Abstract
OBJECTIVE
Sympathetic nervous system (SNS) overactivity contributes to the pathogenesis and target organ complications of obesity. This study was conducted to examine the effects of lifestyle interventions (weight loss alone or together with exercise) on SNS function.
RESEARCH DESIGN AND METHODS
Untreated men and women (mean age 55 ± 1 year; BMI 32.3 ± 0.5 kg/m2) who fulfilled Adult Treatment Panel III metabolic syndrome criteria were randomly allocated to either dietary weight loss (WL, n = 20), dietary weight loss and moderate-intensity aerobic exercise (WL+EX, n = 20), or no treatment (control, n = 19). Whole-body norepinephrine kinetics, muscle sympathetic nerve activity by microneurography, baroreflex sensitivity, fitness (maximal oxygen consumption), metabolic, and anthropometric measurements were made at baseline and 12 weeks.
RESULTS
Body weight decreased by −7.1 ± 0.6 and −8.4 ± 1.0 kg in the WL and WL+EX groups, respectively (both P < 0.001). Fitness increased by 19 ± 4% (P < 0.001) in the WL+EX group only. Resting SNS activity decreased similarly in the WL and WL+EX groups: norepinephrine spillover by −96 ± 30 and −101 ± 34 ng/min (both P < 0.01) and muscle sympathetic nerve activity by −12 ± 6 and −19 ± 4 bursts/100 heart beats, respectively (both P < 0.01), but remained unchanged in control subjects. Blood pressure, baroreflex sensitivity, and metabolic parameters improved significantly and similarly in the two lifestyle intervention groups.
CONCLUSIONS
The addition of moderate-intensity aerobic exercise training to a weight loss program does not confer additional benefits on resting SNS activity. This suggests that weight loss is the prime mover in sympathetic neural adaptation to a hypocaloric diet.
The metabolic syndrome (MetS) is an increasingly prevalent multidimensional risk factor for cardiovascular disease and type 2 diabetes (1). Its etiology is complex and incompletely understood, but thought to involve the interplay between metabolic susceptibility, lifestyle factors, and the acquisition of excess visceral adiposity (2). Scientific studies performed over the last 2 decades strongly support the relevance of the sympathetic nervous system (SNS) in both the pathogenesis and target organ complications of MetS obesity (3).
Several indexes of SNS activity, such as urinary norepinephrine excretion, norepinephrine spillover from sympathetic nerves, and postganglionic muscle sympathetic nerve activity (MSNA) are increased in subjects with MetS, even in the absence of hypertension (4–7). Among the adiposity indexes, abdominal visceral fat is most strongly associated with elevated MSNA (8). Because of the bidirectional relationship between sympathetic activation and insulin resistance, much debate has focused on their chronology. Prospective studies with 10–20 years follow-up indicate that elevated plasma norepinephrine concentration (9) and sympathetic reactivity (10) precede and predict future rise in BMI and development of insulin resistance. Although seemingly counterintuitive, sympathetic activation may be causally linked to obesity via β-adrenoceptor desensitization (11) and insulin resistance (12,13). In established obesity, metabolic, cardiovascular (baroreflex impairment), and medical conditions (obstructive sleep apnea) contribute significantly to sympathetic neural drive and further aggravate insulin resistance, hence establishing a vicious cycle (3,7). Chronic sympathetic activation is associated with an increased prevalence of preclinical cardiovascular and renal changes that are recognized predictors of adverse clinical prognosis (3,14,15).
Weight loss and exercise are recommended as first-line treatments for MetS. The Diabetes Prevention Program and the Oslo Diet and Exercise Study have shown the marked clinical benefits of intensive lifestyle intervention on the resolution of the MetS (16,17). Individually, both weight loss (5) and exercise training (18,19) cause sympathoinhibition and improvement in MetS components. We have previously reported that moderate weight loss (7% of body weight) by diet alone is accompanied by reductions in whole-body norepinephrine spillover and MSNA and improvement in spontaneous cardiac baroreflex function in middle-aged MetS subjects (5). Because exercise is often added to energy restriction in the treatment of obesity, it is pertinent to clarify its additive benefits. Augmented improvements in metabolic, anthropometric, and cardiovascular parameters have been observed after combined exercise training and dietary weight loss in some (17,20,21), but not other studies (22), and there are limited data regarding their combined effect on sympathetic activity (23). Exercise training may potentially augment weight loss induced sympathoinhibition by promoting a greater loss of fat relative to lean mass (20,21), by further improvement in insulin sensitivity (24) and reduction in plasma leptin concentration (21), and by potentiation of baroreceptor sensitivity (18).
The present study was conducted to 1) test the hypothesis that weight loss by combined hypocaloric diet and aerobic exercise training would be associated with greater sympathoinhibition and improvement in MetS components than hypocaloric diet alone and 2) to examine the interrelationships between reduction in sympathetic tone and concurrent changes in anthropometric, metabolic (insulin sensitivity, plasma leptin concentration), and cardiovascular parameters. A moderate-intensity bicycle riding protocol was chosen as the exercise intervention, based on an earlier study that demonstrated attenuation in whole-body and renal norepinephrine spillover rates with this regimen in healthy men (19).
RESEARCH DESIGN AND METHODS
Men and postmenopausal women, aged 45–65 years, who fulfilled Adult Treatment Panel III criteria for the MetS (25) were recruited through newspaper advertisement. To be eligible, candidates had to have central obesity (waist circumference ≥102 cm in men and ≥88 cm in women) and two or more MetS parameters (5,25). All were nonsmokers; sedentary, defined as physical exercise two or less times per week for <20 min per session (26); with a stable body weight (± 1 kg) in the previous 6 months; and willing to accept random assignment. Exclusion criteria included type 2 diabetes (fasting glucose ≥7 mmol/l); a history of secondary hypertension or cardiovascular, cerebrovascular, renal, liver, or thyroid disease; and use of drugs known to affect measured parameters. Participants treated for hypertension (n = 5, monotherapy with angiotensin II receptor antagonist in all cases) or hypercholesterolemia (n = 3, HMG-CoA reductase inhibitor in all cases) were studied after medications had been discontinued for 6 weeks. Screening investigations comprised physical examination, medical and dietary histories, 12-lead electrocardiogram, blood biochemistry, and lipid analyses. Supine blood pressure was measured on three occasions 1 week apart as the average of five readings after a 5-min rest (Dinamap, Model 1846SX; Critikon, Tampa, FL). The third of these measurements was defined as baseline blood pressure. Stratified randomization by sex and hypertensive status in blocks of six was used to allocate subjects to one of three groups: weight loss by caloric restriction alone (WL), weight loss by combined caloric restriction and aerobic exercise (WL+EX), or no treatment (control). Intervention duration was 12 weeks. The study was approved by the Alfred Hospital Ethics Committee, and all subjects gave written informed consent.
