Abstract
OBJECTIVE
The effect of diabetes on neovascularization varies between different organ systems. While excessive angiogenesis complicates diabetic retinopathy, impaired neovascularization contributes to coronary and peripheral complications of diabetes. However, how diabetes influences cerebral neovascularization is not clear. Our aim was to determine diabetes-mediated changes in the cerebrovasculature and its impact on the short-term outcome of cerebral ischemia.
RESEARCH DESIGN AND METHODS
Angiogenesis (capillary density) and arteriogenesis (number of collaterals and intratree anostomoses) were determined as indexes of neovascularization in the brain of control and type 2 diabetic Goto-Kakizaki (GK) rats. The infarct volume, edema, hemorrhagic transformation, and short-term neurological outcome were assessed after permanent middle–cerebral artery occlusion (MCAO).
RESULTS
The number of collaterals between middle and anterior cerebral arteries, the anastomoses within middle–cerebral artery trees, the vessel density, and the level of brain-derived neurotrophic factor were increased in diabetes. Cerebrovascular permeability, matrix metalloproteinase (MMP)-9 protein level, and total MMP activity were augmented while occludin was decreased in isolated cerebrovessels of the GK group. Following permanent MCAO, infarct size was smaller, edema was greater, and there was no macroscopic hemorrhagic transformation in GK rats.
CONCLUSIONS
The augmented neovascularization in the GK model includes both angiogenesis and arteriogenesis. While adaptive arteriogenesis of the pial vessels and angiogenesis at the capillary level may contribute to smaller infarction, changes in the tight junction proteins may lead to the greater edema following cerebral ischemia in diabetes.
Type 2 diabetic patients hold two- to fourfold higher risk for cerebrovascular disease and stroke (1,2), and 70% of patients with a recent stroke have overt diabetes or pre-diabetes distinguished by impaired fasting glucose or impaired glucose tolerance (3). However, the underlying basis of this increased risk remains unclear. Diabetic vascular complications are well studied in diabetic retinopathy, nephropathy, peripheral arterial disease, and coronary artery disease. However, diabetes-induced structural and functional changes in the cerebral vasculature are unknown.
It is becoming clear that the integrity of cerebral blood vessels is critical in the pathophysiology of stroke. While type 2 diabetes accounts for ∼90–95% of all diagnosed cases of diabetes in adult patients, most of the experimental studies are focused on type 1 diabetes induced by streptozotocin (STZ) injection, which is associated with high-level and short-term elevations of blood glucose. We have recently shown that diabetic Goto-Kakizaki (GK) rats develop smaller infarct and greater hemorrhagic transformation after stroke. These animals also showed cerebrovascular remodeling characterized by increased vessel tortuosity, vascular endothelial growth factor (VEGF) expression, and matrix metalloproteinase (MMP)-2 and -9 activity (4). Generated from glucose-intolerant Wistar rats, GK rat is a nonobese model of spontaneous type 2 diabetes with moderately elevated glucose levels (5,6). In light of previous reports that showed greater ischemic damage in acute hyperglycemic models of stroke (7–9), we hypothesized that chronic moderate hyperglycemia as seen in the GK model promotes neovascularization that affects the extent of ischemic injury. Considering that neovascularization may originate from new vessel formation as well as functional remodeling of existing vessels, the goals of the current study were 1) to determine the effect of diabetes on capillary density and arteriogenesis, 2) to determine the influence of diabetes on blood-brain barrier (BBB) baseline permeability and expression of proteins important for vascular integrity, and finally 3) to evaluate the effect of ischemic injury on infarct and hemorrhagic transformation development in diabetic rats with preexisting vascular disease, all of which may impact the outcome of ischemic injury.
RESEARCH DESIGN AND METHODS
Male Wistar (Harlan Laboratories, Indianapolis, IN) and GK (in-house bred, derived from the Tampa colony) rats (10–12 weeks old, 250–270 g) were maintained at a constant temperature (21–23°C) with a 12/12-h light/dark cycle and allowed access to food and water ad libitum. Body weight and blood glucose measurements were performed twice weekly. Glucose measurements were taken from the tail vein and measured using a Free Style glucose meter (Abbott Diabetes Care, Alameda, CA). A1C was measured to evaluate long-term glucose levels with an A1C Now+ kit (Bayer Healthcare, Sunnyvale, CA).
Collateral number and size of pial vessels.
