Abstract
OBJECTIVE
Hyperglycemia impairs angiogenesis in response to ischemia, leading to ventricular remodeling. Although the effects of overexpressing angiogenic growth factors have been studied in inducing angiogenesis, the formation of functional vessels remains a challenge. The present study evaluates the reversal of diabetes-mediated impairment of angiogenesis in the infarcted diabetic rat myocardium by proangiogenic gene therapy.
RESEARCH DESIGN AND METHODS
Ad.VEGF and Ad.Ang1 were intramyocardially administered in combination immediately after myocardial infarction to nondiabetic and diabetic rats. Ad.LacZ was similarly administered to the respective control groups. The hearts were excised for molecular and immunohistochemical analysis at predetermined time points. The myocardial function was measured by echocardiography 30 days after the intervention.
RESULTS
We observed reduced fibrosis and increased capillary/arteriolar density along with reduced ventricular remodeling, as assessed by echocardiography in the treated diabetic animals compared with the nontreated diabetic controls. We also observed increased phosphorylated mitogen-activated protein kinase–activated protein kinase-2, 2 days after the treatment and increased expression of vascular endothelial growth factor (VEGF), Flk-1, angiopoietin-1 (Ang-1), Tie-2, and survivin, 4 days after treatment in the diabetic animals. Gel shift analysis revealed that the combination gene therapy stimulated the DNA binding activity of nuclear factor-κB in the diabetic animals.
CONCLUSIONS
Our preclinical data demonstrate the efficacy of coadministration of adenoviral VEGF and Ang-1 in increasing angiogenesis and reducing ventricular remodeling in the infarcted diabetic myocardium. These unique results call for the initiation of a clinical trial to assess the efficacy of this therapeutic strategy in the treatment of diabetes-related human heart failure.
Diabetic individuals who develop an ischemic heart disease (IHD) sustain an unfavorable prognosis for survival compared with other IHD subjects without diabetes (1). This condition may be attributed to impaired coronary collateral vessel development and reduced myocardial vascular perfusion in response to ischemia, leading to profound ventricular remodeling and subsequent heart failure (2). Various studies have linked diabetes-mediated impaired myocardial angiogenesis to alterations in the delicate balance of angiogenic growth factors and cytokines regulating vascular stability (2–4) and compromised signal transduction (4). Several studies have reported the possible role of decreased vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang-1) in the pathogenesis of diabetes-mediated impairment of angiogenesis in the myocardium (5–7).
There have been several attempts at preclinical and clinical levels to induce angiogenesis by overexpressing angiogenic factors in the peri-infarct zone after myocardial infarction (MI). Most of the studies have approached this issue using a single gene as the therapeutic agent. Delivery of vectors encoding VEGF165 (VEGF) and VEGF-2 was shown to improve collateral vascular perfusion and nourish the oxygen-depleted myocardium, thereby reducing angina and improving heart function in human clinical trials (8–10). However, investigations into the long-term effects of sustained expression of VEGF in mice models revealed deleterious effects due to the formation of leaky immature vessels/hemangiomas and subsequent death of the experimental animal (11,12). Furthermore, transgenic mice overexpressing VEGF revealed lengthy and leaky dermal vessels with evident inflammation (13,14).
On the other hand, the Ang-1 system is known to play a critical role in vascular maturation and stabilization, thereby supporting VEGF-induced neovascularization in a complementary manner (6,14,15). Recently, Ang-1 gene therapy has been shown to support the maturation of the immature vasculature in db/db mice (16).
In the recent past, work has been done to elucidate the synergistic effect of coadministration of VEGF and Ang-1 in ischemic rat myocardium (17–19). Zhou et al. (18) reported that combined gene therapy using VEGF and Ang-1 significantly reduced myocardial infarct size through the induction of the phosphatidylinositol 3-kinase and Bcl-2 survival pathways and nuclear factor-κB (NFκB) activation.
The prospect of a gene therapy using a combination of VEGF and Ang-1 encoding vectors to activate the angiogenic signaling cascade has not yet been explored in the diabetic ischemic myocardium. Diabetes reflects a far more challenging condition, where the VEGF and Ang-1 system is significantly downregulated, hampering the ability of the myocardium to respond to an ischemic stress (2,6), and where the usual revascularization techniques such as coronary artery bypass graft and percutaneous transluminal coronary angioplasty tend to fail, thereby leaving many of the diabetic IHD subjects with no option. Therefore, in this study we aimed at using a combination gene therapy approach involving in vivo adenoviral gene delivery of VEGF and Ang-1, to enhance neoangiogenesis by repairing the impaired angiogenic signaling cascade and thereby reducing ventricular remodeling in streptozotocin (STZ)-induced type 1 diabetic rats. Our findings emphasize the efficacy of coadministration of adenoviral vectors encoding VEGF and Ang-1 in inducing and stabilizing the process of angiogenesis that is impaired in the diabetic myocardium and in reducing ventricular remodeling in the infarcted myocardium in a diabetic milieu, thereby supporting the development of a combination gene therapy for therapeutic myocardial angiogenesis.
RESEARCH DESIGN AND METHODS
Experimental animals.
This study was performed in accordance with the principles of laboratory animal care formulated by the National Society for Medical Research and with the Guide for the Care and Use of Laboratory Animals (20). The experimental protocol was approved by the Institutional Animal Care Committee of the University of Connecticut Health Center (Farmington, CT). Male SD rats (300–325 g) were randomly separated into normal and diabetic rats as they received an intraperitoneal injection of vehicle (0.1 mol/l citrate buffer, pH 4.5) alone or STZ at a dosage of 65 mg/kg body wt dissolved in 0.1 mol/l citrate buffer.
Experimental design/surgical procedure.
MI was induced in the diabetic animals 30 days after the induction of diabetes as previously described (21). Age-matched nondiabetic animals were used as comparable controls. The rats were randomized into six groups: 1) control (nondiabetic) sham (CS), 2) diabetic sham (DS), 3) control (nondiabetic) MI (CMI) + Ad.LacZ (CLZMI), 4) CMI + (Ad.VEGF+Ad.Ang1) (CVAMI), 5) diabetic MI (DMI) + Ad.LacZ (DLZMI), and 6) DMI + (Ad.VEGF+Ad.Ang1) (DVAMI). Ad.VEGF and Ad.Ang1 were generous gifts from Dr. C. Li, East Tennessee State University (18). Dr. Li's groups verified the transfection efficiency of these adenoviral vectors in vitro. Moreover, the authors demonstrated the synergistic effect of coadministration of VEGF and Ang-1 in the ischemic rat myocardium (18). Therefore, in our current investigation, the adenoviral dosage was used based on Zhou et al.'s observations (18).