Dietary protocol.
A modified Dietary Approaches to Stop Hypertension (DASH) diet was used as the background diet (5,27). The macronutrient composition was 30% fat (6% polyunsaturated, 15% monounsaturated, and 9% saturated), 22% protein, and 48% carbohydrate. Basal energy requirements were calculated by indirect calorimetry (Quark b2 breath-by-breath pulmonary gas exchange analyzer; Cosmed, Rome, Italy) using the Weir equation (28). Energy intake was reduced by 600 calories per day. Subjects were provided with 14-day menu plans and recipes and prepared meals in their homes. They attended fortnightly for dietary counseling. Compliance was assessed by prospective 4-day diet records, which were analyzed using Australian Food Composition Tables (FoodWorks Professional Version 3.02; Xyris Software, Highgate Hill, Australia). Sodium, potassium, and protein intake were quantified by 24-h urine collections.
Exercise intervention.
Exercise training comprised 40 min bicycle riding on alternate days at a moderate intensity of 65% of predetermined maximum heart rate (19). This corresponded to target heart rates within the range 120–145 bpm during exercise. Workload was increased as necessary to maintain target heart rate. Once a week, exercise was performed under supervision in the Alfred Hospital Heart Centre. Remaining sessions were performed at the subjects' homes, using provided exercise bicycles and heart rate monitors. Compliance was assessed by the measurement of maximal oxygen consumption (VO2max, expressed as milliliter per kilogram fat-free mass per minute) during a continuous incremental cycle ergometry protocol, during which workload was increased by 20 W/min. Subjects also kept records of average heart rate during each exercise session.
Control group.
Control subjects were instructed to maintain their usual dietary and exercise habits. They attended the Heart Centre every 3 weeks for body weight and blood pressure measurement.
Anthropometric measurements.
Body weight was measured in light indoor clothes without shoes, using a digital scale. Waist circumference was measured at the midpoint between the lowest rib and iliac crest and hip circumference at the level of the greater trochanters. Body composition was determined by dual-energy X-ray absorptiometry scan (GE-LUNAR Prodigy Advance PA+130510; GE Medical Systems, Lunar, Madison, WI). Total body, trunk, abdominal (measured in the abdominal cut at L1–L4 level), and peripheral (arms and legs) fat and lean masses were measured.
Sympathetic nervous system activity.
Subjects attended at 0800 having fasted for 12 h and abstained from caffeine and alcohol for 18 and 36 h, respectively. They were instructed not to exercise on the day before investigations to eliminate acute effects of exercise (29). Subjects collected a 24-h urine specimen immediately before attendance. Measurements of resting SNS activity were performed in a quiet room (temperature 22°C) with subjects lying supine. They voided before commencement. Resting metabolic rate was first determined over a 30-min rest period using breath-by-breath pulmonary gas analysis. Nitrogen excretion was estimated from 24-h urea measurements.
Norepinephrine kinetics.
The dynamic processes of whole-body norepinephrine entry or “spillover” into and removal from the central plasma compartment were determined using the isotope dilution method (30). Tracer doses of tritiated norepinephrine were administered intravenously by constant infusion, after a priming bolus, with steady-state blood sampling from the brachial artery (5).
Muscle sympathetic nerve activity.
Recordings of multi-unit postganglionic MSNA were made from a tungsten microelectrode (FHC, Bowdoinham, ME) inserted into the right peroneal nerve at the fibular head (5). A subcutaneous reference electrode was positioned 2–3 cm away from the recording site. Standard criteria were used to ascertain an MSNA site. The nerve signal was amplified (×50,000), filtered (bandpass, 700–2,000 Hz), and integrated. Intra-arterial blood pressure, electrocardiogram, respiration, and MSNA were digitized with a sampling frequency of 1,000 Hz (PowerLab recording system, model ML 785/8SP; ADI Instruments). Resting measurements were recorded over a 15-min period and averaged. Sympathetic bursts were counted manually and expressed as burst frequency (bursts/min) and burst incidence (bursts/100 heart beats).
Spontaneous cardiac baroreflex sensitivity.
Baroreflex sensitivity was assessed by the sequence method (31). The slope of the regression line between cardiac interval and systolic blood pressure was calculated for each validated sequence and averaged during a 15-min supine recording.
Metabolic measurements.
A standard 75-g oral glucose tolerance test (OGTT) was performed, with blood sampling at 0, 30, 60, 90, and 120 min (Glucaid, Fronine PTY, Australia). Whole-body insulin sensitivity was calculated from OGTT parameters according to the formula of Matsuda and DeFronzo (32). Fasting blood samples were also collected for measurement of plasma leptin, high-sensitivity C-reactive protein (hs-CRP), and lipid profile.
Laboratory measurements.
Plasma norepinephrine was determined by high-performance liquid chromatography with electrochemical detection. Intra-assay coefficients of variation (CVs) in our laboratory are 1.3% for norepinephrine and 2.3% for 3H-norepinephrine; interassay CVs are 3.8 and 4.5%, respectively. Arterial plasma glucose was quantified by enzymatic methods (Architect C18000 analyzer; Abbott Laboratories, IL), insulin, and leptin by radioimmunoassay (Linco Research, MO), lipids by automated enzymatic methods, and hs-CRP by immunoturbidimetric assay.
Statistical methods.
Data are presented as means ± SE. Statistical analysis was performed using SigmaStat Version 3.5 (Systat Software, Point Richmond, CA). Comparisons between baseline and post-intervention data were made by two-way repeated-measure ANOVA. The Holm-Sidak test was used for post hoc comparisons. ANCOVA, with adjustment for baseline values, was also performed for the primary outcome variables (norepinephrine spillover and MSNA). Nonparametric data were log-transformed. Subgroup analyses by sex were performed by two-way repeated-measure ANOVA. Areas under the plasma concentration-time curve (AUC0–120) were calculated by the trapezoidal rule for glucose and insulin. Associations between changes in selected variables were assessed using Pearson's and Spearman's rank correlations. Forward stepwise regressions were carried out with those univariate correlations where P < 0.05. We estimated that a sample size of 20 subjects per group had 80% power at a significance level of 5% (two-tailed) to demonstrate group differences of ≥9% in log norepinephrine spillover and ≥24% in MSNA.
