Abstract
OBJECTIVE
At least 20 type 2 diabetes loci have now been identified, and several of these are associated with altered β-cell function. In this study, we have investigated the combined effects of eight known β-cell loci on insulin secretion stimulated by three different secretagogues during hyperglycemic clamps.
RESEARCH DESIGN AND METHODS
A total of 447 subjects originating from four independent studies in the Netherlands and Germany (256 with normal glucose tolerance [NGT]/191 with impaired glucose tolerance [IGT]) underwent a hyperglycemic clamp. A subset had an extended clamp with additional glucagon-like peptide (GLP)-1 and arginine (n = 224). We next genotyped single nucleotide polymorphisms in TCF7L2, KCNJ11, CDKAL1, IGF2BP2, HHEX/IDE, CDKN2A/B, SLC30A8, and MTNR1B and calculated a risk allele score by risk allele counting.
RESULTS
The risk allele score was associated with lower first-phase glucose-stimulated insulin secretion (GSIS) (P = 7.1 × 10−6). The effect size was equal in subjects with NGT and IGT. We also noted an inverse correlation with the disposition index (P = 1.6 × 10−3). When we stratified the study population according to the number of risk alleles into three groups, those with a medium- or high-risk allele score had 9 and 23% lower first-phase GSIS. Second-phase GSIS, insulin sensitivity index and GLP-1, or arginine-stimulated insulin release were not significantly different.
CONCLUSIONS
A combined risk allele score for eight known β-cell genes is associated with the rapid first-phase GSIS and the disposition index. The slower second-phase GSIS, GLP-1, and arginine-stimulated insulin secretion are not associated, suggesting that especially processes involved in rapid granule recruitment and exocytosis are affected in the majority of risk loci.
Type 2 diabetes is a polygenic disease in which the contribution of a number of detrimental gene variants in combination with environmental factors is thought to be necessary for the development of disease. In the past 2 years, results of several genome-wide association studies (GWASs) have been published (1–5), leading to a rapidly increasing number of detrimental type 2 diabetes susceptibility loci. More recently, it has indeed been shown that combining information from these diabetes loci into a risk allele score for all loci enhances diabetes risk (6–9). However, the predictive power of this combined risk allele score is yet insufficient to substitute or largely improve predictive power of known clinical risk factors (8,9). At present, little is known about how these gene variants in combination affect insulin secretion or insulin resistance. Based on recent data, mainly obtained from oral glucose tolerance tests (OGTTs), it was shown that a combined risk allele score from gene variants associated with type 2 diabetes is associated with insulin secretion and not with insulin sensitivity (10–13). However, the OGTT is unable to distinguish between first- and second-phase insulin secretion. Furthermore, other secretagogues, like glucagon-like peptide (GLP)-1 and arginine, were not included in these studies.
It is thought that the rapid recruitment and release of insulin granules from the readily releasable pool (RRP) is responsible for the first phase of insulin secretion, whereas the slower prolonged second phase involves recruitment to the membrane of more distant granules and de novo insulin synthesis. Although the exact pathways regulating both phases of glucose-stimulated insulin secretion (GSIS) are not completely resolved, it seems logical that they are at least in part different. This is further corroborated by our recent observation that the heritability for both phases of GSIS in twins is derived from partly nonoverlapping sets of genes (13a).
Also, other nonglucose, stimuli-like incretins and amino acids can evoke an insulin response. Detailed phenotypic investigations of the response to these different stimuli may help to elucidate which processes are primarily affected by these loci. Previously, we have already shown that type 2 diabetes genes/loci can have different effects on first- and second-phase GSIS, as measured using hyperglycemic clamps. Also, based on the method of stimulation (i.e., oral versus intravenous), the outcome may differ substantially (14–17), which provides further clues about the mechanism by which they affect insulin secretion.
In this study, we genotyped gene variants in TCF7L2, KCNJ11, HHEX/IDE, CDKAL1, IGF2BP2, SLC30A8, CDKN2A/CDKN2B, and MTNR1B in 447 hyperglycemic clamped subjects (256 with normal glucose tolerance [NGT] and 191 with impaired glucose tolerance [IGT]) from four independent studies in the Netherlands and Germany. These eight loci were chosen based on the fact that they were reproducibly associated with β-cell function in various studies (rev. in 18,19). A combined risk allele score of all eight gene variants was calculated for each individual and tested against the various detailed measurements of β-cell function using the hyperglycemic clamp, generally considered to be the gold standard for quantification of first- and second-phase GSIS (20). Furthermore, we also assessed the combined effect of these eight genes on two other stimuli, GLP-1 and arginine-stimulated insulin secretion during hyperglycemia, in a subset of the study sample (n = 224). The latter test provides an estimation of the maximal insulin secretion capacity of a subject and may, according to animal studies, serve as a proxy for β-cell mass (21).