Immediately after MI, adenoviral vectors encoding for VEGF (Ad.VEGF, 6 × 107 pfu) and Ang-1 (Ad.Ang1, 1.5 × 105 pfu) were intramyocardially administered in combination (in 100 μl of PBS, using a 30-gauge needle) at four sites at the border zone of the ischemic area in the CVAMI and DVAMI groups. Adeno-LacZ (Ad.LacZ, 6 × 107 pfu) was used as the control adenoviral vector in the CLZMI and DLZMI groups, to nullify any possible effects exerted by the vector itself.
For detailed descriptions of the materials and methods, please refer to the supplementary material available in the online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0336/DC1.
Statistical analysis.
All data were analyzed by statistical software GraphPad Prism PC software (San Diego, CA) Version 5.0. Statistical analysis was performed using one-way ANOVA. Post hoc comparisons between the groups were performed by Newman-Keuls multiple comparison test. Results are presented as mean ± SEM with P ≤ 0.05 used to indicate statistical significance.
RESULTS
The explanation of the result demonstrating the efficacy of intramyocardial gene delivery (Fig. 1A) has been included in the supplementary material in the online appendix.
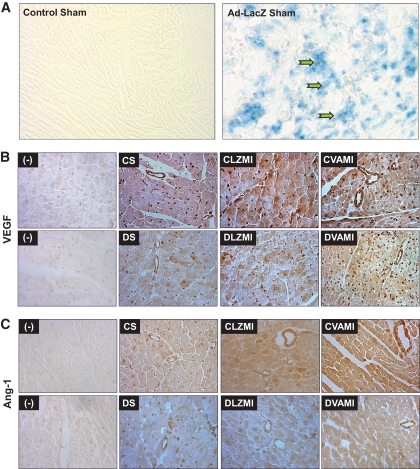
FIG. 1.
A: Representative micrographs showing the in vivo transfection efficiency of Ad.LacZ in the nondiabetic sham-operated groups. Robust infection of the myocardium as assessed by β-galactosidase staining in the viable cardiac muscle surrounding the sites of gene transfer can be seen in the Ad.LacZ-transfected myocardium. B: Expression of VEGF as assessed by immunohistochemical staining. C: Expression of Ang-1 as assessed by immunohistochemical staining. The decrease in the expression of VEGF and Ang-1 is evident in the diabetic DS and DLZMI groups compared with the respective nondiabetic CS and CLZMI groups. Increase in the expression of VEGF and Ang-1 can be seen in the groups that received the combination gene therapy (CVAMI and DVAMI) compared with their respective (CLZMI and DLZMI) Ad.LacZ-treated groups. (−) represents representative micrographs showing the sections in which primary antibody was not added to verify the specificity of the staining protocol. CS, nondiabetic control sham; DS, diabetic sham; CLZMI, nondiabetic control animals that received Ad.LacZ injections; DLZMI, diabetic animals that received Ad.LacZ injections; CVAMI, nondiabetic control animals that received combination gene therapy; and DVAMI, diabetic animals that received combination gene therapy. (A high-quality color digital representation of this figure is available in the online issue.)
Immunohistochemical analysis of VEGF and Ang-1.
There was a decrease in the expression of VEGF and Ang-1 in the DS compared with CS (Fig. 1B and C). The MI induced by left anterior descending artery (LAD) occlusion seemed to trigger the expression of VEGF and Ang-1 in the nondiabetic control (CLZMI) animals to which Ad.LacZ was administered. However, this infarction-triggered expression of the angiogenic growth factors seemed to be impaired in the diabetic animals (DLZMI, Fig. 1B and C). The coadministration of Ad.VEGF and Ad.Ang1 in the nondiabetic (CVAMI) group markedly induced expression of both growth factors. Similarly, the expression of VEGF and Ang-1 was restored markedly upon treatment in the diabetic (DVAMI) myocardium (Fig. 1B and C).
Biological effects of coexpression of VEGF and Ang-1 in the diabetic ischemic myocardium
Myocardial fibrosis (Masson trichrome staining).
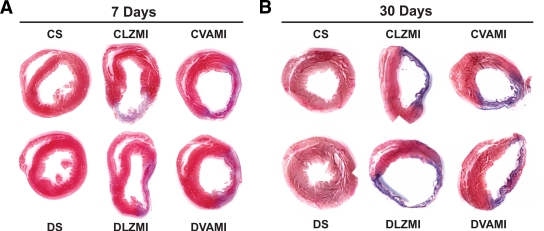
To determine the effect of the combination gene therapy on the extent of myocardial fibrosis, Masson trichrome staining was performed on paraffin-embedded heart tissue sections 7 and 30 days after the surgical intervention. After 7 days of MI, there was significant increase in myocardial fibrosis in the DLZMI (30.8 ± 2.8%) compared with the CLZMI (23.9 ± 0.5%) group. Upon therapy, the nondiabetic CVAMI (20.2 ± 0.4%) group demonstrated reduced collagen deposition and fibrosis compared with the respective Ad.LacZ-treated nondiabetic CLZMI (23.9 ± 0.5%) group. Similarly, the diabetic MI group (DVAMI, 20.1 ± 0.7%) that received the therapy demonstrated significantly reduced myocardial collagen deposition and fibrosis compared with the respective Ad.LacZ-treated diabetic MI group (DLZMI, 30.8 ± 2.8%) (Fig. 2A). Figure 2A shows heart tissue sections of the diabetic MI animals (DVAMI) that received the therapy with less fibrosis compared with the Ad.LacZ-treated diabetic MI group (DLZMI), which showed increased fibrosis and ventricular dilatation. CS and DS (Fig. 2A and B) represent the nondiabetic and diabetic sham-operated groups with no significant fibrosis, respectively. We observed a similar trend after 30 days of intramyocardial gene therapy (Fig. 2B). The diabetic MI group (DVAMI, 16.5 ± 1.9%) that received the therapy showed reduced myocardial collagen fibrosis compared with the respective Ad.LacZ-treated diabetic MI group (DLZMI, 20.1 ± 1.1%). Evidently there was a less prominent scar extension in the treated diabetic rats (DVAMI) compared with the Ad.LacZ-treated (DLZMI) animals (Fig. 2B).