RESULTS
Out of 123 subjects screened for eligibility, 64 were enrolled since they met inclusion criteria. Five dropped out after baseline testing; therefore, 59 subjects completed the study. At baseline, treatment groups were well matched for age, sex, anthropometric, metabolic, blood pressure, and fitness measurements (Tables 1–3) and also for habitual dietary intake (data not shown).
TABLE 1.
Anthropometric responses by treatment group
| Group |
Time × group interaction (P) | |||
|---|---|---|---|---|
| Control | WL | WL+EX | ||
| n | 19 | 20 | 20 | |
| Age (years) | 55 ± 1 | 55 ± 1 | 54 ± 1 | — |
| Sex (male/female) | 11/8 | 12/8 | 12/8 | — |
| BMI (kg/m2) | ||||
| Baseline | 33.0 ± 0.8 | 32.2 ± 0.9 | 31.8 ± 0.8 | |
| Final | 33.4 ± 0.8 | 29.8 ± 0.8†§ | 29.0 ± 0.8†§ | |
| Change | 0.4 ± 0.1 | −2.4 ± 0.2§ | −2.8 ± 0.3§ | <0.001 |
| Body weight (kg) | ||||
| Baseline | 97.6 ± 3.6 | 94.3 ± 2.3 | 92.9 ± 2.9 | |
| Final | 98.6 ± 3.7 | 87.2 ± 2.2†§ | 84.5 ± 2.5†§ | |
| Change | 1.0 ± 0.3 | −7.1 ± 0.6§ | −8.4 ± 1.0§ | <0.001 |
| Waist circumference (cm) | ||||
| Baseline | 109.4 ± 2.5 | 106.5 ± 1.9 | 105.1 ± 2.2 | |
| Final | 109.3 ± 2.5 | 99.8 ± 2.1†§ | 95.3 ± 2.0†§ | |
| Change | −0.1 ± 0.5 | −6.7 ± 0.7§ | −9.8 ± 1.2‡§ | <0.001 |
| Waist-to-hip ratio | ||||
| Baseline | 0.94 ± 0.02 | 0.94 ± 0.02 | 0.91 ± 0.02 | |
| Final | 0.94 ± 0.02 | 0.091 ± 0.01† | 0.88 ± 0.02†§ | |
| Change | 0.0 ± 0.0 | −0.02 ± 0.01 | −0.03 ± 0.01§ | 0.012 |
| Total body fat mass (kg) | ||||
| Baseline | 35.5 ± 2.2 | 36.4 ± 1.8 | 35.4 ± 1.5 | |
| Final | 35.9 ± 2.3 | 31.2 ± 1.9† | 28.5 ± 1.9†§ | |
| Change | 0.3 ± 0.2 | −5.2 ± 0.7§ | −6.9 ± 0.9§ | <0.001 |
| Total body lean mass (kg) | ||||
| Baseline | 56.8 ± 2.7 | 53.7 ± 2.2 | 53.1 ± 2.8 | |
| Final | 57.6 ± 2.7 | 52.2 ± 2.1† | 52.2 ± 2.7* | |
| Change | 0.7 ± 0.2 | −1.5 ± 0.5§ | −0.9 ± 0.4§ | <0.001 |
| Trunk fat mass (kg) | ||||
| Baseline | 21.0 ± 1.2 | 20.6 ± 0.9 | 20.1 ± 0.8 | |
| Final | 21.1 ± 1.2 | 17.5 ± 1.0†§ | 15.7 ± 0.9†§ | |
| Change | 0.2 ± 0.2 | −3.1 ± 0.5§ | −4.4 ± 0.6§ | <0.001 |
| Abdominal fat mass (kg) | ||||
| Baseline | 3.3 ± 0.2 | 3.2 ± 0.2 | 3.0 ± 0.2 | |
| Final | 3.3 ± 0.2 | 2.6 ± 0.2†§ | 2.3 ± 0.1†§ | |
| Change | 0.1 ± 0.1 | −0.5 ± 0.1§ | −0.8 ± 0.1§ | <0.001 |
Data are means ± SE. Baseline values did not differ between groups for any parameter.
*P < 0.05 and
†P < 0.001 vs. baseline;
‡P ≤ 0.01 vs. WL group;
§P < 0.01 vs. control group.
WL, weight loss by caloric restriction; WL+EX, weight loss by caloric restriction and aerobic exercise.
TABLE 2.
Fitness and blood pressure responses by treatment group
| Group |
Time × group interaction (P) | |||
|---|---|---|---|---|
| Control | WL | WL+EX | ||
| n | 19 | 20 | 20 | |
| Vo2max (ml · FFM−1 · min−1) | ||||
| Baseline | 29.3 ± 1.4 | 27.1 ± 1.3 | 29.1 ± 1.4 | |
| Final | 27.6 ± 1.5 | 26.8 ± 1.6 | 34.2 ± 1.4‡§‖ | |
| Change | −1.8 ± 1.0 | −0.3 ± 1.0 | 5.1 ± 1.1§‖ | <0.001 |
| Maximum workload (W) | ||||
| Baseline | 169 ± 9 | 155 ± 9 | 163 ± 10 | |
| Final | 161 ± 10 | 148 ± 9 | 201 ± 12‡§‖ | |
| Change | −7 ± 4 | −7 ± 5 | 38 ± 4§‖ | <0.001 |
| Heart rate (bpm) | ||||
| Baseline | 62 ± 2 | 63 ± 2 | 61 ± 2 | |
| Final | 63 ± 2 | 61 ± 3* | 57 ± 2‡ | |
| Change | 1 ± 1 | −2 ± 2 | −5 ± 1‖ | 0.023 |
| Systolic blood pressure (mmHg) | ||||
| Baseline | 136 ± 4 | 134 ± 4 | 131 ± 3 | |
| Final | 133 ± 4 | 124 ± 4‡ | 121 ± 4‡ | |
| Change | −2 ± 3 | −10 ± 2 | −10 ± 2 | 0.035 |
| Diastolic blood pressure (mmHg) | ||||
| Baseline | 75 ± 2 | 76 ± 2 | 76 ± 2 | |
| Final | 75 ± 2 | 73 ± 2* | 72 ± 2† | |
| Change | 0 ± 1 | −3 ± 1 | −4 ± 1 | 0.222 |
Data are means ± SE. Baseline values did not differ between groups for any parameter.