To determine the effect of diabetes on the pial vessel arteriogenesis, the collateral number and size of cerebrovasculature were measured in 5- and 10-week-old animals. After being anesthetized with sodium pentobarbital (100 mg/kg), the animals were injected with 3 ml freshly prepared polyurethane elastomer PU4ii (vasQtec, Zurich, Switzerland) through the aorta in 1 min. PU4ii mixture was prepared by mixing the blue-stained ethylmethylketone (30% of the final mixture) and 0.8 g of PU4ii hardener shortly before casting as previously described (10). The rats were decapitated immediately, and the brain was removed and immersed in 4% paraformaldehyde for 48 h.
Stereomicroscopic images of the perfused brains were captured, and actual vessel length of cortical branches of middle cerebral arteries (MCAs) starting from its origin was measured using National Institutes of Health Image-J software. Each hemisphere was divided into six grids of equal area, and the total number of collaterals between the anterior cerebral arteries (ACAs), posterior arteries (PCAs), and MCAs were counted manually (11). A collateral was defined as an anastomosis between MCAs and ACAs or PCAs. Arteriole-to-arteriole anastomoses between the MCA branches were also counted and defined as intratree anastomosis. The diameter was measured at the midpoint of the collaterals. At least eight measurements of the diameters were taken on each hemisphere, and the average of all these measurements was considered as the average diameter of the collaterals.
Vascular density.
Cerebral vessel density was measured with modified fluorescein isothiocyanate–dextran assay (12). To stain the vasculature, 1 ml of 5% FITC-dextran (150 kDa; Sigma, St. Louis, MO) in saline was injected through the femoral vein and circulated for 30 min under isoflurane anesthesia. Brains were enucleated and fixed in 4% paraformaldehyde for 48 h. Brain sections (50 μm) with 600-μm intervals were mounted onto slides. By means of a computer-controlled platform, the hemisphere of the sections was stereologically captured with the Lucivid system (MicroBrightField, Williston, VT) and analyzed by the Spaceball protocol of Stereo Investigator software (13) (MicroBrightField). Each section was randomly counted at 12–18 points, and the average number was analyzed for statistics. Vessel density was defined as the length of fluorescence stained capillaries within the observation area (expressed as μm/μm3).
Vascular permeability.
The BBB permeability was assessed by measuring FITC-BSA (Molecular Probes, Carlsbad, CA) extravasations as previously described (14). In brief, 10 mg/kg FITC-BSA was injected through femoral vein 30 min before the rats were killed. Plasma fluorescence intensity in each animal was measured with a fluorescence spectrophotometer (BioTek, Winooski, VT) using standard curves of FITC-BSA in normal rat plasma. Fluorescence intensity in the cortex of brain sections were analyzed with a fluorescence microscope (Carl Zeiss, Thronwood, NY). The average cerebral fluorescence intensity was normalized with the plasma fluorescence intensity of each animal for statistics.
Isolation of cerebral vessels and evaluation of structural proteins.
After the major vessels (basilar, MCA, ACA, PCA, and connecting arteries of the Circle of Willis) were removed and saved as macrovessel preparation, the brain was homogenized with ice-cold PBS (0.01mmol/l, pH 7.4). The homogenate was centrifuged at 2,000g, 4°C for 10 min. The supernatant was saved as brain homogenate (15). The pellet was washed in PBS and gently layered on top of a dextran solution (15%, MW 38,400) and centrifuged at 4,000g, 4°C for 20 min. The final pellet was saved as cerebral microvessel preparation. Both macro- and microvessels were homogenized in radioimmunoprecipitation buffer as previously described (16). Homogenates were immunoblotted with occludin and claudin-5 (Zymed Laboratories, Carlsbad, CA), collagen IV (Abcam, Cambridge, MA), MMP-2, and MMP-9 (Calbiochem, Gibbstown, NJ) antibodies, respectively. All blots were probed with actin (Calbiochem) for loading control. Densities of protein bands were analyzed with Gel Pro Analyzer software (Media Cybernetics, Bethesda, MD). Total MMP activities were measured in macrovessels with gelatin zymography as previously described (16). Brain-derived neurotrophic factor (BDNF) level in the homogenates was measured using an enzyme-linked immunosorbent assay (ELISA) kit from Promega (Madison, WI).
Focal cerebral ischemia.
The unilateral permanent MCAO was achieved by intraluminal filament technique (4,17). The animals were anesthetized with isofluorane inhalation in a glass chamber prior to stroke procedure. The right femoral artery was catheterized for blood sampling for arterial blood gas analysis. The anesthesia was kept at 2% isofluorane in 70% nitrous oxide and 30% oxygen during surgery. The cerebral perfusion was measured with a PIM 3 scanning laser Doppler imaging system (Perimed, Stockholm, Sweden) to evaluate basal perfusion as well as changes in perfusion following MCAO to confirm successful occlusion. Body temperature was maintained constant at 37.5°C with a heating pad and monitored by a rectal probe.
