2009;106:9274–9279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 2001;10:1–8 [DOI] [PubMed] [Google Scholar]
- 35.Kuusniemi AM, Kestilä M, Patrakka J, Lahdenkari AT, Ruotsalainen V, Holmberg C, Karikoski R, Salonen R, Tryggvason K, Jalanko H: Tissue expression of nephrin in human and pig. Pediatr Res 2004;55:774–781 [DOI] [PubMed] [Google Scholar]
- 36.Khoshnoodi J, Sigmundsson K, Ofverstedt LG, Skoglund U, Obrink B, Wartiovaara J, Tryggvason K: Nephrin promotes cell-cell adhesion through homophilic interactions. Am J Pathol 2003;163:2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lahdenperä J, Kilpeläinen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K: Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int 2003;64:404–413 [DOI] [PubMed] [Google Scholar]
- 38.Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS: Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest 2003;112:209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T: Interaction with podocin facilitates nephrin signaling. J Biol Chem 2001;276:41543–41546 [DOI] [PubMed] [Google Scholar]
- 40.Lehtonen S, Lehtonen E, Kudlicka K, Holthöfer H, Farquhar MG: Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol 2004;165:923–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinta-Valkama J, Palmén T, Lassila M, Holthöfer H: Podocyte-associated proteins FAT, alpha-actinin-4 and filtrin are expressed in Langerhans islets of the pancreas. Mol Cell Biochem 2007;294:117–125 [DOI] [PubMed] [Google Scholar]
- 42.Leibiger IB, Leibiger B, Berggren PO: Insulin feedback action on pancreatic beta-cell function. FEBS Lett 2002;532:1–6 [DOI] [PubMed] [Google Scholar]
- 43.Orci L, Gabbay KH, Malaisse WJ: Pancreatic beta-cell web: its possible role in insulin secretion. Science 1972;175:1128–1130 [DOI] [PubMed] [Google Scholar]
- 44.Jewell JL, Luo W, Oh E, Wang Z, Thurmond DC: Filamentous actin regulates insulin exocytosis through direct interaction with Syntaxin 4. J Biol Chem 2008;283:10716–10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bubb MR, Spector I, Beyer BB, Fosen KM: Effects of jasplakinolide on the kinetics of actin polymerization: an explanation for certain in vivo observations. J Biol Chem 2000;275:5163–5170 [DOI] [PubMed] [Google Scholar]
- 46.Gruden G, Perin PC, Camussi G: Insight on the pathogenesis of diabetic nephropathy from the study of podocyte and mesangial cell biology. Curr Diabetes Rev 2005;1:27–40 [DOI] [PubMed] [Google Scholar]