*P < 0.05,
†P < 0.01, and
‡P < 0.001 vs. baseline;
§P ≤ 0.05 vs. WL group;
‖P < 0.01 vs. control group.
Blood pressure and heart rate represent the average of five supine readings measured by Dinamap monitor. WL, weight loss by caloric restriction; WL+EX, weight loss by caloric restriction and aerobic exercise.
TABLE 3.
Metabolic responses by treatment group
| Group |
Time × group interaction (P) | |||
|---|---|---|---|---|
| Control | WL | WL+EX | ||
| n | 19 | 20 | 20 | |
| Fasting glucose (mmol/l) | ||||
| Baseline | 5.5 ± 0.1 | 5.7 ± 0.2 | 5.6 ± 0.1 | |
| Final | 5.3 ± 0.1 | 5.1 ± 0.1‡ | 5.0 ± 0.1‡ | |
| Change | −0.2 ± 0.1 | −0.6 ± 0.2§ | −0.6 ± 0.1§ | 0.022 |
| Fasting insulin (mU/l) | ||||
| Baseline | 15.8 ± 1.0 | 18.3 ± 1.1 | 15.8 ± 1.3 | |
| Final | 17.8 ± 2.0 | 12.9 ± 1.0‡§ | 12.9 ± 1.2‡§ | |
| Change | 2.1 ± 1.3 | −5.4 ± 1.2§ | −2.9 ± 0.8§ | <0.001 |
| HOMA-IR | ||||
| Baseline | 4.10 ± 0.31 | 4.91 ± 0.31 | 4.09 ± 0.38 | |
| Final | 4.60 ± 0.56 | 3.25 ± 0.29‡§ | 3.20 ± 0.32‡§ | |
| Change | 0.50 ± 0.34 | −1.66 ± 0.32§ | −0.89 ± 0.23§ | <0.001 |
| Insulin AUC0–120 (mU · l−1 · min−1) | ||||
| Baseline | 9,541 ± 807 | 10,250 ± 1,038 | 9,382 ± 940 | |
| Final | 9,640 ± 693 | 7,851 ± 902‡ | 7,343 ± 870† | |
| Change | 99 ± 444 | −2,340 ± 819§ | −2,039 ± 606§ | 0.024 |
| ISI | ||||
| Baseline | 2.36 ± 0.17 | 2.13 ± 0.15 | 2.74 ± 0.29 | |
| Final | 2.33 ± 0.18 | 3.13 ± 0.33‡ | 3.85 ± 0.59‡§ | |
| Change | −0.04 ± 0.13 | 1.00 ± 0.27§ | 1.10 ± 0.42§ | 0.001 |
| HDL cholesterol (mmol/l) | ||||
| Baseline | 1.21 ± 0.06 | 1.19 ± 0.05 | 1.28 ± 0.07 | |
| Final | 1.18 ± 0.06 | 1.12 ± 0.05* | 1.19 ± 0.06* | |
| Change | −0.02 ± 0.03 | −0.07 ± 0.03 | −0.09 ± 0.04 | 0.394 |
| Triglycerides (mmol/l) | ||||
| Baseline | 2.1 ± 0.3 | 1.8 ± 0.3 | 2.0 ± 0.2 | |
| Final | 2.0 ± 0.3 | 1.3 ± 0.2‡§ | 1.4 ± 0.2‡§ | |
| Change | −0.1 ± 0.2 | −0.5 ± 0.2§ | −0.7 ± 0.2§ | 0.048 |
| Fasting leptin (ng/ml) | ||||
| Baseline | 13.3 ± 1.8 | 17.8 ± 3.7 | 15.3 ± 3.2 | |
| Final | 15.3 ± 2.7 | 10.3 ± 2.4‡ | 9.5 ± 2.3‡§ | |
| Change | 2.0 ± 1.2 | −7.5 ± 1.8§ | −5.8 ± 1.5§ | <0.001 |
| hs-CRP (mg/l) | ||||
| Baseline | 3.2 ± 0.5 | 2.4 ± 0.4 | 2.7 ± 0.4 | |
| Final | 3.2 ± 0.5 | 2.3 ± 0.4 | 1.8 ± 0.3†§ | |
| Change | 0.0 ± 0.4 | −0.2 ± 0.2 | −0.9 ± 0.3§ | 0.035 |
| Urinary sodium (mmol/day) | ||||
| Baseline | 180 ± 21 | 145 ± 16 | 147 ± 16 | |
| Final | 143 ± 10 | 108 ± 10* | 125 ± 11 | |
| Change | −37 ± 18 | −37 ± 10 | −22 ± 16 | 0.622 |
| RMR (cal/24 h) | ||||
| Baseline | 1,704 ± 92 | 1,651 ± 106 | 1,585 ± 130 | |
| Final | 1,646 ± 98 | 1,515 ± 76 | 1,528 ± 89 | |
| Change | −57 ± 86 | −136 ± 78 | −57 ± 75 | 0.719 |
| RMR (cal · 24 h−1 · FFM−1) | ||||
| Baseline | 28.1 ± 1.0 | 29.3 ± 1.5 | 27.8 ± 1.1 | |
| Final | 27.2 ± 1.0 | 27.9 ± 1.3 | 27.9 ± 1.3 | |
| Change | −0.9 ± 1.3 | −1.4 ± 1.5 | 0.1 ± 1.2 | 0.674 |
Data are means ± SE. Baseline values did not differ between groups for any parameter.
*P < 0.05,
†P < 0.01, and
‡P < 0.001 vs. baseline;
§P < 0.05 vs. control group.
HOMA-IR, homeostasis model assessment insulin resistance index; ISI, whole-body Matsuda (32) insulin sensitivity index; RMR, resting metabolic rate.
Dietary and fitness parameters.
Daily energy intake decreased by 600 ± 100 and 560 ± 90 calories in the WL and WL+EX groups, respectively (P both <0.001). Macronutrient changes from baseline included reductions in fat (by 3 ± 1 and 5 ± 1% of total energy, respectively, P both <0.05) and saturated fat (by 4 ± 1% in both groups, P both <0.001) and an increase in relative protein consumption (by 4 ± 1 and 3 ± 1%, respectively, P both <0.001). There were no significant dietary changes in the control group. Mean urinary sodium excretion decreased by 22 to 37 mmol/day across the three groups (Table 3, group effect, P = 0.12; group by time interaction, P = 0.62). Potassium excretion did not change. Aerobic capacity increased by 19 ± 4% in the WL+EX group only, as did maximum workload by 38 ± 4 W (P both <0.001, Table 2).
Body weight and composition.