FIG. 2.
Effect of combination gene therapy on myocardial fibrosis 7 and 30 days after gene therapy. A: Representative images show myocardial infarct and fibrosis after 7 days of MI and combination gene therapy. B: Representative images show myocardial infarct and fibrosis after 30 days of MI and combination gene therapy. There is no evident fibrosis in the CS and DS groups. A thinner infarct and significant fibrosis is evident in the CLZMI and DLZMI groups. Combination gene therapy in the CVAMI and DVAMI groups resulted in a thicker infarct containing islands of viable cardiac tissue. CS, nondiabetic control sham; DS, diabetic sham; CLZMI, nondiabetic control animals that received Ad.LacZ injections; DLZMI, diabetic animals that received Ad.LacZ injections; CVAMI, nondiabetic control animals that received combination gene therapy; and DVAMI, diabetic animals that received combination gene therapy. (A high-quality color digital representation of this figure is available in the online issue.)
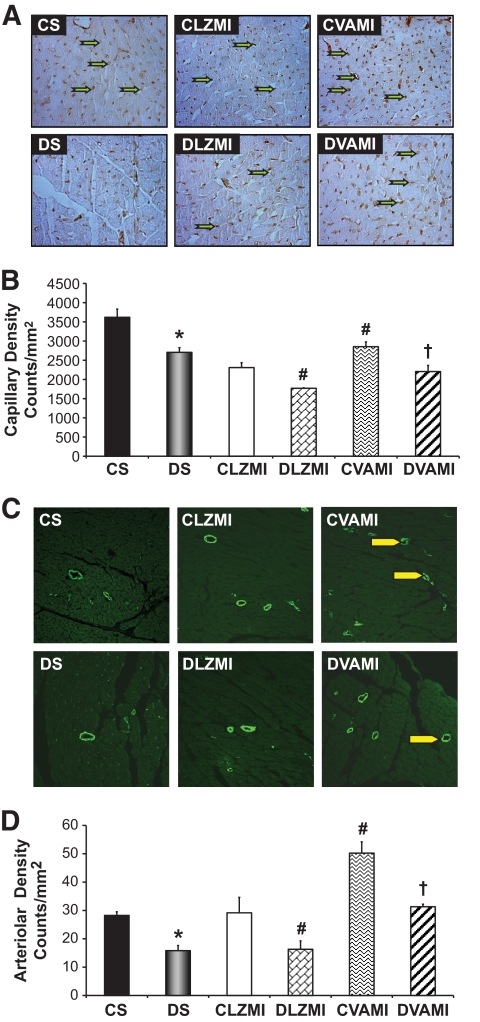
Capillary density and arteriolar density.
To understand whether the reduction in fibrosis was paralleled by neoangiogenesis, we performed immunohistochemical analysis for CD31 (endothelial cell marker) after 7 days of the therapy, allowing the estimation of the density of CD31-positive vessels (capillary density in counts/mm2) (Fig. 3A and B). There was a significant decrease in the capillary density in the DS-operated (2,706 ± 122) and Ad.LacZ-treated diabetic MI (DLZMI, 1,769 ± 12) groups compared with the CS-operated (3,619 ± 214) and Ad.LacZ-treated nondiabetic MI (CLZMI, 2,308 ± 130) groups, respectively. In the present study, we observed an increase in the myocardial capillary vessel density in the diabetic MI animals (DVAMI, 2,206 ± 159) that received the adenoviral vectors encoding VEGF and Ang-1 in combination compared with the Ad.LacZ-treated diabetic MI (DLZMI, 1,769 ± 12) animals. Similar results were observed in the nondiabetic MI (CVAMI) group that received the therapy (2,853 ± 123) compared with the Ad.LacZ-treated nondiabetic MI (CLZMI, 2,308 ± 130) group.
FIG. 3.
Effect of combination gene therapy on capillary and arteriolar density 7 days after the intervention. Representative images (A) and graphical representation (B) of capillary density analysis among the different groups (counts/mm2). Representative images (C) and graphical representation (D) of arteriolar density analysis among the different groups (counts/mm2). There was a significant increase in the capillary and arteriolar density in the CVAMI and DVAMI groups compared with the CLZMI and DLZMI groups, respectively. CS (black solid bar), nondiabetic control sham; DS (gray solid bar), diabetic sham; CLZMI (white bar), nondiabetic control animals that received Ad.LacZ injections; DLZMI (diagonal brick bar), diabetic animals that received Ad.LacZ injections; CVAMI (wave bar), nondiabetic control animals that received combination gene therapy; and DVAMI (wide upward diagonal bar), diabetic animals that received combination gene therapy. Values are presented as mean ± SEM. *P ≤ 0.05 when DS is compared with CS, #P ≤ 0.05 compared with CLZMI, †P ≤ 0.05 when DVAMI is compared with DLZMI. (A high-quality color digital representation of this figure is available in the online issue.)
To analyze the angiogenic response to the therapy, the arteriolar density (in counts/mm2) was measured by immunostaining the heart tissue sections (Fig. 3C and D) after 7 days of gene therapy for α-smooth muscle actin. There was a decrease in the arteriolar density in the DS-operated (15.8 ± 1.8) and Ad.LacZ-treated diabetic MI (DLZMI, 16.3 ± 2.9) groups compared with the CS-operated (28.3 ± 1.3) and Ad.LacZ-treated nondiabetic MI (CLZMI, 29.2 ± 5.5) groups, respectively. In the present study, we observed a significant increase in the number of arterioles (arteriolar density) in the diabetic MI (DVAMI) animals that received the therapy (31.3 ± 0.9) compared with the Ad.LacZ-treated diabetic MI (DLZMI, 16.3 ± 2.9) group. Similar results were observed in the nondiabetic MI (CVAMI) group that received the therapy (50.2 ± 3.9) compared with the Ad.LacZ-treated nondiabetic MI (CLZMI, 29.2 ± 5.5) group.
Molecular basis for the angiogenic and cardioprotective effect of VEGF–Ang-1 therapy in the diabetic ischemic myocardium
Effect of gene therapy on the expression of VEGF and Flk-1.