RESEARCH DESIGN AND METHODS
Hyperglycemic clamp cohorts.
Four independent studies from the Netherlands (NL) and Germany (D) were used. The clinical characteristics of the study groups are given in Table 1. Details of three of four samples have previously been described (Hoorn [NL, 137 with IGT]; Utrecht [NL, 60 with NGT/12 with IGT]; Tübingen [D, 83 with NGT/35 with IGT]) (17). We have extended our study sample with a cohort selected from the Netherlands Twin Register (NTR; 113 with NGT/7 with IGT) (22). This cohort consists of a mixed sample of twins and nontwin sibs recruited from 50 families (family size 1–9). In total, the NTR twin sample includes 66 monozygotic twins (31 pairs), 25 dizygotic twins (11 pairs), and 29 nontwin sibs (14 twin, nontwin sib pairs; 1 single nontwin sib).
TABLE 1.
Clinical characteristics of the hyperglycemic clamp cohorts
| The Netherlands |
Germany (Tübingen)* | |||
|---|---|---|---|---|
| Hoorn* | Utrecht* | NTR twins* | ||
| n (NGT/IGT) | 137 (0/137) | 72 (60/12) | 120 (113/7) | 118 (83/35) |
| Sex (M/F) | 64/73 | 17/55 | 55/65 | 51/67 |
| Age (years) | 60.5 ± 8.7 | 46.6 ± 6.7 | 31.6 ± 6.4 | 39.2 ± 13.2 |
| BMI (kg/m2) | 28.0 ± 4.0 | 25.9 ± 3.8 | 24.1 ± 3.5 | 25.5 ± 5.4 |
| Fasting plasma glucose (mmol/l) | 6.3 ± 0.7 | 4.7 ± 0.5 | 4.6 ± 0.4 | 5.1 ± 0.7 |
| 2-h plasma glucose (mmol/l) | 8.8 ± 1.7 | 5.7 ± 1.6 | 5.4 ± 1.2 | 6.5 ± 2.0 |
| Fasting plasma insulin (pmol/l) | 62 (46–91) | 36 (24–54) | 40 (26–47) | 43 (30–66) |
Hyperglycemic clamp procedure.
All participants underwent a hyperglycemic clamp at 10 mmol/l glucose for at least 2 h (21,23–26). After a priming infusion of glucose to acutely raise blood glucose levels, blood glucose levels were measured with a glucose analyzer and kept constant at 10 mmol/l during the whole clamp. Insulin levels were measured with immunoassays as previously described (21,23–26). To correct for this and possible other differences between centers, we introduced a dummy variable (study center) in our statistical analyses. First-phase insulin secretion was determined as the sum of the insulin levels during the first 10 min of the clamp. Second-phase insulin secretion was determined as the mean of the insulin levels during the last 40 min of the second hour of the clamp (80–120 min). The insulin sensitivity index (ISI) was calculated by relating the glucose infusion rate (M) to the plasma insulin concentration (I) during the last 40 min of the second hour of the clamp (M/I). Mitrakou et al. (27) compared the ISI determined with a hyperglycemic clamp with insulin sensitivity as determined using the euglycemic-hyperinsulinemic clamp in the same subjects and found a good agreement between the two methods. The disposition index (DI) was calculated by multiplication of first-phase insulin secretion and ISI in order to quantify insulin secretion in relation to the ambient insulin sensitivity (28,29).
Subjects from Tübingen and the NTR twin sample both underwent an extended clamp using additional GLP-1 and arginine stimulation as described previously (21). GLP-1–stimulated insulin release was measured as the mean incremental area under the curve (160–180 min) following GLP-1 stimulation (4.5 pmol/kg bolus for 1 min at t = 120 followed by a continuous infusion of 1.5 pmol · kg−1 · min−1. In the Dutch NTR twin cohort, slightly lower GLP-1 concentrations were used (1.5 pmol/kg and 0.5 pmol · kg−1 · min−1, respectively). Arginine-stimulated acute insulin release was measured by injecting a bolus of 5 g arginine hydrochloride at t = 180 as described previously (21). The acute insulin response to arginine was calculated as the mean incremental area under the curve from 182 to 185 min.