At 24 h after occlusion, cerebral blood perfusion was evaluated with PIM 3 again and the animal was immediately killed. Brains were enucleated and sliced in the coronal plane with 2-mm intervals. Section images were scanned before and after 2,3,5-triphenyltetrazolium chloride (TTC) staining. The infarct size was evaluated as previously described (4). Hemorrhagic transformation was assessed macroscopically in a binary fashion since our previous study showed overt hematoma formation but not diffuse bleeding in GK rats. Edema was determined as the difference in volume of ischemic and nonischemic hemispheres and normalized to infarct volume.
Neurobehavioral tests.
Neurological outcome of ischemic injury was assessed by a Bederson test (16) and elevated-body swing test (EBST). The Bederson test was combined with contralateral hind-limb retraction, beam walking ability, and bilateral forepaw grasp tests (18). Scores were given to each item from 0 to 3 for a total of 12 for maximal deficit. The animals with a score lower than six after MCAO were excluded for analysis. These tests were repeated at 24 h before the animals were killed. EBST was assessed to evaluate motor asymmetry (19).
Statistical analysis.
A two-tailed unpaired t test, 95% CI, was used to compare the average data for all the studies between two groups. Data are expressed as means ± SE. Differences were considered significant at P < 0.05.
RESULTS
Physiological parameters.
Metabolic parameters are summarized in Table 1. A1C and blood glucose was significantly higher in diabetic animals. There was no significant difference in arterial blood gases and body weight.
TABLE 1.
Physiological parameters of control and diabetic rats in the study
| Control | Diabetes | |
|---|---|---|
| n | 12 | 9 |
| Body weight (g) | 262 ± 6 | 255 ± 8 |
| Blood glucose (mg/dl) | 106 ± 3 | 145 ± 6* |
| A1C (%) | 4.6 ± 0.1 | 7.3 ± 0.7* |
| pH | 7.44 ± 0.01 | 7.39 ± 0.06 |
| pCO2 (mmHg) | 44.7 ± 0.8 | 49.1 ± 6.6 |
| pO2 (mmHg) | 168 ± 5 | 159 ± 4 |
Data are means ± SE.
*P < 0.0001 vs. control.
Effect of diabetes on cerebral neovascularization.
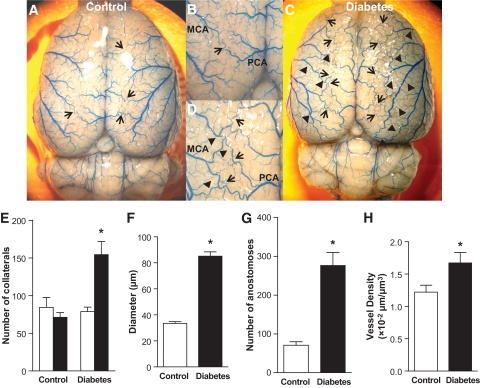
Neovascularization may result from angiogenesis (capillary sprouting) and/or arteriogenesis (collateral formation and growth as a result of remodeling of native collaterals to functional arterioles). Number and diameter of collaterals formed between pial MCAs and ACAs were evaluated as measures of arteriogenesis. In addition, number of anostomoses within the MCA tree was determined. Visualization of the surface vasculature demonstrated that cerebral vessels display increased tortuosity and typical corkscrew pattern in 10-week-old GK rats as we previously reported (Fig. 1). Diabetes significantly improved the number (Fig. 1E) and diameter (Fig. 1F) of the collaterals and intratree arteriole-to-arteriole anastomoses (Fig. 1G) between MCA branches. To determine whether these changes are inherent to the GK model or develop as a result of diabetes, we evaluated collateral numbers in younger (5-week-old) control and GK rats before the onset of diabetes and no difference was found between the groups (Fig. 1E). Capillary density was measured as an index of angiogenesis. There was prominent microvessel stained with FITC-dextran and the quantification using unbiased stereological analysis with the Spaceball protocol in Stereo Investigator software demonstrated increased capillary density in diabetic animals (Fig. 1H).
FIG. 1.
Increased angiogenesis and arteriogenesis in diabetic GK rats. A–D: Representative brain images of 10-week-old control (A and B, n = 9) and diabetic GK (C and D, n = 7) rats infused with Pu4ii indicate increased vascular tortuosity (corkscrew pattern) indicative of vascular remodeling and collateralization in diabetes. Arrow: collaterals between MCA and PCA or ACA; arrow head: anastomoses between MCA branches. E: Number of collaterals was significantly increased in 10-week-old GK rats compared with control but not in younger 5-week-old animals (n = 5 per group) before the onset of diabetes. In 10-week-old animals, the diameter of the collaterals (F), the anastomoses within the MCA tree (G), and the total microvessel density (H) were all increased in diabetes (n = 6 per group). *P < 0.05 vs. control. □, 5 weeks; ■, 10 weeks. (A high-quality color digital representation of this figure is available in the online issue.)