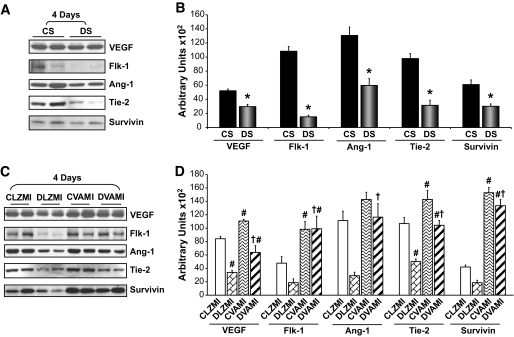
We observed a significant reduction in the expression of VEGF (1.75-fold) and Flk-1 (7.1-fold) 4 days after the surgical procedure in the DS-operated animals compared with the CS-operated animals (Fig. 4A and B). Similarly, there was a significant reduction in the expression of VEGF (2.5-fold) and Flk-1 (2.5-fold) in the DLZMI group compared with the CLZMI group (Fig. 4C and D). However, there was a significant increase (1.6-fold) in VEGF expression in the nondiabetic Ad.LacZ-treated MI (CLZMI) group compared with the CS-operated animals. This MI-induced increase in the expression of VEGF was compromised in the diabetic MI (DLZMI) group that was treated with Ad.LacZ. Upon treatment with a combination of adenoviral vectors encoding VEGF and Ang-1 in the nondiabetic MI (CVAMI) animals, we documented a significant increase in the levels of VEGF (1.3-fold) and Flk-1 (2.1-fold) 4 days after the gene transfer compared with the Ad.LacZ-treated nondiabetic MI (CLZMI) group (Fig. 4C and D). Similarly, we observed a significant increase in the expression of VEGF (1.9-fold) and Flk-1 (5.3-fold) in the diabetic animals that received the therapy (DVAMI), 4 days after the combination gene transfer compared with the Ad.LacZ-treated diabetic MI (DLZMI) group (Fig. 4C and D).
FIG. 4.
Effect of combination gene therapy on the expression of VEGF, Flk-1, Ang-1, Tie-2, and survivin 4 days after the intervention. A: Representative Western blots of VEGF, Flk-1, Ang-1, Tie-2, and survivin comparing CS and DS groups. B: Bar graphs show the quantitative difference in expression of VEGF, Flk-1, Ang-1, Tie-2, and survivin between the CS and DS groups. C: Representative Western blots for VEGF, Flk-1, Ang-1, Tie-2, and survivin comparing the CLZMI, DLZMI, CVAMI, and DVAMI groups, 4 days after the therapy. D: Bar graphs show the quantitative difference in expression of VEGF, Flk-1, Ang-1, Tie-2, and survivin among the CLZMI, DLZMI, CVAMI, and DVAMI groups. There was a significant increase in the expression of these proteins in the CVAMI and DVAMI groups compared with the CLZMI and DLZMI groups, respectively. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control. CS (black solid bars), nondiabetic control sham; DS (gray solid bars), diabetic sham; CLZMI (white bar), nondiabetic control animals that received Ad.LacZ injections; DLZMI (diagonal brick bar), diabetic animals that received Ad.LacZ injections; CVAMI (wave bar), nondiabetic control animals that received combination gene therapy; and DVAMI (wide upward diagonal bar), diabetic animals that received combination gene therapy. Values given as mean ± SEM. *P ≤ 0.05 when DS is compared with CS, #P ≤ 0.05 compared with CLZMI, †P ≤ 0.05 when DVAMI is compared with DLZMI.
Effect of gene therapy on the expression of Ang-1 and Tie-2.
In the present study, we observed a significant reduction in the expression of Ang-1 (2.2-fold) and Tie-2 (3.1-fold) 4 days after the surgical procedure, in the DS-operated animals compared with CS-operated animals (Fig. 4A and B). Upon treatment with a combination of adenoviral vectors encoding VEGF and Ang-1 in the nondiabetic MI (CVAMI) animals, we documented an increase in the levels of Ang-1 (1.3-fold) and Tie-2 (1.3-fold) 4 days after the gene transfer compared with the Ad.LacZ-treated nondiabetic MI (CLZMI) group (Fig. 4C and D). Similarly, we observed a significant increase in the expression of Ang-1 (4.0-fold) and Tie-2 (2.1-fold) in the diabetic animals that received the therapy (DVAMI), 4 days after the treatment, compared with the Ad.LacZ-treated diabetic MI (DLZMI) group (Fig. 4C and D).
Effect of gene therapy on the expression of survivin.
There was a marked decrease in the expression of antiapoptotic protein survivin in the DS-treated (2.0-fold) and diabetic Ad.LacZ-treated (DLZMI, 2.3-fold) MI groups compared with the CS-operated and Ad.LacZ-treated nondiabetic MI (CLZMI) groups, respectively (Fig. 4A and B). We observed a significant increase in the expression of survivin in the nondiabetic animals that received the therapy (CVAMI, 3.7-fold) 4 days after the treatment compared with the Ad.LacZ-treated nondiabetic MI (CLZMI) group (Fig. 4C and D). Similarly, we observed a significant increase in the expression of survivin in the diabetic animals that received the therapy (DVAMI, 7.2-fold) 4 days after the treatment compared with the Ad.LacZ-treated diabetic MI (DLZMI) group (Fig. 4C and D).
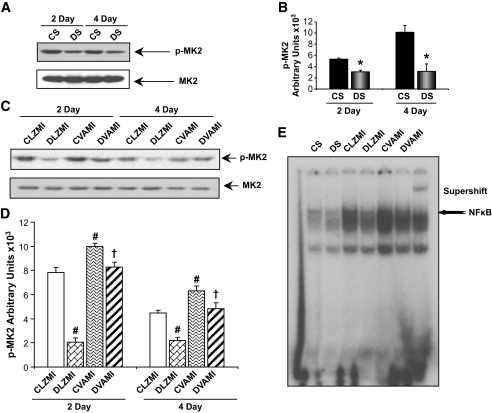
Effect of gene therapy on the phosphorylation of MK2.
There was a marked decrease in the phosphorylation of mitogen-activated protein kinase–activated protein kinase-2 (MK2, at Threonine 334) in the DS-treated (1.7-fold) and diabetic Ad.LacZ-treated (DLZMI, 3.8-fold) MI groups compared with the CS-operated and Ad.LacZ-treated nondiabetic MI (CLZMI) groups, respectively, 2 days after the surgical procedure (Fig. 5A–D). We observed a significant increase in the phosphorylation of MK2, 2 days after the treatment in the nondiabetic animals that received the therapy (CVAMI, 1.3-fold), compared with the Ad.LacZ-treated nondiabetic MI (CLZMI) group (Fig. 5C and D). Similarly, we observed a significant increase in the phosphorylation of MK2, 2 days after the treatment in the diabetic animals that received the therapy (DVAMI, 3.9-fold), compared with the Ad.LacZ-treated diabetic MI (DLZMI) group (Fig. 5C and D).