Genotyping.
Based on the available literature regarding GSIS and the novel type 2 diabetes genes, we selected gene variants in TCF7L2 (rs7903146), KCNJ11 (rs5219), CDKAL1 (rs7754840), IGF2BP2 (rs4402960), HHEX/IDE (rs1111875), SLC30A8 (rs13266634), CDKN2A/B (rs10811661), and MTNR1B (rs10830963) for genotyping. Results of the association analysis of the effect of the individual genes on GSIS during hyperglycemic clamps in the Dutch Hoorn and Utrecht study and the German Tübingen study have been published previously (14–17). β and P values for all four samples combined, including the Dutch NTR twins, are given in supplementary Table 1 (available in the online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0736/DC1). All single nucleotide polymorphisms (SNPs) were measured using either the Sequenom platform (Sequenom, San Diego, CA) or Taqman SNP genotyping assays (Applied Biosystems, Foster City, CA) in all subjects. The genotyping success rate was >97% for all SNPs, and samples measured in duplicate (∼5%) revealed no errors.
Statistics.
We combined the information of the SNPs using an allele count model (6). We summed the number of risk alleles carried by each individual, assuming an equal and additive effect of each allele. The effect of the sum score of risk alleles on the responses was examined by calculating the β values for the risk allele score with linear generalized estimating equations, which takes into account the family relatedness when computing the standard errors (i.e., in the twin sample). For ease of interpretation, the exponent β values (10β) are given throughout the article. For analyses of first- and second-phase GSIS, GLP-1 and arginine-stimulated insulin secretion adjustment for age, sex, BMI, study center, glucose tolerance status, and ISI were used. For the analysis of ISI and DI, ISI was removed from the model. All outcome variables were log-transformed prior to analysis. Logistic regression with adjustment for age, sex, and BMI was used to test associations with dichotomous end points like the absence of a first-phase insulin peak and type 2 diabetes. A priori power calculations showed that the design used in this study would allow the detection of a difference in insulin secretion between 10% (glucose) to 25% (GLP-1, arginine) with 80% power (α < 0.05), depending on the stimulus used and allele frequency. All data are given as estimated mean (95% CI) unless otherwise stated. After correction for multiple hypothesis testing, results were regarded significant at P < 0.008 (six tests). For all statistical analyses, SPSS version 16.0 software (SPSS, Chicago, IL) was used.
RESULTS
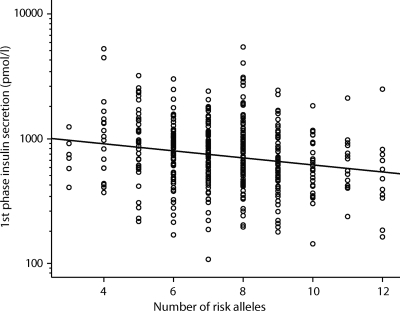
The risk allele counts for the eight β-cell genes were normally distributed in our participants (supplementary Fig. 1). There was a significant inverse correlation between the number of risk alleles and first-phase GSIS (β = 0.95 [95% CI 0.93–0.97]; P = 7.1 × 10−6) (Fig. 1), indicating that first-phase GSIS decreases with a factor of 0.95 with each additional risk allele. The observed effect size on first-phase GSIS was equal in both subjects with NGT and IGT (βNGT = 0.95, P = 4.6 × 10×5, and βIGT = 0.95, P = 0.015, respectively). Furthermore, the effect was present in each of the separate study samples (β range 0.93–0.96, all P ≤ 0.08). There was no significant effect of the number of risk alleles on second-phase GSIS or ISI (both P ≥ 0.13). However, there was also an inverse correlation with the DI as measured by the clamp (β = 0.96 [95% CI 0.94–0.99]; P = 1.6 × 10−3). The risk allele score explains 4% of the variance in first-phase GSIS and 5% of the variance in the DI.
FIG. 1.
First-phase GSIS in relation to the risk allele counts for the eight loci. Each circle represents an independent participant. The line represents the regression line after adjustment for age, sex, BMI, study center, glucose tolerance status, and ISI. β = 0.95 (95% CI 0.93–0.97); P = 7.1 × 10−6.
