Abstract
OBJECTIVE
To examine the sensitivity and specificity of A1C as a diagnostic test for type 2 diabetes in older adults.
RESEARCH DESIGN AND METHODS
Cross-sectional study of community-dwelling adults without known diabetes who had an oral glucose tolerance test and A1C measured on the same day.
RESULTS
Mean age of the 2,107 participants was 69.4 ± 11.1 years; 43% were men. Based on the American Diabetes Association (ADA) criteria, 198 had previously undiagnosed type 2 diabetes. The sensitivity/specificity of A1C cut point of 6.5% was 44/79%. Results were similar in age- and sex-stratified analyses. Given the A1C cut point of 6.5%, 85% of participants were classified as nondiabetic by ADA criteria.
CONCLUSIONS
The limited sensitivity of the A1C test may result in delayed diagnosis of type 2 diabetes, while the strict use of ADA criteria may fail to identify a high proportion of individuals with diabetes by A1C ≥6.5% or retinopathy.
The current criteria for a diagnosis of type 2 diabetes (1) require a fasting plasma glucose (FPG) test and/or a 75-g oral glucose tolerance test (OGTT), a diagnostic method that is time-consuming, requires fasting, and is affected by acute perturbations in glucose levels and short-term lifestyle changes. Even though the time for onset has been shortened in the last decades, the onset of type 2 diabetes occurs years before clinical diagnosis (2,3).
A1C has been suggested as a useful tool for type 2 diabetes screening and diagnosis (4–6); it does not require fasting, reflects the usual 3–4 months prior glycemia, has less intraindividual variability, and may better predict diabetes-related complications (7,8). Recent reports have stated that the cut point of ≥6.5% would be diagnostic if confirmed by a repeated test (4,5). Further investigation of A1C diagnostic performance in specific age and sex groups is still lacking.
We designed the present study to determine the sensitivity and specificity of A1C for type 2 diabetes diagnosis compared with the current OGTT gold standard as well as diabetic retinopathy (DR) in a cohort of older adults from the Rancho Bernardo Study.
RESEARCH DESIGN AND METHODS
Participants included 2,107 adults without known type 2 diabetes or anemia who had an OGTT and A1C test between 1984 and 1987. Glucose tolerance status was defined by the American Diabetes Association (ADA) OGTT criteria as 1) normoglycemia, FPG <100 mg/dl and 2-h postchallenge glucose <140 mg/dl; 2) pre-diabetes, FPG ≥100 mg/dl and <126 mg/dl or 2-h postchallenge glucose ≥140 mg/dl and <200 mg/dl; and 3) type 2 diabetes, FPG ≥126 mg/dl or 2-h postchallenge glucose ≥200 mg/dl (1). Anemia was assessed by history.
All participants provided written informed consent. The study protocol was approved by the University of California, San Diego Human Research Protection Program.
Laboratory and anthropometric data were performed as previously described (9). A1C was measured with high-performance liquid chromatography using an automated analyzer (normal range 4.5–6.5%) (SmithKline, Van Nuys, CA). Ophthalmologic evaluation was performed by nonmydriatic retinal photography (10).
All analyses were performed using SPSS version 13.1 (SPSS, Chicago, IL). Receiver operating characteristic curves were constructed to calculate sensitivity/specificity of A1C cut points for type 2 diabetes diagnosis, and κ coefficients were used to test for agreement between A1C values and diabetes status.
RESULTS
Mean age was 69.4 ± 11.1 years; 43% were men. There were 198 participants with previously undiagnosed diabetes who had type 2 diabetes by ADA criteria. At the time of diabetes diagnosis, mean A1C was 6.5 ± 1.07% compared with 5.9 ± 0.73% and 6.06 ± 0.75% in participants with normal glucose and pre-diabetes, respectively (P < 0.001).
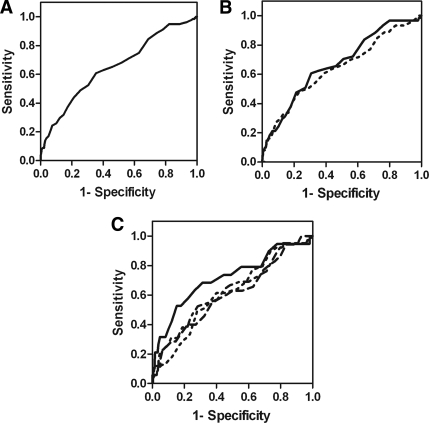
Overall, the A1C cut point of 6.5% had a sensitivity/specificity of 44/79% (area under receiver operating characteristic curve 0.65) (Fig. 1A). The A1C cut point of 6.15% yielded the highest combination of sensitivity (63%) and specificity (60%) but would miss one-third of those with type 2 diabetes by ADA criteria and misclassify one-third of those without. Results in sex-stratified analysis were similar (Fig. 1B). In analysis stratified by quartiles of age, the sensitivity/specificity of A1C cut point of 6.5% was up to 52/95% (Fig. 1C).
Figure 1.
A1C receiver operating characteristic curve for type 2 diabetes diagnosis: (A) whole cohort; (B) men (—) and women (---); and (C) by quartiles of age: first quartile (—), second quartile (---), third quartile (---), and fourth quartile (----).