FIG. 5.
Effect of combination gene therapy on phosphorylation of MK2 (Western blot) and DNA binding activity of NFκB (electrophoretic mobility shift assay [EMSA]). A: Representative Western blots for p-MK2 comparing CS and DS groups, 2 and 4 days after the surgery. B: Graphical representation of p-MK2 in the CS and DS groups, 2 and 4 days after the therapy. C: Representative Western blots for p-MK2 comparing CLZMI, DLZMI, CVAMI, and DVAMI groups, 2 and 4 days after the therapy. D: Graphical representation of p-MK2 in the CLZMI, DLZMI, CVAMI, and DVAMI groups, 2 and 4 days after the therapy. The gene therapy significantly increased the levels of p-MK2, 2 and 4 days after the therapy. E: EMSA analysis reveals increased DNA binding activity of NFκB in the CVAMI and DVAMI groups compared with the CLZMI and DLZMI groups, respectively. CS (black solid bars), nondiabetic control sham; DS (gray solid bars), diabetic sham; CLZMI (white bar), nondiabetic control animals that received Ad.LacZ injections; DLZMI (diagonal brick bar), diabetic animals that received Ad.LacZ injections; CVAMI (wave bar), nondiabetic control animals that received combination gene therapy; and DVAMI (wide upward diagonal bar), diabetic animals that received combination gene therapy. Values given as mean ± SEM. *P ≤ 0.05 when DS is compared with CS, #P ≤ 0.05 compared with CLZMI, †P ≤ 0.05 when DVAMI is compared with DLZMI.
Similarly, the therapy significantly increased the levels of phosphorylated MK2 (p-MK2) 4 days after intervention in both the DVAMI and CVAMI groups compared with the DLZMI and CLZMI groups, respectively. However, there was an evident decrease in the levels of p-MK2 in all the groups 4 days after the intervention compared with 2 days after the intervention.
Effect of gene therapy on the DNA binding activity of NFκB.
Gel shift analysis 2 days after the surgical procedure revealed significant decrease in the DNA binding activity of NFκB in the DS animals compared with the nondiabetic CS animals (Fig. 5E). NFκB DNA binding activity was significantly elevated in the nondiabetic control animals that received the therapy (CVAMI) compared with the Ad.LacZ-treated nondiabetic MI (CLZMI) group (Fig. 5E). Similarly, the DNA binding activity of NFκB was significantly restored upon coadministration of adenoviral VEGF and Ang-1 in the diabetic animals (DVAMI) compared with their respective Ad.LacZ-treated diabetic controls (DLZMI) (Fig. 5E).
Preservation of myocardial functions after MI in the diabetic myocardium through VEGF/Ang-1 combination gene therapy.
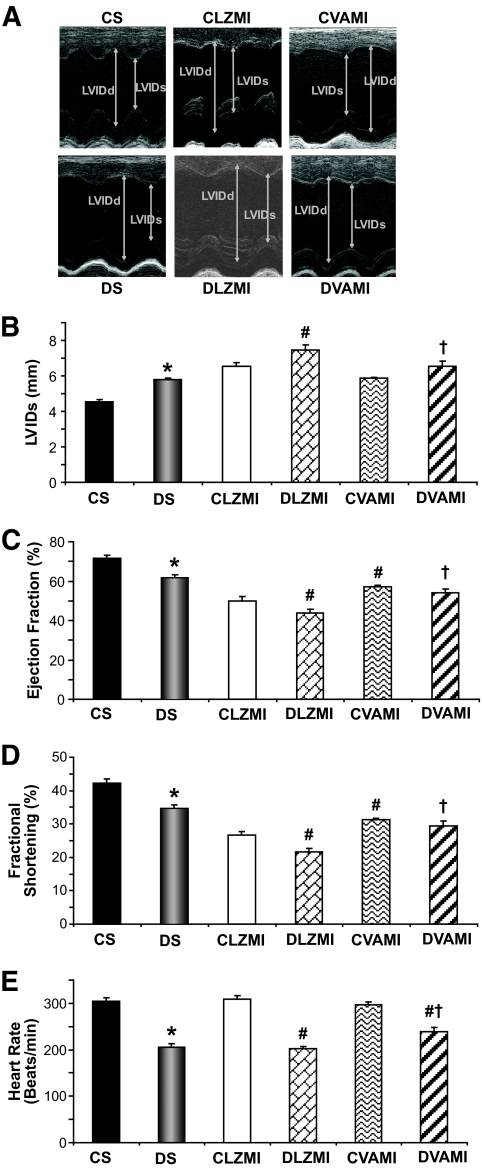
To evaluate the in vivo functional consequences of intramyocardial combination gene therapy, we performed echocardiographic analysis on the experimental animals in the different groups 30 days after the gene transfer. Anterior wall thickness showed a decrease in all the groups subjected to MI compared with the sham group, and the thinning of the anterior wall is greater in the DLZMI group compared with the diabetic group treated with VEGF and Ang-1 combination gene therapy (data not shown). Larger left ventricular (LV) dimensions at systole were also detected in diabetic sham (5.8 ± 0.1) compared with nondiabetic sham (4.5 ± 0.1) groups. Four weeks after LAD ligation, the left ventricular inner diameters (LVIDs; mm) were significantly larger in DLZMI rats (7.4 ± 0.3) compared with nondiabetic CLZMI (6.5 ± 0.2) rats (Fig. 6A and B). However, this increase in LV dimensions was found to be prevented in the DVAMI (6.5 ± 0.3) groups compared with DLZMI (7.4 ± 0.3) groups. Also, nondiabetic CVAMI (5.9 ± 0.1) groups showed better improvement compared with nondiabetic CLZMI (6.5 ± 0.3) groups (Fig. 6A and B). Along with the LV dimensions, we also noticed better systolic functions measured by ejection fraction (in %) and fractional shortening (in %) in all the control groups compared with their respective diabetic groups. We observed a significant increase in the ejection fraction (%) in the combination gene therapy–treated nondiabetic MI (CVAMI) group (57.3 ± 0.8 vs. 50.1 ± 2) and diabetic MI (DVAMI) group (53.9 ± 2.2 vs. 43.8 ± 2) compared with their respective Ad.LacZ-treated nondiabetic (CLZMI) and diabetic (DLZMI) groups (Fig. 6C). Similarly, there was a significant increase in fractional shortening (%) in the combination gene therapy–treated nondiabetic MI (CVAMI) group (31.2 ± 0.5 vs. 26.6 ± 1.2) and diabetic MI (DVAMI) group (29.3 ± 1.5 vs. 21.6 ± 1.1) compared with their respective Ad.LacZ-treated nondiabetic (CLZMI) and diabetic (DLZMI) groups (Fig. 6D). There were evident signs of bradycardia in all the diabetic groups compared with their respective control groups. In the present study, we observed improvement in the heart rate (in beats/min) in the combination gene therapy–treated diabetic MI (DVAMI) group (239.2 ± 8.5) compared with the Ad.LacZ-treated diabetic (DLZMI) group (201.8 ± 5.4) (Fig. 6E).