To examine whether our results can be attributed to the effect of one or more single loci, we also added the single loci to the model with the risk allele score; however, none of the single loci remained significant in this analysis (all P > 0.3). Previously, we showed that three single loci are significantly associated with first-phase GSIS (CDKAL1, IGF2BP2, and MTNR1B) (supplementary Table 1) (14–17,30). Therefore, we also tested a model that includes the three significant single loci and a combined risk allele score for the remaining five loci (TCF7L2, KCNJ11, HHEX, SLC30A8, and CDKN2A/B). In this analysis, the five-gene risk allele score still added significant information to the model (P < 0.05).
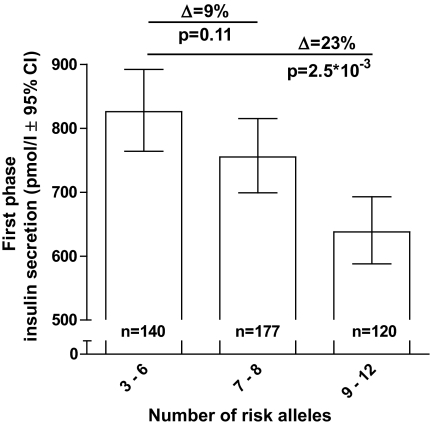
For ease of interpretation, we next stratified the participants into three approximately equally sized strata; carriers of a low (less than seven risk alleles, n = 141, 32%), medium (7–8 risk alleles, n = 183, 40%), and high number of risk alleles (more than eight risk alleles, n = 123, 28%). The characteristics of the three groups are given in Table 2 and the results per study sample in supplementary Table 2. Analysis of the difference in first-phase GSIS between these different strata showed a 9 and 23% lower first GSIS in the medium and high strata compared with the reference group (low) (Ptrend = 5.9 × 10−6) (Fig. 2). Analysis of the differences in DI between these groups showed a 9 and 17% reduction in DI (Ptrend = 2.9 × 10−3) (Table 2). Again, no significant difference between the strata was found for second-phase GSIS or ISI (both P > 0.16). We did not observe an association of the number of risk alleles and GLP-1–stimulated insulin release during the clamp (Table 2). Furthermore, the maximal insulin secretion capacity as measured by arginine stimulation was not affected by the number of risk alleles present (P = 0.65) (Table 2).
TABLE 2.
Clinical characteristics of three stratified groups for number of risk alleles
| Group | n | Sex (M/F) | Age (years) | BMI (kg/m2) | First-phase insulin response (pmol/l) | Second-phase insulin response (pmol/l) | ISI (μmol · min−1 · kg−1 · pmol/l−1) | DI (μmol · min−1 · kg−1) | GLP-1–stimulated insulin release (pmol/l)* | Arginine-stimulated insulin release (pmol/l)* |
|---|---|---|---|---|---|---|---|---|---|---|
| Low | 141 | 58/83 | 45 ± 15 | 26.0 ± 4.6 | 826 (764–892) | 248 (232–265) | 0.142 (0.130–0.156) | 118 (108–129) | 1,792 (1,541–2,084) | 2,145 (1,930–2,385) |
| Medium | 183 | 88/95 | 45 ± 15 | 25.8 ± 4.5 | 755 (699–815) | 252 (236–269) | 0.140 (0.128–0.153) | 108 (100–116) | 1,698 (1,441–2,002) | 1,982 (1,747–2,249) |
| High | 123 | 42/81 | 45 ± 13 | 26.1 ± 4.4 | 638 (588–693) | 239 (221–258) | 0.158 (0.142–0.174) | 98 (90–107) | 1,614 (1,354–1,923) | 2,080 (1,855–2,332) |
| β1 | 0.88 (0.83–0.93) | 0.98 (0.93–1.03) | ND | ND | 0.95 (0.84–1.07) | 0.98 (0.91–1.06) | ||||
| P | 5.9 × 10−6 | 0.50 | 0.38 | 0.65 | ||||||
| β2 | 0.87 (0.81–0.93) | 0.96 (0.90–1.03) | 1.05 (0.98–1.13) | 0.91 (0.86–0.97) | 0.92 (0.83–1.06) | 0.97 (0.89–1.06) | ||||
| P | 1.8 × 10−5 | 0.27 | 0.16 | 2.9 × 10−3 | 0.28 | 0.48 |
Data are means ± SD or estimated means using model 1 (95% CI). Low = carriers of less than seven risk alleles, medium = carriers of seven or eight risk alleles, high = carriers of more than eight risk alleles. All variables were log-transformed before analysis. P values were computed for different additive models using linear generalized estimating equations, which takes into account the family relatedness when computing the standard errors. Model 1: adjusted for study center, glucose tolerance status, age, sex, BMI, and ISI. Model 2: adjusted for study center, glucose tolerance status, age, sex, and BMI.