Effect of diabetes on cerebrovascular integrity.
Since there was an increase in capillary density, in order to determine whether the vascular structure alterations induced by diabetes contribute to increased leakiness, the BBB permeability was examined with the FITC-BSA extravasation. There was a 1.5-fold increase in fluorescence intensity in diabetes compared with control indicating increased baseline permeability in diabetes (Fig. 2A). Next, tight junction proteins occludin and claudin-5 as well as collagen IV, a key component of basal lamina, were measured in cerebral micro and macrovessels isolated from control and diabetic animals. A 65-kDa band corresponding to occludin was detected in all samples and it was decreased in the microvasculature of diabetic animals as compared with controls (Fig. 2B). There was no difference in occludin levels in the macrovessels. A lower band around 63 kDa was also detected in both vascular beds with no difference between groups (data not shown). Claudin-5 and collagen IV levels were similar in micro- and macrovessels from both experimental groups (Figs. 2C and D).
FIG. 2.
Cerebrovascular permeability and matrix proteins are altered in diabetic GK rats. A: BBB permeability was significantly increased in diabetes (n = 6 per group). *P < 0.01. B: Occludin protein levels, evaluated by immunoblotting of brain microvessel and macrovessel homogenates and normalized to actin levels, were decreased in the microvasculature of diabetic rats (n = 8 per group). *P < 0.05 vs. control. There was no difference in microvessels or microvessel claudin-5 levels (C) or microvascular collagen IV levels (D) in control and diabetic animals (n = 8 per group). □, microvessel; ■, macrovessel.
MMPs are involved in the regulation of vascular remodeling and BBB breakdown following ischemic injury. Thus, MMP-2 and MMP-9 proteins were measured in the same macro- and microvessel preparations used for evaluation of tight junction proteins. MMP-9 was more abundant in both micro- and macrovessels from diabetic GK rats than in controls (Fig. 3A). MMP-2, on the other hand, was significantly higher in the macrovessels, but not microvessels, of diabetic rats (Fig. 3B). MMP-2 and MMP-9 activity of macrovessels was assessed by gelatin zymography. Despite increases in MMP-2 protein levels in diabetic rats, there was no difference in MMP-2 activity between control and GK rats. MMP-9 activity, on the other hand, was increased in diabetic rats. Microvessel MMP-2 activity was also similar between groups (data not shown). Lytic bands corresponding to microvessel MMP-9 were very faint, so they were not quantified. To determine whether increased MMP activities were developed as a result of diabetes, we also measured macrovessel MMP activities in 5-week-old rats before the onset of diabetes. Total MMP activities were 108 ± 15 vs. 99 ± 11 pixels in control and diabetic rats (n = 4), respectively.
FIG. 3.
Effect of diabetes on extracellular matrix proteins and BDNF levels. MMP proteins are differentially regulated in the cerebrovasculature of diabetic rats. A: MMP-9 protein was increased in both vascular beds in diabetes (n = 6 per group). *P < 0.05 vs. control. □, microvessel; ■, macrovessel. B: MMP-2 protein was more abundant in the macrovessels and significantly increased in diabetes (n = 6 per group). *P < 0.05 vs. control; **P < 0.01 vs. microvessel. □, microvessel; ■, macrovessel. C: MMP-9 activity was increased in the macrovessels from diabetic rats. *P < 0.05 vs. control. □, MMP-2; ■, MMP-9. D: BDNF levels were higher in diabetes in both vascular beds (n = 5 per group). *P < 0.05 vs. control; **P < 0.05 vs. microvessel. □, microvessel; ■, macrovessel.
A recent study (20) provided evidence that BDNF released from endothelial cells protects the neurons from a wide array of insults. Accordingly, BDNF levels in the vessel preparations were measured by ELISA. The macrovessels of both control and diabetic animals showed high levels of BDNF, while the microvessels in diabetic group had higher level than controls (Fig. 3D).
Infarct size, hemorrhagic transformation, and edema.
Infarct size was smaller (29%) in diabetic animals than in controls (49%) (Fig. 4A). Consistent with our previous findings, the infarcts were mainly in the striatum in GK rats, while that of Wistar rats had both cortical and subcortical localization. Edema on the other hand was higher in diabetes (Fig. 4B). In contrast to our previous finding of overt hematoma formation following 3 h MCAO/21 h reperfusion (4), there was no macroscopic hemorrhagic transformation after 24 h permanent MCAO in either strain.