FIG. 6.
Effect of combination gene therapy on left ventricular myocardial functions (echocardiography). A: Representative echocardiograph pictures of parasternal short axis images after 30 days of surgery and therapy. Bar graphs represent LVIDs in systole (in mm) (B), % ejection fraction (C), % fractional shortening (D), and heart rate in beats/min (E). There was a significant increase in the myocardial functions in the CVAMI and DVAMI groups compared with the CLZMI and DLZMI groups, respectively. CS (black solid bars), nondiabetic control sham; DS (gray solid bars), diabetic sham; CLZMI (white bar), nondiabetic control animals that received Ad.LacZ injections; DLZMI (diagonal brick bar), diabetic animals that received Ad.LacZ injections; CVAMI (wave bar), nondiabetic control animals that received combination gene therapy; and DVAMI (wide upward diagonal bar), diabetic animals that received combination gene therapy. Values given as mean ± SEM. *P ≤ 0.05 when DS is compared with CS, #P ≤ 0.05 compared with CLZMI, †P ≤ 0.05 when DVAMI is compared with DLZMI.
DISCUSSION
The angiogenic growth factors are downregulated in the diabetic myocardium, in turn hampering myocardial collateral vessel formation as an adaptation to ischemia (2). Therefore, supporting the overexpression of these factors by means of gene therapy might aid in repairing the process of impaired angiogenesis in the diabetic ischemic myocardium. However, therapy using vectors encoding a single angiogenic factor has shown less significant improvement than what was expected, mainly because the biological system requires a cascade of growth factors and responsive intracellular signaling mechanisms for the development of a fully functional vascular system (22–24). Therefore, therapeutic angiogenesis is currently targeting combinations of angiogenic molecules as a therapeutic measure to induce myocardial angiogenesis (22). Ang-1 is known to modify VEGF responses to neovascularization by positively affecting vessel maturation and stability (25). A strategy to locally overexpress VEGF and Ang-1 in combination would prove beneficial because, although VEGF can take the lead in the process of neovascularization, Ang-1 would be expected to support the maturation of the newly formed vessels.
We observed significant reduction in the expression of VEGF and Ang-1 in the diabetic myocardium. However, MI induced the expression of VEGF in the Ad.LacZ-treated nondiabetic MI group. This increase in VEGF in the nondiabetic MI group can be explained as an early adaptive response to the myocardial ischemia caused by LAD ligation (26,27). This response was not seen in the diabetic MI animals, confirming the impairment of the angiogenic machinery in the diabetic myocardium (28). In our present study, the strategy of combination gene therapy markedly increased the expression of VEGF and Ang-1 in the diabetic ischemic heart compared with the Ad.LacZ-treated diabetic MI group.
The impairment of myocardial angiogenesis in a diabetic condition has also been associated with compromised signaling mechanisms through the receptors, Flk-1 for VEGF and Tie-2 for Ang-1 (6,28,29). It is also known that expression of these receptors is regulated by a positive-feedback signaling mechanism where the ligand itself controls the levels of expression of its own receptors (6,30). The downregulation in the expression of the receptors that we observed in the diabetic myocardium corresponds to the impaired angiogenic signaling mechanisms associated with a diabetic milieu. The increase in the expression of the receptors Flk-1 and Tie-2 that we observed upon gene therapy can be correlated to the increased expression of their ligands: VEGF and Ang-1, respectively. Therefore, the therapy might have been effective in correcting the angiogenic machinery directly associated with VEGF and Ang-1 that is impaired in the diabetic myocardium.
The VEGF/Flk-1–induced effects on endothelial cell migration through p38 mitogen-activated protein kinase (MAPK) activation are mediated primarily by phosphorylation and activation of the p38MAPK substrate MK2 (31,32). It was also reported that MK2 activation plays an essential role in VEGF-induced actin reorganization, migration, and tubule formation in endothelial cells (33). Recently, we have shown that ischemic preconditioning induces VEGF expression followed by Flk-1 activation, thereby activating an angiogenic signaling cascade by the activation of p38MAPK and MK2 leading to the activation of NFκB (34). To elucidate the mechanism, we used heterozygous Flk-1+/− and homozygous MK2−/− knockout mice. Ischemic preconditioning in the Flk-1+/− mice failed to bring about significant phosphorylation of MK2 and increase DNA binding activity of NFκB compared with the wild-type mice myocardium subjected to ischemic preconditioning. Similarly, when we studied the effects of ischemic preconditioning in MK2−/− knockout mice, we observed that the ischemic preconditioning–mediated activation of NFκB and the effective angiogenic response were significantly impaired when MK2 was knocked down (34). Thus, we were able to confirm that downstream of Flk-1, activation of MK2 plays a crucial role in NFκB activation and angiogenesis in the myocardium. Our treatment strategy significantly increased the phosphorylation of MK2, which can be associated with the increase in the expression of VEGF and its receptor Flk-1, thereby triggering the angiogenic signaling pathway in the diabetic ischemic myocardium.
We have previously documented the effect of hypoxic preconditioning on NFκB activation and the role of NFκB in myocardial angiogenesis (35,36). Both VEGF and Ang-1 have been shown to increase the DNA binding activity of NFκB, thereby modulating endothelial cell proliferation, survival, and migration (31). NFκB has been shown to activate several prosurvival genes, cytokines, and inhibitors of apoptosis necessary for endothelial cell survival (37). It was recently shown that combined gene therapy using VEGF165 and Ang-1 significantly reduced myocardial infarct size through the induction of NFκB activation (18). Our treatment strategy has significantly improved the DNA binding activity of NFκB in the diabetic ischemic myocardium, thereby confirming improvement in the angiogenic signaling mechanism associated with increase in the expression of VEGF and Ang-1 and phosphorylation of MK2.