*Available for 224 subjects from the Tübingen and NTR sample. ND, not determined.
FIG. 2.
Mean estimated first-phase GSIS in three different risk allele strata. Those with three to six risk alleles were used as a reference group.
Recently, we have shown that a four-gene risk allele score alters the age-related decline in β-cell function in obese subjects as measured by OGTT (11). Although we have a limited number of obese subjects in the present study (BMI ≥30 kg/m2, n = 66), we noted a similar increased decline in β-cell function in obese subjects with a higher number of risk alleles (first-phase GSIS: βlow = 1.01 [95% CI 0.99–1.03], P = 0.46; βmedium = 0.98 [0.96–0.99], P = 1.1 × 10×3; βhigh = 0.97 [0.96–0.99], P = 5.5 × 10−3).
Previously, we have shown that the absence of a first-phase insulin peak is a strong predictor of future development of type 2 diabetes in subjects with IGT (26). In the present study, subjects with IGT without a first-phase peak had on average 1.28 (95% CI 0.71–1.85) more risk alleles than those with a peak (P = 1.0 × 10−5). In three strata, the frequency of an absent first-phase peak increased from 12% in the low group to 40% in the high stratum (Ptrend = 6.9 × 10−4, adjusted for age, sex, and BMI) (Table 3). Those with a medium or high number of risk alleles also had an increased risk of conversion to type 2 diabetes during follow-up; however, due to the small numbers, this was not significant (Table 3).
TABLE 3.
Impaired glucose-tolerant group details and follow-up
| Group (number of risk alleles) | n | First-phase peak absent/present | Type 2 diabetes during follow-up (n = 93) (yes/no) |
|---|---|---|---|
| Low (≤6) | 51 | 6/45 (0.12) | 9/20 (0.31) |
| Medium (7–8) | 75 | 21/54 (0.28) | 14/24 (0.37) |
| High (≥9) | 47 | 20/30 (0.40) | 13/13 (0.50) |
| P | 4.7 × 10−3 | 0.16 | |
| Pmodel 1 | 6.9 × 10−4 | 0.19 |
Stratification according to the number of risk alleles in subjects with IGT only. Absence of the first-phase peak was defined according to the method of Nijpels et al (26). Numbers in parentheses are percentages of total. P = unadjusted; Pmodel 1 is P value after logistic regression analysis adjusted for age, sex, and BMI.
DISCUSSION
In this study, we have shown that a risk allele score for eight β-cell loci is associated with lower glucose-stimulated first-phase insulin secretion but not with other measures of β-cell function. Previously, three other groups investigated the relationship between a risk allele score of β-cell loci and GSIS. Pascoe et al. (10) used a risk allele score of seven loci (TCF7L2, KCNJ11, HHEX/IDE, CDKAL1, IGF2BP2, SLC30A8, and CDKN2A/B), whereas Haupt and colleagues (10–12) used four loci for his main analyses (TCF7L2, CDKAL1, HHEX/IDE, and SLC30A8). Finally, Stančáková et al. (13) recently reported the results of a risk allele score identical to the one used in this study. All three groups mainly used data from OGTTs in nondiabetic volunteers and were able to show that their risk allele scores are inversely correlated with β-cell function. The novelty of our study is the fact that we used hyperglycemic clamps with three different stimuli and the extended risk allele score including eight proven β-cell loci (TCF7L2, KCNJ11, HHEX/IDE, CDKAL1, IGF2BP2, SLC30A8, CDKN2A/B, and MTNR1B, a gene for which it has recently been shown that it is associated with type 2 diabetes and reduced GSIS) (30–33). We were able to show that only the first-phase GSIS is associated with our combined risk allele score. In contrast, the other measures of β-cell function and insulin sensitivity were not associated. Furthermore, we noted a significant association with a lower DI (which is the product of first-phase GSIS × ISI), suggesting that the investigated subjects are unable to compensate adequately for a diminished insulin sensitivity (29). Previously, it has been shown that a low DI is associated with glucose intolerance and highly predictive for future diabetes (34). Remarkably, the alterations in first-phase GSIS and DI are already present in subjects with NGT, suggesting that these defects are either present from birth on or develop well before the onset of hyperglycemia. Interestingly, it appears from our previous (11) and current data that environmental and/or genetic factors acting on obesity interact with the genetic effects on β-cell function by altering the rate of the age-related decline in β-cell function.