FIG. 4.
Infarct size is smaller in diabetic rats after permanent MCAO. A: Summary of cerebral infarct size from TTC-stained brain sections of control and diabetic rats. Infarct size was calculated as percentage of contralateral hemisphere (n = 13 for control and 9 for diabetes). *P = 0.001. B: Edema formation was assessed as the volume difference between ischemic and nonischemic hemispheres and normalized to infarct volume. *P < 0.05. There was a small but significant difference in Bederson score (C) but not in EBST (D) between the groups. *P < 0.05. D: □, before; ■, after. (A high-quality color digital representation of this figure is available in the online issue.)
Neurobehavioral outcome.
The modified Bederson test score was 0 in both groups before surgery and increased to 10.8 ± 0.3 in control and to 9.7 ± 0.4 in diabetic rats at 24 h after MCAO (Fig. 4C). As shown in Fig. 4D, MCAO induced a significant increase in left-swing responses in both groups with no obvious difference between the groups.
Cerebral perfusion.
Single-point laser Doppler probe has been widely used at monitoring cerebral blood perfusion in MCAO experiments. However, the single-point blood flow alteration is less representative for the overall perfusion. In this study, we used the scanning laser Doppler imaging system to monitor the real time subcranial cerebral blood perfusion. At 5 min after MCAO, the extent of perfusion decrease was similar in both control and diabetic rats (Fig. 5A), indicating that the extent of occlusion was comparable between the groups. However, at 24 h and immediately before they were killed, GK rats showed a slight recovery of perfusion, whereas there was no change in control rats (Fig. 5B).
FIG. 5.
Cerebral perfusion before and after MCAO. A: Cerebral perfusion was decreased to the same extent in both groups following MCAO. Percent decrease (5 min post-MCAO versus baseline) in perfusion was summarized in the bar graph. B: Cerebral perfusion was reevaluated before sacrifice at 24 h after occlusion and percent change (24 h post-MCAO versus 5 min post-MCAO) indicated recovery of flow in diabetic rats (n = 13 for control and 9 for diabetes). *P < 0.001. (A high-quality color digital representation of this figure is available in the online issue.)
DISCUSSION
Both clinical and experimental studies have shown that elevations in blood glucose due to preexisting diabetes or the acute stress response at the time of stroke is associated with a higher incidence and severity of cerebral infarction and an increased risk of hemorrhagic transformation secondary to acute ischemic stroke (21–26). The emerging neurovascular unit concept underlies the important contribution of cerebral blood vessels to the pathophysiology of stroke (27). Diabetes, although endocrine in origin, increases mortality and morbidity due to its vascular complications. It is a well-established fact that adaptive neovascularization of the coronary and peripheral circulations is impaired in diabetes, resulting in increased coronary artery disease and peripheral vascular disease risk, respectively (28,29). On the other hand, excessive angiogenesis occurs in the retinal circulation leading to diabetic retinopathy. The effect of diabetes on cerebral vasculature was unknown. Accordingly, this study investigated the early effects of diabetes (5-week period) on cerebrovascular density, neovascularization patterns, permeability, and perfusion in a type 2 diabetes model that presents with increased vascular but decreased neuronal damage following I/R injury (4). The main findings are 1) cerebrovascular density and collateralization was increased in diabetic rats providing evidence of increased angiogenesis and arteriogenesis; 2) BBB integrity was compromised even before an ischemic insult in diabetes; 3) 24 h after permanent occlusion, there was small but significant recovery of perfusion in diabetic rats, suggesting collateral flow; 4) consistent with our previous results with temporary MCAO, permanent occlusion also caused smaller infarcts in diabetes, suggesting neuroprotection in early moderate diabetes; and 5) there was no macroscopic hemorrhage after permanent occlusion, suggesting that bleeding is result a reperfusion injury of the newly formed/remodeled vessels in this model.