Body weight decreased by 7.6 ± 0.7 and 8.8 ± 0.9% in the WL and WL+EX groups, respectively (P = 0.20 between groups) and there were concomitant reductions in fat mass (Table 1). The reduction in waist circumference was significantly greater in the WL+EX compared with the WL group (P = 0.01), and this was also reflected in the change in trunk fat mass, which tended to decrease more in the former group (P = 0.06). Lean body mass declined significantly in both lifestyle intervention groups. Change in lean body mass correlated with absolute change in dietary protein intake (g/day, r = 0.47, P = 0.002), indicating that reduction in protein consumption during weight loss was associated with loss of lean mass. Subgroup analysis by sex showed that men lost more weight (8.8 ± 0.8 vs. 6.2 ± 0.8 kg), total body fat (7.2 ± 0.8 vs. 4.3 ± 0.6 kg), and trunk fat (4.7 ± 0.5 vs. 2.3 ± 0.4 kg) than women (P all <0.05). Women in the WL+EX group maintained their lean body mass (mean change was −0.5 ± 0.5 kg, P = 0.26), whereas the men in the WL+EX group tended to lose lean mass (mean change was −1.1 ± 0.6 kg, P = 0.07).
Metabolic variables.
Fasting plasma glucose and insulin levels were reduced and by similar magnitude in both lifestyle groups (P all≤0.001), whereas glucose tolerance (glucose AUC0–120 and 2-h glucose concentration) did not change. Whole-body insulin sensitivity index increased by 49 ± 11% in the WL group and 45 ± 12% in the WL+EX group (P both <0.001). Fasting triglycerides, HDL cholesterol, and plasma leptin levels decreased significantly and by similar magnitude in both lifestyle groups (Table 3). High-sensitivity CRP decreased significantly only in the WL+EX group. Resting metabolic rate tended to decrease (time effect, P = 0.08) but when normalized to fat-free mass, there were no significant group effects or interactions.
Cardiovascular parameters.
Resting blood pressure decreased by similar magnitude in both lifestyle groups (Table 2). Spontaneous cardiac baroreflex sensitivity increased by 50 ± 20% in the WL group and 54 ± 20% in the WL+EX group (P both <0.05). The increase in baroreflex sensitivity was greater in men than women (6.3 ± 1.6 vs. 1.3 ± 1.2 ms/mmHg, P = 0.02).
Sympathetic activity.
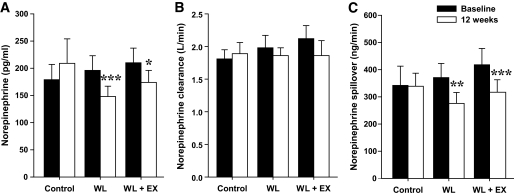
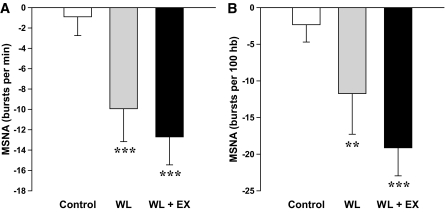
Because of difficulties with arterial line placement in four subjects, paired norepinephrine kinetics data were available for 55 participants (17 control, 20 WL, and 18 WL+EX). Arterial norepinephrine concentration and calculated norepinephrine spillover rates were significantly reduced after both WL and WL+EX treatment, whereas no changes were noted in norepinephrine plasma clearance (Fig. 1). The percentage change in norepinephrine spillover rate averaged −22 ± 6% for the WL group and −22 ± 7% for the WL+EX group (P both <0.01). After adjustment for baseline values by ANCOVA, between-group differences in the final value were significant for the WL and WL+EX groups versus the control group (P both <0.01). Acceptable paired MSNA recordings were obtained in 46 subjects (15 control, 15 WL, and 16 WL+EX). Both the WL and WL+EX interventions were associated with reductions in MSNA (Fig. 2): burst frequency decreased by 25 ± 9 and 29 ± 7% (P both ≤0.001), respectively, and burst incidence by 16 ± 12% and 27 ± 5%, respectively (P both <0.01). After adjustment for baseline values by ANCOVA, between-group differences in the final value were significant for the WL and WL+EX groups versus the control group (P all <0.05), whereas differences between the WL and WL+EX group were not significant.
FIG. 1.
Whole-body norepinephrine kinetics (means ± SE). Arterial plasma norepinephrine concentration (A), norepinephrine plasma clearance (B), and calculated NE spillover rate (C) before and after 12 weeks of lifestyle intervention with weight loss by caloric restriction (WL, n = 20), weight loss by caloric restriction and aerobic exercise (WL+EX, n = 18), or no treatment (control, n = 17). *P < 0.05, **P < 0.01, and ***P < 0.001 versus baseline. Plasma NE: time effect, P = 0.004; group × time interaction, P = 0.006. NE plasma clearance: no significant time, group, or interaction effects. Whole-body NE spillover: time effect, P = 0.002; group × time interaction, P = 0.004.
FIG. 2.
Absolute changes (means ± SE) in muscle sympathetic nerve activity (MSNA) after 12 weeks of weight loss by caloric restriction (WL, n = 15), caloric restriction and aerobic exercise (WL+EX, n = 16), or no treatment (control, n = 15). Multi-unit MSNA is expressed as burst frequency (A) and burst incidence (B). **P < 0.01 and ***P < 0.001 versus baseline. Burst frequency: time effect, P < 0.001; group × time interaction, P = 0.02. Burst incidence: time effect, P < 0.001; group × time interaction, P = 0.03. hb, heart beats.
No significant sex effects were observed for the change in sympathetic activity after lifestyle interventions.
Correlation and regression analysis.
Change in waist-to-hip ratio was the strongest correlate of change in whole-body norepinephrine spillover rate after lifestyle interventions for the whole group (r = 0.31, P = 0.06). This was also the case in men (r = 0.36, P = 0.08), whereas in women, change in total body fat mass (r = 0.52, P = 0.06), trunk fat mass (r = 0.55, P = 0.04), abdominal fat mass (r = 0.60, P = 0.02), and HDL cholesterol levels (r = −0.60, P = 0.02) were the strongest correlates. The reduction in MSNA burst incidence after lifestyle interventions correlated significantly with anthropometric changes in the whole-group and in both sexes (Table 4). Improvement in individual MetS components (fasting glucose, insulin sensitivity, and HDL cholesterol) were also associated with the reduction in MSNA in men. Increases in baroreflex sensitivity and fitness level were not associated with change in either whole-body norepinephrine spillover rate or MSNA. Stepwise linear regression analysis of the whole-group showed that change in total body fat mass (P = 0.03) and plasma leptin concentration (P = 0.01) were the strongest independent predictors of change in MSNA burst incidence, explaining 33 and 21% of the variance, respectively. Change in whole-body norepinephrine spillover was predicted by change in abdominal fat mass (P = 0.02) in women, which explained 36% of the variance.