Our data highlight the importance of using different methods to investigate various aspects of insulin secretion. Whereas previous studies have shown that these genes together can affect overall insulin secretion during OGTTs, this report refines this important observation by showing that mainly the first phase of GSIS is affected. This suggests that their combined effect primarily involves processes regulating the rapid recruitment and exocytosis of insulin granules following glucose stimulation. SLC30A8 encodes a β-cell–specific Zn transporter important for insulin storage, stability, and granule exocytosis, which may fit well with the observed defect (35). For the other genes, it is less clear how they may affect the first phase of GSIS. However, for one of the genes present in our risk allele score, TCF7L2, its role in insulin granule recruitment and exocytosis was recently supported by cell-based studies using overexpression or knock down of the gene (36).
As we and others have shown previously, the genetic variation in TCF7L2 mainly affects GLP-1–induced insulin secretion (16,37). In our current analysis, no resistance to GLP-1–induced insulin secretion with increasing number of risk alleles could be detected. This may have several reasons. First, this incretin resistance mediated by variation in TCF7L2 is likely to be masked in the present analysis by the other seven risk loci that have no known effect on incretin-induced insulin secretion. This also suggests that the association of the risk allele score with first-phase GSIS is not dominated by the effects of a single locus but rather reflects the addition of independent risk mechanisms from all loci together. This is further corroborated by the fact that when we tested for dominance of single genes, by adding them to the model, there were no associations with the single loci. Second, the power of the present analysis may be too low considering the relatively small subgroup in which we assessed the GLP-1–induced insulin secretion (n = 224).
Several of the loci present in our risk allele score are putatively involved in transcriptional and/or cell cycle control, and it has been suggested that they may cause a reduced β-cell mass leading to the observed β-cell defects (19,38,39). However, our data show that our risk allele score of eight proven β-cell genes is not associated with arginine-induced insulin secretion during hyperglycemia, a marker of (near) maximal insulin secretion capacity, which has been proposed as a proxy for β-cell mass (21).
The finding that a higher risk allele score has no effect on second-phase GSIS, incretin-induced insulin secretion, or maximal insulin secretion capacity in subjects with NGT and IGT does not exclude a relevant role of these mechanisms in the β-cell defects leading to type 2 diabetes. However, we may conclude that the reduced first-phase GSIS is the first and prominent β-cell defect leading to type 2 diabetes. This is in accordance with our recent finding that the absence of a first-phase insulin peak during hyperglycemic clamps was the best predictor of future development of type 2 diabetes in subjects with IGT (hazard ratio 5.74 [95% CI 2.60–12.67]) (26). The strong correlation we observe between our risk allele score and the absence of a first-phase peak in our subjects with IGT suggests that the eight genes we tested might be a better predictor of future type 2 diabetes compared with the generally used risk allele score of all known type 2 diabetes genes. However, due to the very small number of converters in our study, this hypothesis should be tested in larger, more suitable, prospective study samples.
One of the strong aspects of our studies is the fact that we use four independent study samples from the Netherlands and Germany, which largely reduced the chance of false-positive findings. However, although this is the largest study sample available using hyperglycemic clamps to test associations between diabetes loci and β-cell function, we cannot exclude that we have missed some of the more subtle alterations. Larger samples including type 2 diabetic subjects and perhaps other sophisticated tests of β-cell function would be needed to fully explore all aspects of β-cell function regarding these diabetes loci.
In conclusion, we show that a combined score of risk alleles for eight β-cell loci is associated with reduced first- but not second-phase GSIS or maximal insulin secretion capacity. Furthermore, in subjects with IGT, there was a strong correlation with the absence of a first-phase insulin peak, which is a strong predictor of future development of type 2 diabetes. Our data provide evidence that the β-cell loci identified thus far act mainly via detrimental effects on processes involved in the early, rapid recruitment and exocytosis of insulin granules after glucose stimulation rather than altering maximal insulin secretion capacity.
Supplementary Material
Acknowledgments
The Dutch work of this study was supported by the Dutch Diabetes Research Foundation (Amersfoort, the Netherlands), the Netherlands Organization for Health Research and Development, and the Research Institute for Diseases in the Elderly (RIDE Program). The German work was supported by a grant from the Deutsche Forschungsgemeinschaft (KFO 114, Fr 1561/5-1 and MSD).
No potential conflicts of interest relevant to this article were reported.