Survivin (an inhibitor of apoptosis) is upregulated by VEGF and Ang-1 in the endothelial cells, thereby decreasing apoptosis (38–41). It has been reported that activation of NFκB signaling contributes to endothelial cell survival and tubular morphogenesis by the transactivation and upregulation of the prosurvival protein survivin (42). The reduced expression of survivin in the diabetic myocardium can be correlated to the increased myocardial fibrosis as evidenced by trichrome staining. Our treatment strategy leading to the coexpression of VEGF and Ang-1 might have led to the increased expression of survivin through the activation of NFκB signaling in the diabetic ischemic myocardium in the present study.
The cooperative effect of VEGF and Ang-1 coexpression and the increase in the expression of their respective receptors due to the therapy in mediating neovascularization were confirmed by a marked increase in the capillary density and arteriolar density in the DVAMI group compared with the Ad.LacZ-treated diabetic MI group. This increase in vascularization as a result of the therapy might have led to the significant reduction in the myocardial fibrosis and marked increase in the islands of viable cardiac tissue in the infarct and peri-infarct regions of the diabetic infarcted heart upon therapy. Although stabilization of the VEGF-induced neovascularization by Ang-1 is the most likely reason for the observed increase in capillary and arteriolar density in the surviving myocardium immediately adjacent to the infarct, the possibility of VEGF- and Aug-1–induced cell survival, preservation of existing vessels, and a resultant direct effect on myocardial repair cannot be ruled out. Moreover, there is evidence that suggests that post-MI vascular repair is not dependent solely on the activation and participation of resident endothelial cells, but may also reflect an increased bone marrow–derived endothelial progenitor cell mobilization and homing, which are in turn activated by overexpression of both VEGF and Ang-1 (43,44). Therefore, there is a possibility that our combination gene therapy might have induced endothelial progenitor cell homing to the diabetic infarcted myocardium aiding in the reparative process.
A number of preclinical and clinical studies have shown structural changes in parallel with the functional changes of diabetic heart disease (45). After MI, the surviving myocardium of nondiabetic subjects exhibits hyperkinesia to compensate for the infarcted myocardium in an attempt to maintain cardiac output. When it comes to a diabetic subject, in addition to the already compromised cardiac functions, after an MI, the myocardium is unable to achieve this compensatory enhancement in function due to significant ventricular remodeling, myocardial fibrosis, and collagen deposition, which in turn further reduces myocardial functional capability (45). In our current study, the reduced fibrosis and improved vascularization as a result of the therapy might have caused the improvement in the myocardial functions 30 days after MI in the diabetic rats. The therapy resulted in preservation of myocardial thickness and less prominent collagen deposition. Consistent with other reports, though bradycardia was evident in all the diabetic groups, the heart rate significantly improved upon treatment (46). The treatment resulted in better contractile function and suppression of the progressive cardiac failure that is associated with ventricular remodeling in a diabetic infarcted heart.
To the best of our knowledge our results have documented for the first time that intramyocardial coadministration of adenoviral vectors encoding VEGF and Ang-1 induces angiogenesis and vessel maturation, thereby rendering cardioprotection against the ischemic stress induced by MI in STZ-induced type 1 diabetic rats. The therapy significantly reduced the ventricular remodeling as evidenced by the significant reduction in the collagenous fibrotic tissue and improvement in the myocardial functions in conjunction with significant increase in the levels of VEGF and its receptor Flk-1, Ang-1 and its receptor Tie-2, p-MK2, and antiapoptotic survivin. Our unique and promising preclinical findings therefore support the development of a combination gene therapy for therapeutic myocardial angiogenesis and call for the initiation of a clinical trial to assess the efficacy of this unique therapeutic strategy in the treatment of diabetes-related human heart failure.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grants HL-56803 and HL-69910 to N.M.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR: Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999;100:1134–1146 [DOI] [PubMed] [Google Scholar]
- 2.Martin A, Komada MR, Sane DC: Abnormal angiogenesis in diabetes mellitus. Med Res Rev 2003;23:117–145 [DOI] [PubMed] [Google Scholar]
- 3.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM: Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol 1999;154:355–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waltenberger J: Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res 2001;49:554–560 [DOI] [PubMed] [Google Scholar]
- 5.Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW: Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 2005;111:2073–2085 [DOI] [PubMed] [Google Scholar]
- 6.Chen JX, Stinnett A: Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol 2008;28:1606–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N: Resveratrol alleviates cardiac dysfunction in streptozotocin inudced diabetes: role of nitric oxide, thioredoxin and heme oxygenase. Free Radic Biol Med 2007;43:720–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM: Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 1998;98:2800–2804 [DOI] [PubMed] [Google Scholar]
- 9.Reilly JP, Grise MA, Fortuin FD, Vale PR, Schaer GL, Lopez J, Van Camp JR, Henry T, Richenbacher WE, Losordo DW, Schatz RA, Isner JM: Long-term (2-year) clinical events following transthoracic intramyocardial gene transfer of VEGF-2 in no-option patients. J Interv Cardiol 2005;18:27–31 [DOI] [PubMed] [Google Scholar]
- 10.Gyöngyösi M, Khorsand A, Zamini S, Sperker W, Strehblow C, Kastrup J, Jorgensen E, Hesse B, Tägil K, Bøtker HE, Ruzyllo W, Teresiñska A, Dudek D, Hubalewska A, Rück A, Nielsen SS, Graf S, Mundigler G, Novak J, Sochor H, Maurer G, Glogar D, Sylven C: NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation 2005;112:I157–I165 [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P: VEGF gene therapy: stimulating angiogenesis or angioma-genesis?. Nat Med 2000;6:1102–1103 [DOI] [PubMed] [Google Scholar]