Using the A1C cut point of 6.5%, the agreement with type 2 diabetes diagnosis was low (the κ coefficient was 0.119), and 85% of participants with A1C ≥6.5% were classified as non-type 2 diabetes by OGTT ADA criteria of whom 34% were normoglycemic. When compared with type 2 diabetes diagnosis based only on FPG ≥126 mg/dl, the agreement was also low (κ coefficient 0.061). The same pattern was observed considering type 2 diabetes diagnosis based only on postchallenge glucose ≥200 mg/dl (κ coefficient 0.112).
In order to compare A1C and ADA criteria with a type 2 diabetes complication, we considered their prevalent retinopathy. Only 1.8% (n = 38) of these individuals without known diabetes had any degree of DR; of those, 40% had A1C ≥6.5% and none had type 2 diabetes by ADA criteria.
CONCLUSIONS
In this cohort of older adults, the suggested A1C cut point of 6.5% had relatively low sensitivity and specificity for type 2 diabetes diagnosis in all age-groups and in both sexes. There was low agreement between type 2 diabetes diagnosis made by A1C and ADA criteria. However, A1C criteria were met in 40% of the participants with prevalent DR, while OGTT criteria were met in none.
In a recent systematic review of nine studies, Bennett et al. (5) reported that the A1C cut point of ≥6.1% had sensitivity of 78–91% and specificity of 79–84% compared with OGTT. In contrast, data from the National Health and Nutrition Examination Survey (NHANES III) showed that A1C ≥6.5% had sensitivity/specificity of 44/99% (11). The present study showed sensitivity similar to that in the NHANES reports; however, our specificity of 79% for A1C ≥6.5% was much lower than previously reported. This is likely because these studies included much younger populations.
The recently published International Expert Committee Report on the role of A1C for type 2 diabetes diagnosis (6) states that there is no single assay for hyperglycemia that can be considered the gold standard. In the present study, 85% of participants with A1C ≥6.5% were not classified as diabetic by ADA criteria and one-third of the participants with diabetes by ADA criteria would be classified as normoglycemic by A1C, i.e., a significant proportion of misclassification. These observations raise two concerns: it would not be desirable to miss those with high A1C, considering that the burden of DR correlates better with A1C than with FPG or OGTT and that the prevalence of DR increases substantially when A1C values exceed 7% (6,8). On the other hand, performing A1C instead of OGTT would miss 30% of those who are already diabetic and those with pre-diabetes. Failing to identify pre-diabetes would miss interventional opportunities to prevent or delay type 2 diabetes (12).
To our knowledge, this is the first report of A1C diagnostic performance in the elderly. Our findings are important because the elderly in the U.S. have the greatest current burden and are expected to have the greatest increase in the prevalence of type 2 diabetes (13,14).
We conclude that the limited sensitivity of the A1C test may result in missed or delayed diagnosis of type 2 diabetes, whereas the use of current OGTT criteria will fail to identify a high proportion of individuals with A1C >6.5%. Further studies and discussion are needed before revising guidelines for type 2 diabetes diagnosis.
Acknowledgments
The Rancho Bernardo Study was funded by the National Institutes of Health/National Institute on Aging Grants AG07181 and AG028507 and the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK31801. C.K.K. was a recipient of a grant from Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES) Brazil (Programa de Doutorado no Pais com Estagio no Exterior [PDEE] sandwich).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus (Position Statement). Diabetes Care 2009; 32( Suppl. 1): S62– S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harris MI, Klein R, Welborn TA, Knuiman MW: Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care 1992; 15: 815– 819 [DOI] [PubMed] [Google Scholar]
- 3. Norris SL, Kansagara D, Bougatsos C, Fu R: U.S. Preventive Services Task Force. Screening adults for type 2 diabetes: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2008; 148: 855– 868 [DOI] [PubMed] [Google Scholar]
- 4. Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB: A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 2008; 93: 2447– 2453 [DOI] [PubMed] [Google Scholar]
- 5. Bennett CM, Guo M, Dharmage SC: HbA(1c) as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med 2007; 24: 333– 343 [DOI] [PubMed] [Google Scholar]
- 6. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327– 1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selvin E, Crainiceanu CM, Brancati FL, Coresh J: Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 2007; 167: 1545– 1551 [DOI] [PubMed] [Google Scholar]
- 8. Tapp RJ, Tikellis G, Wong TY, Harper CA, Zimmet PZ, Shaw JE: Australian Diabetes Obesity and Lifestyle Study Group. Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care 2008; 31: 1349– 1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kramer CK, von Mühlen D, Gross JL, Laughlin GA, Barrett-Connor E: Blood pressure and fasting plasma glucose rather than metabolic syndrome predict coronary artery calcium progression: the Rancho Bernardo Study. Diabetes Care 2009; 32: 141– 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klein R, Klein BE, Neider MW, Hubbard LD, Meuer SM, Brothers RJ: Diabetic retinopathy as detected using ophthalmoscopy, a nonmydriatic camera and a standard fundus camera. Ophthalmology 1985; 92: 485– 491 [DOI] [PubMed] [Google Scholar]
- 11. Buell C, Kermah D, Davidson MB: Utility of A1C for diabetes screening in the 1999–2004 NHANES population. Diabetes Care 2007; 30: 2233– 2235 [DOI] [PubMed] [Google Scholar]
- 12. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS: Diabetes trends in the U.S.: 1990–1998. Diabetes Care 2000; 23: 1278– 1283 [DOI] [PubMed] [Google Scholar]
- 14. Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ: Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care 2001; 24: 1936– 1940 [DOI] [PubMed] [Google Scholar]