The authors thank the participants for their kind cooperation.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, et al. : A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007;445:881–885 [DOI] [PubMed] [Google Scholar]
- 2.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, et al. : Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316:1331–1336 [DOI] [PubMed] [Google Scholar]
- 3.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, et al. : A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007;316:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, et al. : Replication of genome-wide association signals in U.K. samples reveals risk loci for type 2 diabetes. Science 2007;316:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, et al. : Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 2008;40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weedon MN, Mccarthy MI, Hitman G, Walker M, Groves CJ, Zeggini E, Rayner NW, Shields B, Owen KR, Hattersley AT, Frayling TM: Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med 2006;3:e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauchi S, Meyre D, Durand E, Proenca C, Marre M, Hadjadj S, Choquet H, De GF, Gaget S, Allegaert F, et al. : Post genome-wide association studies of novel genes associated with type 2 diabetes show gene-gene interaction and high predictive value. PLoS ONE 2008;3:e2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lango H, Palmer CN, Morris AD, Zeggini E, Hattersley AT, Mccarthy MI, Frayling TM, Weedon MN: Assessing the combined impact of 18 common genetic variants of modest effect sizes on type 2 diabetes risk. Diabetes 2008;57:3129–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L: Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 2008;359:2220–2232 [DOI] [PubMed] [Google Scholar]
- 10.Pascoe L, Frayling TM, Weedon MN, Mari A, Tura A, Ferrannini E, Walker M: Beta cell glucose sensitivity is decreased by 39% in non-diabetic individuals carrying multiple diabetes-risk alleles compared with those with no risk alleles. Diabetologia 2008;51:1989–1992 [DOI] [PubMed] [Google Scholar]
- 11.Haupt A, Staiger H, Schafer SA, Kirchhoff K, Guthoff M, Machicao F, Gallwitz B, Stefan N, Haring HU, Fritsche A: The risk allele load accelerates the age-dependent decline in beta cell function. Diabetologia 2009;52:457–462 [DOI] [PubMed] [Google Scholar]
- 12.Haupt A, Guthoff M, Schafer SA, Kirchhoff K, Machicao F, Gallwitz B, Staiger H, Stefan N, Fritsche A, Haring HU: The inhibitory association of recent type 2 diabetes risk loci on insulin secretion is modulated by insulin sensitivity. J Clin Endocrinol Metab 2009;94:1775–1780 [DOI] [PubMed] [Google Scholar]
- 13.Stančáková A, Kuulasmaa T, Paananen J, Jackson AU, Bonnycastle LL, Collins FS, Boehnke M, Kuusisto J, Laakso M: Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes 2009;58:2129–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Simonis-Bik AM, Eekhoff EM, de Moor MH, Kramer MH, Boomsma DI, Heine RJ, Dekker JM, Maassen JA, 't Hart LM, Diamant M, de Geus EJ: Genetic influences on the insulin response of the beta cell to different secretagogues. Diabetologia 2009;52:2570–2577 [DOI] [PubMed] [Google Scholar]
- 14.Tschritter O, Stumvoll M, Machicao F, Holzwarth M, Weisser M, Maerker E, Teigeler A, Haring H, Fritsche A: The prevalent Glu23Lys polymorphism in the potassium inward rectifier 6.2 (KIR6.2) gene is associated with impaired glucagon suppression in response to hyperglycemia. Diabetes 2002;51:2854–2860 [DOI] [PubMed] [Google Scholar]
- 15.'t Hart LM, Van Haeften TW, Dekker JM, Bot M, Heine RJ, Maassen JA: Variations in insulin secretion in carriers of the E23K variant in the KIR6.2 subunit of the ATP-sensitive K(+) channel in the β-cell. Diabetes 2002;51:3135–3138 [DOI] [PubMed] [Google Scholar]
- 16.Schafer SA, Tschritter O, Machicao F, Thamer C, Stefan N, Gallwitz B, Holst JJ, Dekker JM, 't Hart LM, Nijpels G, et al. : Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia 2007;50:2443–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groenewoud MJ, Dekker JM, Fritsche A, Reiling E, Nijpels G, Heine RJ, Maassen JA, Machicao F, Schafer SA, Haring HU, et al. : Variants of CDKAL1 and IGF2BP2 affect first-phase insulin secretion during hyperglycaemic clamps. Diabetologia 2008;51:1659–1663 [DOI] [PubMed] [Google Scholar]
- 18.Florez JC: Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia 2008;51:1100–1110 [DOI] [PubMed] [Google Scholar]