In contrast to previous reports that hyperglycemia promotes infarct expansion as mentioned above, we have reported smaller infarction but greater and more frequent hemorrhagic transformation occurrence in GK rats, a lean model of type 2 diabetes, after ischemic reperfusion injury induced by 3 h MCAO and 21 h reperfusion (4). It was also observed that cerebrovascular tortuosity and MMP activity in MCA were increased prior to I/R injury, providing evidence for cerebrovascular remodeling after a short 5- to 6-week period of diabetes as GK rats develop diabetes around 6 weeks of age and MCAO was performed when animals were 11–12 weeks old. These results suggested that there may be vascular and neuronal components contributing to differences observed in patterns of ischemic injury in control versus diabetic rats. Thus, the current study focused on early changes induced by diabetes on the cerebrovasculature from a structural point of view. It is well established that diabetes modulates neovascularization depending on the vascular bed. While excessive pathological angiogenesis leads to diabetic retinopathy (28,30), neovascularization is impaired in the myocardium and skeletal muscle contributing to coronary artery disease and peripheral arterial disease, respectively. Accordingly, we first addressed the question whether there was neovascularization in diabetic rats. Neovascularization may result from vasculogenesis (new vessel formation from progenitor cells), angiogenesis (capillary sprouting), collateral growth, and/or arteriogenesis defined as remodeling of native collaterals to functional arterioles (31–33). As the vessels remodel and collaterals form, vessels present with increased diameter and a typical corkscrew pattern as we detect in the pial vessels of the GK model (34). Accordingly, we evaluated capillary density as a measure of angiogenesis and pial collateral number and diameter as a measure of arteriogenesis in our model, all of which were increased in diabetes. These findings suggest that cerebrovasculature undergoes adaptive arteriogenesis and angiogenesis in moderate diabetes.
We next evaluated permeability as a measurement of cerebrovascular integrity in diabetes prior to an ischemic event. Increased permeability may be due to diabetes-induced damage to the BBB function and/or due to the immature nature of the newly formed vessels. Cerebrovascular permeability was increased in diabetes as reported in early diabetic retinopathy (14,35). Since tight junction proteins such as occludin and claudin-5 have essential roles in regulating BBB stability (36), we evaluated abundance of occludin, claudin-5, and collagen IV in the isolated micro- and macrovascular vessels from the brain. Occludin was lower in the microvasculature but not macrovasculature of the diabetic group. Chehade et al. (37) reported that occludin but not zona occludens-1 levels are decreased at 2 weeks after the induction of diabetes by STZ injection. Another study reported increased MMP-2 and MMP-9 activity and rapid degradation of occludin after temporary focal ischemia (38). In the current study, MMP-9 was increased in microvessel preparation along with decreased occludin. Taken together, these changes in the structural components of microvessels may be contributing to increased permeability in the diabetic GK rats. While we do not have direct evidence on the regulation of these proteins following an ischemic insult in our model, it is highly possible that these changes also play a role in the development of increased edema formation following permanent focal MCAO as we report in the current study as well as the development of overt hematomas following temporary occlusion in the GK rats as we reported previously (4).
Cerebral perfusion is a key determinant of the extent of ischemic injury. Thus, we confirmed that the extent of MCA occlusion was similar in control and diabetic rats using a scanning-laser Doppler imaging system. While it is recognized that cerebral blood flow needs to be assessed by more quantitative approaches, we found that 24 h after permanent MCAO, there was restoration of cerebral perfusion in the diabetic group consistent with increased collateralization in GK rats.
Numerous past experimental studies have reported exacerbation of the ischemic damage by hyperglycemia (7,21,24,25,39). These studies also reported increased hemorrhagic transformation only if blood flow was reestablished following occlusion. Consistent with these results, in the current study we did not observe any hematoma formation in either group. The interesting finding of the current study is consistent with our previous finding that infarct size is smaller in diabetic GK rats even after permanent occlusion. Careful review of the literature on diabetes and focal brain ischemia demonstrated that most studies used animal models in which blood glucose was elevated acutely by glucose injection just prior to occlusion or diabetes was induced by STZ injection a few days prior to surgery. In leptin receptor–deficient db/db mice, edema and infarct size after hypoxic-ischemic injury was increased as compared with nondiabetic animals (40), but it has to be recognized that this model is associated with obesity, and leptin is neuroprotective. An earlier study by Warner et al. reported that acutely hyperglycemic, but not diabetic, rats are more vulnerable to global ischemia despite similar levels of glycemia, suggesting some degree of protection in diabetes (41). Observational studies support this concept. Hyperglycemic nondiabetic acute ischemic stroke patients appear to suffer the most from stroke (26,42). Interestingly, myocardium is reported to be resistant to ischemic injury in diabetes as a result of metabolic preconditioning (43). We detected higher BDNF levels in the vessels of diabetic rats. A recent in vitro study (20) provided very intriguing evidence for neuroprotection by endothelium-derived BDNF secretion mediated by integrin signaling. It is possible that in our model, all the neovascularization events taking place may stimulate BDNF synthesis and release. Based on our intriguing results, we speculate that longer duration of hyperglycemia as seen in our GK model preconditions the brain, although mechanisms by which this occurs remain to be determined.