- 12.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM: VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation 2000;102:898–901 [DOI] [PubMed] [Google Scholar]
- 13.Jain RK, Munn LL: Leaky vessels? Call Ang1! Nat Med 2000;6:131–132 [DOI] [PubMed] [Google Scholar]
- 14.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD: Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000;6:460–463 [DOI] [PubMed] [Google Scholar]
- 15.Thurston G: Complementary actions of VEGF and angiopoietin-1 on blood vessel growth and leakage. J Anat 2002;200:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JX, Stinnett A: Ang-1 gene therapy inhibits hypoxia-inducible factor-1alpha (HIF-1alpha)-prolyl-4-hydroxylase-2, stabilizes HIF-1alpha expression, and normalizes immature vasculature in db/db mice. Diabetes 2008;57:3335–3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Chen Y, Zhang F, Chen L, Ha T, Gao X, Li C: Synergistically therapeutic effects of VEGF165 and angiopoietin-1 on ischemic rat myocardium. Scand Cardiovasc J 2007;41:95–101 [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Ma W, Yang Z, Zhang F, Lu L, Ding Z, Ding B, Ha T, Gao X, Li C: VEGF165 and angiopoietin-1 decreased myocardium infarct size through phosphatidylinositol-3 kinase and Bcl-2 pathways. Gene Ther 2005;12:196–202 [DOI] [PubMed] [Google Scholar]
- 19.Su H, Takagawa J, Huang Y, Arakawa-Hoyt J, Pons J, Grossman W, Kan YW, Su H, Takagawa J, Huang Y, Arakawa-Hoyt J, Pons J, Grossman W, Kan YW: Additive effect of AAV-mediated angiopoietin-1 and VEGF expression on the therapy of infarcted heart. Int J Cardiol 2009;133:191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Academy of Sciences Guide for the Care and Use of Laboratory Animals Bethesda, MD, National Institutes of Health, 1985. (NIH publ. no. 85–23) [Google Scholar]
- 21.Kaga S, Zhan L, Matsumoto M, Maulik N: Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol 2005;39:813–822 [DOI] [PubMed] [Google Scholar]
- 22.Markkanen JE, Rissanen TT, Kivelä A, Ylä-Herttuala S: Growth factor-induced therapeutic angiogenesis and arteriogenesis in the heart–gene therapy. Cardiovasc Res 2005;65:656–664 [DOI] [PubMed] [Google Scholar]
- 23.Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;6:389–395 [DOI] [PubMed] [Google Scholar]
- 24.Risau W: Mechanisms of angiogenesis. Nature 1997;386:671–674 [DOI] [PubMed] [Google Scholar]
- 25.Ylä-Herttuala S, Alitalo K: Gene transfer as a tool to induce therapeutic vascular growth. Nat Med 2003;9:694–701 [DOI] [PubMed] [Google Scholar]
- 26.Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E: Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia: implications for coronary angiogenesis. Cardiovasc Res 1994;28:1176–1179 [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA: Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med 2000;342:626–633 [DOI] [PubMed] [Google Scholar]
- 28.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, Clements RT, Sellke FW: Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation 2007;116:I31–I37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, King GL: Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: a possible explanation for impaired collateral formation in cardiac tissue. Circulation 2002;105:373–379 [DOI] [PubMed] [Google Scholar]
- 30.Shen BQ, Lee DY, Gerber HP, Keyt BA, Ferrara N, Zioncheck TF: Homologous up-regulation of KDR/Flk-1 receptor expression by vascular endothelial growth factor in vitro. J Biol Chem 1998;273:29979–29985 [DOI] [PubMed] [Google Scholar]
- 31.Muñoz-Chápuli R, Quesada AR, Angel Medina M: Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci 2004;61:2224–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousseau S, Houle F, Kotanides H, Witte L, Waltenberger J, Landry J, Huot J: Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J Biol Chem 2000;275:10661–10672 [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K: MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J 2006;25:713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thirunavukkarasu M, Akita Y, Samuel SM, Zhan L, Huang CK, Gaestel M, Maulik N: Sequencial activation of VEGF/Flk-1/MK2/NFkappaB signaling in ischemic preconditioning induced neovascularization: a study with Flk-1+/- and MKK2-/- knockout mice. Circulation 2008;118:S575–S576 [Google Scholar]
- 35.Sasaki H, Ray PS, Zhu L, Otani H, Asahara T, Maulik N: Hypoxia/reoxygenation promotes myocardial angiogenesis via an NF kappa B-dependent mechanism in a rat model of chronic myocardial infarction. J Mol Cell Cardiol 2001;33:283–294 [DOI] [PubMed] [Google Scholar]
- 36.Sasaki H, Zhu L, Fukuda S, Maulik N: Inhibition of NF kappa B activation by pyrrolidine dithiocarbamate prevents in vivo hypoxia/reoxygenation-mediated myocardial angiogenesis. Int J Tissue React 2000;22:93–100 [PubMed] [Google Scholar]
- 37.Romashkova JA, Makarov SS: NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 1999;401:86–90 [DOI] [PubMed] [Google Scholar]
- 38.Beierle EA, Nagaram A, Dai W, Iyengar M, Chen MK: VEGF-mediated survivin expression in neuroblastoma cells. J Surg Res 2005;127:21–28 [DOI] [PubMed] [Google Scholar]
- 39.Mesri M, Morales-Ruiz M, Ackermann EJ, Bennett CF, Pober JS, Sessa WC, Altieri DC: Suppression of vascular endothelial growth factor-mediated endothelial cell protection by survivin targeting. Am J Pathol 2001;158:1757–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS: A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci U S A 2002;99:4349–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, Altieri DC, Sessa WC: Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem 2000;275:9102–9105 [DOI] [PubMed] [Google Scholar]
- 42.Zhu L, Fukuda S, Cordis G, Das DK, Maulik N: Anti-apoptotic protein survivin plays a significant role in tubular morphogenesis of human coronary arteriolar endothelial cells by hypoxic preconditioning. FEBS Lett 2001;508:369–374 [DOI] [PubMed] [Google Scholar]
- 43.Szmitko PE, Fedak PW, Weisel RD, Stewart DJ, Kutryk MJ, Verma S: Endothelial progenitor cells: new hope for a broken heart. Circulation 2003;107:3093–3100 [DOI] [PubMed] [Google Scholar]
- 44.Chavakis E, Urbich C, Dimmeler S: Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol 2008;45:514–522 [DOI] [PubMed] [Google Scholar]
- 45.Fang ZY, Prins JB, Marwick TH: Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 2004;25:543–567 [DOI] [PubMed] [Google Scholar]
- 46.Buñag RD, Tomita T, Sasaki S: Streptozotocin diabetic rats are hypertensive despite reduced hypothalamic responsiveness. Hypertension 1982;4:556–565 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.