- 19.Perry JR, Frayling TM: New gene variants alter type 2 diabetes risk predominantly through reduced beta-cell function. Curr Opin Clin Nutr Metab Care 2008;11:371–377 [DOI] [PubMed] [Google Scholar]
- 20.Defronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 21.Fritsche A, Stefan N, Hardt E, Schutzenauer S, Haring H, Stumvoll M: A novel hyperglycaemic clamp for characterization of islet function in humans: assessment of three different secretagogues, maximal insulin response and reproducibility. Eur J Clin Invest 2000;30:411–418 [DOI] [PubMed] [Google Scholar]
- 22.Simonis-Bik AM, Eekhoff EM, Diamant M, Boomsma DI, Heine RJ, Dekker JM, Willemsen G, van Leeuwen M, de Geus EJ: The heritability of HbA1c and fasting blood glucose in different measurement settings. Twin Res Hum Genet 2008;11:597–602 [DOI] [PubMed] [Google Scholar]
- 23.Ruige JB, Dekker JM, Nijpels G, Poppsnijders C, Stehouwer CDA, Kostense PJ, Bouter LM, Heine RJ: Hyperproinsulinaemia in impaired glucose tolerance is associated with a delayed insulin response to glucose. Diabetologia 1999;42:177–180 [DOI] [PubMed] [Google Scholar]
- 24.Van Haeften TW, Zonderland ML, Dubbeldam S, Erkelens DW: Insulin secretion in normal glucose-tolerant relatives of type 2 diabetic subjects: assessments using hyperglycemic glucose clamps and oral glucose tolerance tests. Diabetes Care 1998;21:278–282 [DOI] [PubMed] [Google Scholar]
- 25.Fritsche A, Stefan N, Hardt E, Haring H, Stumvoll M: Characterisation of beta-cell dysfunction of impaired glucose tolerance: evidence for impairment of incretin-induced insulin secretion. Diabetologia 2000;43:852–858 [DOI] [PubMed] [Google Scholar]
- 26.Nijpels G, Boorsma W, Dekker JM, Hoeksema F, Kostense PJ, Bouter LM, Heine RJ: Absence of an acute insulin response predicts onset of type 2 diabetes in a Caucasian population with impaired glucose tolerance. J Clin Endocrinol Metab 2008;93:2633–2638 [DOI] [PubMed] [Google Scholar]
- 27.Mitrakou A, Vuorinen-Markkola H, Raptis G, Toft I, Mokan M, Strumph P, Pimenta W, Veneman T, Jenssen T, Bolli G, et al. : Simultaneous assessment of insulin secretion and insulin sensitivity using a hyperglycemic clamp. J Clin Endocrinol Metab 1992;75:379–382 [DOI] [PubMed] [Google Scholar]
- 28.Bergman RN, Phillips LS, Cobelli C: Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and B-cell glucose sensitivity from the response to intraveneous glucose. J Clin Invest 1981;68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, et al. : Quantification of the relationship between insulin sensitivity and beta cell function in human subjects: evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 30.Staiger H, Machicao F, Schafer SA, Kirchhoff K, Kantartzis K, Guthoff M, Silbernagel G, Stefan N, Haring HU, Fritsche A: Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine beta-cell function. PLoS ONE 2008;3:e3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, et al. : Variants in MTNR1B influence fasting glucose levels. Nat Genet 2009;41:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, et al. : Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 2009;41:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, et al. : A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet 2009;41:89–94 [DOI] [PubMed] [Google Scholar]
- 34.Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissen M, Isomaa B, Forsen B, Homstrom N, Saloranta C, et al. : Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005;54:166–174 [DOI] [PubMed] [Google Scholar]
- 35.Chimienti F, Devergnas S, Favier A, Seve M: Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 2004;53:2330–2337 [DOI] [PubMed] [Google Scholar]
- 36.da Silva X, Loder MK, McDonald A, Tarasov AI, Carzaniga R, Kronenberger K, Barg S, Rutter GA: TCF7L2 regulates late events in insulin secretion from pancreatic islet β-cells. Diabetes 2009;58:894–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyssenko V, Lupi R, Marchetti P, Del GS, Orho-Melander M, Almgren P, Sjogren M, Ling C, Eriksson KF, Lethagen AL, et al. : Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007;117:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mccarthy MI, Hattersley AT: Learning from molecular genetics: novel insights arising from the definition of genes for monogenic and type 2 diabetes. Diabetes 2008;57:2889–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridderstrale M, Groop L: Genetic dissection of type 2 diabetes. Mol Cell Endocrinol 2009;297:10–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.