There are limitations to our study. First of all, the GK rats are a model of moderate type 2 diabetes without confounding factors like obesity and dyslipidemia that are frequently found in stroke patients. Howewer, diabetes is a risk factor for stroke independent of these comorbidities, and thus GK rats allow us to study effects of hyperglycemia alone. GK rats are an in-bred strain, and vascular changes may be inherent to the model. However, the fact that there was no difference in collateral numbers and MMP activity in younger GK rats before the onset of diabetes as compared with control rats at that age argues against this possibility. We also used relatively young animals. As with any animal model of disease, the diversity of acute ischemic stroke patients in clinical setting cannot be replicated with this model. Second, this study included only a short-term functional evaluation after stroke. Edema and microvascular responses may still be evolving at this point and functional recovery at later time points need to be assessed. Finally, we only studied the effect of short and moderate elevations in blood glucose, but duration and degree of hyperglycemia in diabetes may be critical for neurovascular outcomes following ischemic injury. Nevertheless, our results provide direct evidence that diabetes alters cerebrovascular density, neovascularization patterns, and microvascular permeability, all of which affect the neuronal and vascular damage following ischemic brain injury. When compared with the literature, these findings also suggest that the pattern of ischemic injury under diabetic and hyperglycemic conditions differs. Given that therapeutic angiogenesis after stroke is an active area of research, an enhanced understanding of cerebrovascular networking in the setting of diabetes is fundamentally important to develop preventive and therapeutic strategies for stroke in high-risk patients as well as improving therapeutic angiogenesis after stroke. The effect of longer duration of diabetes on cerebrovascular function, structure, and ultimately on ischemia/reperfusion injury requires further study.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK074385 and NS054688), Philip Morris Inc. and Philip Morris International, the American Heart Association Established Investigator Award (0740002N) (to A.E.), and the Department of Veterans Affairs Merit Award (to S.C.F.).
No potential conflicts of interest relevant to this article were reported.
The authors thank Erin M. Mezzetti, Jimmie R. Hutchinson, and Dr. Vera Portik-Dobos for expert technical assistant.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American Diabetes Association National diabetes fact sheet [article online], 2007. Available at http://www.diabetes.org/diabetes-statistics.jsp Accessed 8 August 2009
- 2.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR: Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999;100:1134–1146 [DOI] [PubMed] [Google Scholar]
- 3.Kernan WN, Inzucchi SE: Type 2 diabetes mellitus and insulin resistance: stroke prevention and management. Curr Treat Options Neurol 2004;6:443–450 [DOI] [PubMed] [Google Scholar]
- 4.Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC: Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol 2007;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farese RV, Standaert ML, Yamada K, Huang LC, Zhang C, Cooper DR, Wang Z, Yang Y, Suzuki S, Toyota T, et al. : Insulin-induced activation of glycerol-3-phosphate acyltransferase by a chiro-inositol-containing insulin mediator is defective in adipocytes of insulin-resistant, type II diabetic, Goto-Kakizaki rats. Proc Natl Acad Sci U S A 1994;91:11040–11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV, Jr, Farese RV: Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver: contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem 2004;279:24929–24934 [DOI] [PubMed] [Google Scholar]
- 7.Kamada H, Yu F, Nito C, Chan PH: Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke 2007;38:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner KR, Kleinholz M, de Courten-Myers GM, Myers RE: Hyperglycemic versus normoglycemic stroke: topography of brain metabolites, intracellular pH, and infarct size. J Cereb Blood Flow Metab 1992;12:213–222 [DOI] [PubMed] [Google Scholar]
- 9.Bomont L, MacKenzie ET: Neuroprotection after focal cerebral ischaemia in hyperglycaemic and diabetic rats. Neurosci Lett 1995;197:53–56 [DOI] [PubMed] [Google Scholar]
- 10.Krucker T, Lang A, Meyer EP: New polyurethane-based material for vascular corrosion casting with improved physical and imaging characteristics. Microsc Res Tech 2006;69:138–147 [DOI] [PubMed] [Google Scholar]
- 11.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE: Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics 2007;30:179–191 [DOI] [PubMed] [Google Scholar]
- 12.Kikano GE, LaManna JC, Harik SI: Brain perfusion in acute and chronic hyperglycemia in rats. Stroke 1989;20:1027–1031 [DOI] [PubMed] [Google Scholar]
- 13.Mouton PR, Gokhale AM, Ward NL, West MJ: Stereological length estimation using spherical probes. J Microsc 2002;206:54–64 [DOI] [PubMed] [Google Scholar]