TABLE 4.
Univariate correlates with change in muscle sympathetic nervous activity burst incidence (bursts/100 heart beats)
| Whole group (n = 31) |
Men (n = 18) |
Women (n = 13) |
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| ΔWeight (kg) | 0.38 | 0.04 | 0.51 | 0.03 | 0.37 | 0.21 |
| ΔBMI (kg/m2) | 0.39 | 0.03 | 0.51 | 0.03 | 0.34 | 0.26 |
| ΔTotal body fat mass (kg) | 0.40 | 0.03 | 0.49 | 0.04 | 0.52 | 0.07 |
| ΔAbdominal fat L1–L4 (kg) | 0.43 | 0.08 | ||||
| ΔTrunk fat mass (kg) | 0.33 | 0.07 | 0.48 | 0.04 | ||
| ΔFasting insulin (mU/l) | 0.39 | 0.03 | 0.41 | 0.09 | ||
| ΔFasting glucose (mmol/l) | 0.56 | 0.02 | ||||
| ΔLog ISI | −0.31 | 0.097 | −0.50 | 0.04 | ||
| ΔHOMA-IR | 0.44 | 0.07 | ||||
| ΔHDL cholesterol (mmol/l) | −0.54 | 0.02 | ||||
| Log Δleptin (ng/ml) | 0.46 | 0.01 | 0.62 | 0.02 | ||
Whole-group data represent pooled correlates of WL and WL+EX groups.
DISCUSSION
The main finding of this study is that incorporation of regular, moderate-intensity aerobic exercise training during a dietary weight loss program does not confer additional benefits on resting sympathetic neural activity, compared with weight loss alone, in middle-aged subjects with MetS obesity. Body weight reduction of 8–9% was accompanied by a 22% reduction in whole-body norepinephrine spillover and comparable attenuation of MSNA in both lifestyle groups. Similarly, we identified no further enhancement of exercise training on MetS components (blood pressure, fasting plasma glucose, triglyceride, and HDL cholesterol levels or insulin sensitivity), despite a 19% increase in fitness and a significantly greater reduction in central adiposity in the WL+EX group. However, combined exercise and weight loss was associated with a reduction of plasma hs-CRP concentrations that was not observed in the WL group.
Our findings are in agreement with those of Trombetta et al. (23), who conducted the only other comparable study in premenopausal obese women (albeit using nonadherent participants as control subjects) and observed similar reductions in resting MSNA after 4 months hypocaloric diet or hypocaloric diet and exercise training. Our metabolic results also concur with those of the CALERIE study, which identified no incremental benefit of weight loss through increased energy expenditure via exercise as opposed to weight loss by hypocaloric diet alone, on insulin action and coronary heart disease risk factors, when caloric deficit was matched in the two treatment groups (33,34). On the other hand, the Oslo Diet and Heart Study showed that 1-year intervention with combined diet and exercise was more effective than diet alone in the treatment of the MetS (17). It is likely that a combination of factors, including weight loss, negative energy balance, dietary composition, metabolic changes, and increased fitness in the WL+EX group, contributed to the observed sympathoinhibition after lifestyle intervention in the present study.
Considerable evidence exists that dietary-induced reductions in body weight are sympathoinhibitory: reductions in whole-body norepinephrine spillover (5), MSNA (5,23,35), and an increase in the parasympathetic indexes of heart rate variability (36) have previously been reported. Similarly, exercise intervention alone, using the same bicycle riding protocol as in the present study, has been shown to lower whole-body norepinephrine spillover by 24% and renal norepinephrine spillover by 41% in healthy young men independent of changes in body weight (19). It has been hypothesized that changes in central sympathetic outflow associated with body weight modification or increased fitness may have a reflex origin (35). The cardiac baroreflex is a compound reflex, where ∼70% of both vagal and sympathetic components of heart rate range are mediated by the arterial baroreceptors and ∼30% by cardiopulmonary baroreceptors. Both weight loss (5,35,37) and exercise training (38) are known to potentiate cardiovagal baroreflex sensitivity and the baroreceptor-sympathetic reflex (18,35); however, the present study is the first to examine their combined effects. Our results show no additive effect of exercise training and hypocaloric diet, beyond that attained by hypocaloric diet alone. The results do, however, emphasize that weight loss is a highly effective strategy to improve baroreflex function, as spontaneous cardiac baroreflex sensitivity increased by ∼50% in both lifestyle groups. Potential contributing mechanisms include increased arterial distensibility or improved neural transduction of barosensory vessel stretch into vagal outflow (39).
Sympathoinhibition after lifestyle intervention correlated positively with change in anthropometric variables in the present study. Change in waist-to-hip ratio and abdominal fat mass were most strongly associated with reduction in whole-body norepinephrine spillover, whereas changes in body weight, total body, and trunk fat masses and plasma leptin concentration were the strongest predictors of change in MSNA. The subcutaneous fat depot is the major source of leptin in humans, owing to the combination of a mass effect and a higher secretion rate in the subcutaneous than visceral adipose region (40). Experimental evidence in obese rodents supports the notion of selective leptin resistance in obesity, with preservation of leptin-dependent sympathoexcitation, but resistance to its anorexigenic effects (41). Although no definitive leptin administration studies have been performed in humans to characterize its effect on SNS activity, both the present study and an earlier weight loss trial performed by our group (5) suggest that reduction in plasma leptin is a significant independent predictor of sympathoinhibition after lifestyle intervention. Improvement in insulin resistance as indicated by decreased fasting insulin concentration and increased whole-body insulin sensitivity index also correlated with change in MSNA and support the notion that hyperinsulinemia enhances central sympathetic outflow (42). Changes in electrolyte status that coincide with energy restriction can modulate the response of the SNS. In particular, sodium depletion to ≤80 mmol/day has been shown to override the suppressive effect of energy restriction and instead trigger sympathetic activation and baroreflex impairment (43). In the present study, we chose not to supplement with sodium, as we felt this was more representative of weight loss in the community at large. Average 24-h urinary sodium excretion decreased modestly to levels commensurate (at week 12) with intermediate sodium intake in the DASH-Sodium trial (44). SNS activation would not be expected at this level of sodium intake; however, some contribution to sympathoinhibition versus baseline intake cannot be ruled out. Consumption of the DASH dietary pattern, which is rich in potassium and magnesium and reduced in total and saturated fat, may have also contributed to the observed reductions in blood pressure in the present study. Overall, however, absolute potassium intake did not change in our study, because of relatively high baseline consumption and use of the DASH diet at hypocaloric levels.
In our study, both WL and WL+EX produced comparable changes in metabolic risk factors, which were in the direction associated with reduced coronary heart disease risk. One exception was the change in plasma HDL cholesterol, which decreased significantly and by similar magnitude in both lifestyle groups. The impact of weight loss on lipids depends on a number of factors including energy balance, dietary composition, and concomitant exercise level (45). Using the same moderate-intensity exercise protocol, Reid et al. (46) demonstrated a significant increase in HDL cholesterol; however, this was diminished when exercise was prescribed together with weight loss. The relative reduction in total and saturated fat intake from baseline may have contributed to the decline in HDL cholesterol in our study (45). Change in HDL cholesterol correlated inversely with change in sympathetic activity, which likely reflects favorable alterations in HDL metabolism with loss of visceral fat mass, since change in HDL also related inversely to change in abdominal fat. It is also possible that reduction in central sympathetic outflow per se may have increased levels of HDL by increasing blood flow to peripheral vascular beds, thereby enhancing lipoprotein lipase activity (47). In our study, hs-CRP improved only in the WL+EX group, which was unexpected in light of previous work, including our own, which consistently shows that levels of this acute-phase reactant decrease after dietary weight loss (5,48).
The strengths of the present study are its randomized controlled design, which accounted for the effects of familiarization on sympathetic measurements; the use of both norepinephrine kinetics methodology and direct measurement of postganglionic MSNA to quantify sympathetic neural drive; and the close individualized supervision of each participant. Our study also has some limitations. First, only a subset of subjects had paired MSNA data and hence the sample size precludes demonstration of differences smaller than 30% between groups. Second, exercise training has many different facets, including frequency, duration, intensity, and exercise type. Our exercise protocol was based on moderate-intensity aerobic training on alternate days over a 12-week period, and thus further studies are required to examine whether higher intensity or frequency training or the inclusion of resistance exercise has additional benefits on neuroadrenergic function. For instance moderate-intensity exercise training 7 days per week has been associated with greater reduction in norepinephrine spillover than the same protocol 3 days per week (49,19). Resistance exercise training improves postexercise heart rate recovery and heart rate variability, reflecting improved cardiac vagal activity (50), but there is a paucity of data to date using robust measurements of sympathetic activity in this setting.
In conclusion, this study provides evidence that both hypocaloric diet and hypocaloric diet with exercise training elicit significant improvements in resting sympathetic neural drive and MetS components. The results suggest that weight loss, and in particular abdominal fat loss, is the prime mover in sympathetic neural adaptation to a hypocaloric diet. These findings support the adoption of lifestyle changes for the prevention of cardiovascular sequelae of obesity.
Acknowledgments
This study was supported by Diabetes Australia Research Trust Grants (2005–2008), a Future Forum Research Grant, and a Bennelong Foundation Grant to N.E.S. The research group also hold a Heart Foundation Grant-in-Aid and a National Health and Medical Research Council Project Grant (472604) from the Australian Government. E.A.L., M.P.S., G.W.L., and M.D.E. are supported by National Health and Medical Research Council fellowships.
No potential conflicts of interest relevant to this article were reported.
We thank the study participants for their cooperation and effort, radiographer Dianne Payne for dual-energy X-ray absorptiometry scan analyses, and Donna Vizi and Jenny Starr for their excellent nursing assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ford ES: Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 2005;28:1769–1778 [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM: Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab 2007;92:399–404 [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, Reid J, Van Zwieten PA: The sympathetic nervous system and the metabolic syndrome. J Hypertens 2007;25:909–920 [DOI] [PubMed] [Google Scholar]
- 4.Lee ZS, Critchley JA, Tomlinson B, Young RP, Thomas GN, Cockram CS, Chan TY, Chan JC: Urinary epinephrine and norepinephrine interrelations with obesity, insulin, and the metabolic syndrome in Hong Kong Chinese. Metabolism 2001;50:135–143 [DOI] [PubMed] [Google Scholar]
- 5.Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ: Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab 2005;90:5998–6005 [DOI] [PubMed] [Google Scholar]
- 6.Grassi G, Dell'Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G: Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia 2005;48:1359–1365 [DOI] [PubMed] [Google Scholar]
- 7.Straznicky NE, Eikelis N, Lambert EA, Esler MD: Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep 2008;10:440–447 [DOI] [PubMed] [Google Scholar]
- 8.Alvarez GE, Beske SD, Ballard TP, Davy KP: Sympathetic neural activation in visceral obesity. Circulation 2002;106:2533–2536 [DOI] [PubMed] [Google Scholar]
- 9.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML: Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 2003;42:474–480 [DOI] [PubMed] [Google Scholar]
- 10.Flaa A, Aksnes TA, Kjeldsen SE, Eide I, Rostrup M: Increased sympathetic reactivity may predict insulin resistance: an 18-year follow-up study. Metabolism 2008;57:1422–1427 [DOI] [PubMed] [Google Scholar]
- 11.Julius S, Valentini M, Palatini P: Overweight and hypertension: a 2-way street? Hypertension 2000;35:807–813 [DOI] [PubMed] [Google Scholar]
- 12.Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO: Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension 1993;21:618–623 [DOI] [PubMed] [Google Scholar]
- 13.Howard BV, Adams-Campbell L, Allen C, Black H, Passaro M, Rodaberough RJ, Rodriguez BL, Safford M, Stevens VJ, Wagenknecht LE: Insulin resistance and weight gain in postmenopausal women of diverse ethnic groups. Int J Obes 2004;28:1039–1047 [DOI] [PubMed] [Google Scholar]
- 14.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD: Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation 2003;108:560–565 [DOI] [PubMed] [Google Scholar]
- 15.Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW: Sympathetic activation in chronic renal failure: causes, consequences and therapeutic implications. J Am Soc Nephrol 2009;20:933–939 [DOI] [PubMed] [Google Scholar]