- 14.El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI: Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol 2006;168:235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun H, Patel KP, Mayhan WG: Tetrahydrobiopterin, a cofactor for NOS, improves endothelial dysfunction during chronic alcohol consumption. Am J Physiol Heart Circ Physiol 2001;281:H1863–H1869 [DOI] [PubMed] [Google Scholar]
- 16.Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A: Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes 2005;54:2638–2644 [DOI] [PubMed] [Google Scholar]
- 17.Elewa HF, Kozak A, Johnson MH, Ergul A, Fagan SC: Blood pressure lowering after experimental cerebral ischemia provides neurovascular protection. J Hypertens 2007;25:855–859 [DOI] [PubMed] [Google Scholar]
- 18.Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF: Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke 2004;35:2206–2210 [DOI] [PubMed] [Google Scholar]
- 19.Borlongan CV, Sanberg PR: Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci 1995;15:5372–5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH: Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci U S A 2008;105:7582–7587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez-Sabin J, Molina CA, Ribo M, Arenillas JF, Montaner J, Huertas R, Santamarina E, Rubiera M: Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke 2004;35:2493–2498 [DOI] [PubMed] [Google Scholar]
- 22.Martini SR, Kent TA: Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab 2007;27:435–451 [DOI] [PubMed] [Google Scholar]
- 23.Bruno A, Biller J, Adams HP, Jr, Clarke WR, Woolson RF, Williams LS, Hansen MD: Acute blood glucose level and outcome from ischemic stroke: trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology 1999;52:280–284 [DOI] [PubMed] [Google Scholar]
- 24.Ennis SR, Keep RF: Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab 2007;27:1573–1582 [DOI] [PubMed] [Google Scholar]
- 25.Kawai N, Keep RF, Betz AL, Nagao S: Hyperglycemia induces progressive changes in the cerebral microvasculature and blood-brain barrier transport during focal cerebral ischemia. Acta Neurochir Suppl 1998;71:219–221 [DOI] [PubMed] [Google Scholar]
- 26.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC: Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001;32:2426–2432 [DOI] [PubMed] [Google Scholar]
- 27.Lo EH: Experimental models, neurovascular mechanisms and translational issues in stroke research. Br J Pharmacol 2008;153(Suppl. 1):S396–S405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvestre JS, Levy BI: Molecular basis of angiopathy in diabetes mellitus. Circ Res 2006;98:4–6 [DOI] [PubMed] [Google Scholar]
- 29.Celik T, Berdan ME, Iyisoy A, Kursaklioglu H, Turhan H, Kilic S, Gulec M, Ozturk S, Isik E: Impaired coronary collateral vessel development in patients with proliferative diabetic retinopathy. Clin Cardiol 2005;28:384–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW: Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets 2005;6:511–524 [DOI] [PubMed] [Google Scholar]
- 31.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W: Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med 2006;10:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silvestre JS, Levy BI, Tedgui A: Mechanisms of angiogenesis and remodelling of the microvasculature. Cardiovasc Res 2008;78:201–202 [DOI] [PubMed] [Google Scholar]
- 33.Silvestre JS, Mallat Z, Tedgui A, Levy BI: Post-ischaemic neovascularization and inflammation. Cardiovasc Res 2008;78:242–249 [DOI] [PubMed] [Google Scholar]
- 34.Clayton JA, Chalothorn D, Faber JE: Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res 2008;103:1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB: Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol 2003;162:1995–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins BT, Davis TP: The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005;57:173–185 [DOI] [PubMed] [Google Scholar]
- 37.Chehade JM, Haas MJ, Mooradian AD: Diabetes-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1) expression. Neurochem Res 2002;27:249–252 [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA: Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 2007;27:697–709 [DOI] [PubMed] [Google Scholar]
- 39.Quast MJ, Wei J, Huang NC, Brunder DG, Sell SL, Gonzalez JM, Hillman GR, Kent TA: Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab 1997;17:553–559 [DOI] [PubMed] [Google Scholar]
- 40.Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA: Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab 2001;21:52–60 [DOI] [PubMed] [Google Scholar]
- 41.Warner TD, Schmidt HHHW, Murad F: Interactions of endothelins and EDRF in bovine native endothelial cells; selective effects of endothelins-3. Am J Physiol 1992;262:H1600–H1605 [DOI] [PubMed] [Google Scholar]
- 42.McCormick MT, Muir KW, Gray CS, Walters MR: Management of hyperglycemia in acute stroke: how, when, and for whom? Stroke 2008;39:2177–2185 [DOI] [PubMed] [Google Scholar]
- 43.Galagudza MM, Nekrasova MK, Syrenskii AV, Nifontov EM: Resistance of the myocardium to ischemia and the efficacy of ischemic preconditioning in experimental diabetes mellitus. Neurosci Behav Physiol 2007;37:489–493 [DOI] [PubMed] [Google Scholar]