- 16.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler SDiabetes Prevention Program Research Group The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005;142:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderssen SA, Carroll S, Urdal P, Holme I: Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: results from the Oslo Diet and Exercise Study. Scand J Med Sci Sports 2007;17:687–695 [DOI] [PubMed] [Google Scholar]
- 18.Grassi G, Seravalle G, Calhoun DA, Mancia G: Physical training and baroreceptor control of sympathetic nerve activity in humans. Hypertension 1994;23:294–301 [DOI] [PubMed] [Google Scholar]
- 19.Meredith IT, Friberg P, Jennings GL, Dewar EM, Fazio VA, Lambert GW, Esler MD: Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension 1991;18:575–582 [DOI] [PubMed] [Google Scholar]
- 20.Svendsen OL, Hassager C, Christiansen C: Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med 1993;95:131–140 [DOI] [PubMed] [Google Scholar]
- 21.Reseland JE, Anderssen SA, Solvoll K, Hjermann I, Urdal P, Holme I, Drevon CA: Effect of long-term changes in diet and exercise on plasma leptin concentrations. Am J Clin Nutr 2001;73:240–245 [DOI] [PubMed] [Google Scholar]
- 22.Weinstock RS, Dai H, Wadden TA: Diet and exercise in the treatment of obesity: effects of 3 interventions on insulin resistance. Arch Intern Med 1998;158:2477–2483 [DOI] [PubMed] [Google Scholar]
- 23.Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FHS, Gowdak MMG, Barretto ACP, Halpern A, Villares SMF, Negrao CE: Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Heart Circ Physiol 2003;285:H974–H982 [DOI] [PubMed] [Google Scholar]
- 24.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP: Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol 1996;81:318–325 [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa FAmerican Heart Association, National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 26.King AC, Haskell WL, Young DR, Oka RK, Stefanick ML: Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation 1995;91:2596–2604 [DOI] [PubMed] [Google Scholar]
- 27.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N: A clinical trial of the effects of dietary patterns on blood pressure: DASH Collaborative Research Group. N Engl J Med 1997;336:1117–1124 [DOI] [PubMed] [Google Scholar]
- 28.Weir JB, de V: New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poehlman ET, LaChance P, Tremblay A, Nadeau A, Dussault J, Thériault G, Després JP, Bouchard C: The effect of prior exercise and caffeine ingestion on metabolic rate and hormones in young adult males. Can J Physiol Pharmacol 1989;67:10–16 [DOI] [PubMed] [Google Scholar]
- 30.Esler M, Jackman G, Bobik A, Kelleher D, Jennings G, Leonard P, Skews H, Korner P: Determination of norepinephrine apparent release rate and clearance in humans. Life Sci 1979;25:1461–1470 [DOI] [PubMed] [Google Scholar]
- 31.Parati G, Saul JP, Castiglioni P: Assessing arterial baroreflex control of heart rate: new perspectives. J Hypertens 2004;22:1259–1263 [DOI] [PubMed] [Google Scholar]
- 32.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 33.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JOWashington University School of Medicine CALERIE Group Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 2006;84:1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JOWashington University School of Medicine CALERIE Group Calorie restriction or exercise: effects on coronary heart disease risk factors: a randomized, controlled trial. Am J Physiol Endocrinol Metab 2007;293:E197–E202 [DOI] [PubMed] [Google Scholar]
- 35.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G: Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 1998;97:2037–2042 [DOI] [PubMed] [Google Scholar]
- 36.Akehi Y, Yoshimatsu H, Kurokawa M, Sakata T, Eto H, Ito S, Ono J VLC: D-induced weight loss improves heart rate variability in moderately obese Japanese. Exp Biol Med 2001;226:440–445 [DOI] [PubMed] [Google Scholar]
- 37.Alvarez GE, Davy BM, Ballard TP, Beske SD, Davy KP: Weight loss increases cardiovagal baroreflex function in obese young and older men. Am J Physiol Endocrinol Metab 2005;289:E665–E669 [DOI] [PubMed] [Google Scholar]
- 38.Somers VK, Conway J, Johnston J, Sleight P: Effects of endurance training on baroreflex sensitivity and blood pressure in borderline hypertension. Lancet 1991;337:1363–1368 [DOI] [PubMed] [Google Scholar]
- 39.Hunt BE, Farquhar WB, Taylor JA: Does reduced vascular stiffening fully explain preserved cardiovagal baroreflex function in older, physically active men? Circulation 2001;103:2424–2427 [DOI] [PubMed] [Google Scholar]
- 40.Van Harmelen V, Reynisdottir S, Eriksson P, Thörne A, Hoffstedt J, Lönnqvist F, Arner P: Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998;47:913–917 [DOI] [PubMed] [Google Scholar]
- 41.Grassi G: Leptin, sympathetic nervous system, and baroreflex function. Curr Hypertens Rep 2004;6:236–240 [DOI] [PubMed] [Google Scholar]
- 42.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL: Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 1991;87:2246–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grassi G, Dell'Oro R, Seravalle G, Foglia G, Trevano FQ, Mancia G: Short- and long-term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation 2002;106:1957–1961 [DOI] [PubMed] [Google Scholar]
- 44.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PHDASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet: DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10 [DOI] [PubMed] [Google Scholar]
- 45.Noakes M, Clifton PM: Weight loss and plasma lipids. Curr Opin Lipidol 2000;11:65–70 [DOI] [PubMed] [Google Scholar]
- 46.Reid CM, Dart AM, Dewar EM, Jennings GL: Interactions between the effects of exercise and weight loss on risk factors, cardiovascular haemodynamics and left ventricular structure in overweight subjects. J Hypertens 1994;12:291–301 [PubMed] [Google Scholar]
- 47.Nash DT: Alpha-adrenergic blockers: mechanisms of action, blood pressure control, and effects of lipoprotein metabolism. Clin Cardiol 1990;13:764–772 [DOI] [PubMed] [Google Scholar]
- 48.Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, Palla S, Bleecker E, Pahor M: Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr 2004;79:544–551 [DOI] [PubMed] [Google Scholar]
- 49.Jennings G, Nelson L, Nestel P, Esler M, Korner P, Burton D, Bazelmans J: The effects of changes in physical activity on major cardiovascular risk factors, hemodynamics, sympathetic function, and glucose utilization in man: a controlled study of four levels of activity. Circulation 1986;73:30–40 [DOI] [PubMed] [Google Scholar]
- 50.Heffernan KS, Jae SY, Vieira VJ, Iwamoto GA, Wilund KR, Woods JA, Fernhall B: C-reactive protein and cardiac vagal activity following resistance exercise training in young African-American and white men. Am J Physiol Regul Integr Comp Physiol 2009;296:R1098–R1105 [DOI] [PubMed] [Google Scholar]