Abstract
OBJECTIVE
Zinc transporter 8 (ZnT8) is an islet β-cell secretory granule membrane protein recently identified as an autoantibody antigen in type 1 diabetes. The aim of this study was to determine the prevalence and role of antibodies to ZnT8 (ZnT8As) in adult-onset diabetes.
RESEARCH DESIGN AND METHODS
ZnT8As were measured by a radioimmunoprecipitation assay using recombinant ZnT8 COOH-terminal or NH2-terminal proteins in 193 patients with adult-onset autoimmune diabetes having antibodies to either GAD (GADAs) or IA-2 (IA-2As) and in 1,056 antibody-negative patients with type 2 diabetes from the Non Insulin Requiring Autoimmune Diabetes (NIRAD) study.
RESULTS
ZnT8As-COOH were detected in 18.6% patients with autoimmune diabetes and 1.4% with type 2 diabetes. ZnT8As-NH2 were rare. ZnT8As were associated with younger age and a high GADA titer. The use of GADAs, IA-2As, and ZnT8As in combination allowed a stratification of clinical phenotype, with younger age of onset of diabetes and characteristics of more severe insulin deficiency (higher fasting glucose and A1C, lower BMI, total cholesterol, and triglycerides) in patients with all three markers, with progressive attenuation in patients with two, one, and no antibodies (all Ptrend < 0.001). Autoantibody titers, association with high-risk HLA genotypes, and prevalence of thyroid peroxidase antibodies followed the same trend (all P < 0.001).
CONCLUSIONS
ZnT8As are detectable in a proportion of patients with adult-onset autoimmune diabetes and seem to be a valuable marker to differentiate clinical phenotypes.
Zinc transporter 8 (ZnT8) is a pancreatic β-cell secretory granule membrane protein that has been recently identified as a target of humoral immunity in type 1 diabetes (1). Autoantibodies to ZnT8 (ZnT8As) constitute an additional marker of autoimmune diabetes, which complement the established antibodies to insulin (IAAs) (2), GAD (GADAs) (3), and protein tyrosine IA-2 (IA-2As) (4). In the first report, ZnT8As were detected in 63% of young patients at onset of disease, overlapping with, but also independent of, GADAs, IAAs, and IA-2As, and the combined use of these four antibody markers raised the detection rate of autoimmunity to 94% in new-onset cases of type 1 diabetes. Moreover, ZnT8As could be detected also in the preclinical phase of type 1 diabetes, showing a trend to a later appearance relative to IAAs, GADAs, and IA-2As but with the ability to identify individuals with a more rapid progression to clinical disease. Although islet autoimmunity is responsible for the large majority of childhood- and adolescent-onset diabetes, it can be found also in 4–10% of adult-onset diabetes. This subgroup of patients test positive for humoral markers of islet autoreactivity, despite having clinical features indistinguishable from those of classic type 2 diabetes, and are characterized as having latent autoimmune diabetes of adult (LADA). Patients with LADA are identified solely by the detection of circulating islet autoantibodies, with islet cell antibodies (ICAs) and GADAs being the antibody markers with the highest prevalence (5,6), followed by IA-2As, which are detected in a minority of case subjects and are almost invariably associated with GADAs (7), whereas insulin autoantibodies, which constitute a specific marker of juvenile diabetes inversely related to age and rare in adults, are unlikely to be useful for LADA screening (8–10). The aim of this study was to evaluate the prevalence of ZnT8As in adult-onset diabetes and establish their potential use as an additional marker of autoimmunity and phenotype characterization in this patient population.
RESEARCH DESIGN AND METHODS
All patients investigated participated in the Non Insulin Requiring Autoimmune Diabetes (NIRAD) study, a nationwide survey based in Italy, conducted with the aim of assessing the prevalence and characteristics of adult-onset autoimmune diabetes (11). Inclusion criteria were 1) diagnosis of diabetes according to the American Diabetes Association, with no insulin requirement and no evidence of ketosis from diagnosis to screening time, and 2) disease duration between 6 months and 5 years. Exclusion criteria included prior insulin therapy, pregnancy, and the presence of any other severe disease. The study was approved by the ethics committees of all participating centers and written informed consent was obtained by all patients before screening. Of the original NIRAD cohort of 4,250 subjects with adult-onset of initially non–insulin-requiring diabetes we studied all 193 patients (4.5% overall prevalence) with autoimmune diabetes defined as having either GADAs or IA-2As (aged 50.3 ± 12.8 years; mean duration of diabetes 2.3 years, range 0.6–4.8 years) (11) and 1,056 patients with type 2 diabetes (aged 51.8 ± 11.8 years, mean duration of diabetes 2.4 years, range 0.5–5 years). For the comparison of clinical phenotypes a subset of 348 age- and sex-matched antibody-negative patients with type 2 diabetes (aged 51.1 ± 10.8 years; mean duration of diabetes 2.2 years, range 0.5–5 years) was selected. Previous patient assessment included the following measurements: anthropometrics; fasting glucose, total cholesterol, HDL cholesterol, triglycerides, uric acid and A1C; GADAs, IA-2As, and thyroid peroxidase (TPO) antibodies; and HLA-DRB1 and DQB1 typing (11,12). The distribution of GADA titers in patients with autoimmune diabetes was independent of diabetes duration and showed a bimodal distribution. Consistent with this observation, patients with autoimmune diabetes were divided into subgroups representing the two distributions, namely low (≤32 arbitrary units, equivalent to 300 World Health Organization units) and high (>32 arbitrary units) GADA titers (11,13).
ZnT8 cDNA cloning
Total RNA was extracted from isolated human pancreatic islets with the Mirvana kit (Applied Biosystems, Foster City, CA) according to the total RNA isolation protocol and reverse transcribed to cDNA using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). The coding regions of ZnT8 corresponding to amino acids 1–74 (ZnT8-NH2) and amino acids 268–369 (ZnT8-COOH R325) were amplified by PCR using PfuUltra II Fusion HS DNA Polymerase (Stratagene, La Jolla, CA) with specific primers containing EcoRI restriction sites and for the forward primers an in-frame start codon within the context of a canonical Kozak sequence. Amplified PCR products were purified with Montage-PCR filter units (Millipore, Billerica, MA) and cut with the restriction enzyme EcoRI (Roche Diagnostics, Basel, Switzerland). Digested PCR fragments were purified with Micropure-EZ spin columns (Millipore), ligated into the EcoRI site of the pTnT plasmid vector (Promega, Hercules, CA) and transformed by electroporation in TOP10 Escherichia coli bacterial cells. Plasmid DNA was extracted from the clones obtained with GenElute spin columns (Sigma-Aldrich, St. Louis, MO), and the cDNA insert was verified by sequencing on an ABI3130 automated sequencer (Applied Biosystems). For large-scale plasmid DNA preparations, Qiagen Midi columns were used (Qiagen, Hilden, Germany). A clone containing a cDNA encoding for the polymorphic residue tryptophan in position 325 of ZnT8 (ZnT8-COOH W325) was obtained from the ZnT8-COOH R325 by site-directed mutagenesis according to the QuickChange protocol (Stratagene).
ZnT8A assay
ZnT8As in patient sera were measured by immunoprecipitation of radiolabeled recombinant ZnT8 antigens. ZnT8 ZnT8-NH2 and ZnT8-COOH proteins were expressed in vitro in a rabbit reticulocyte lysate using the TNT Quick Coupled Transcription/Translation System SP6 kit (Promega) in the presence of 40 μCi of 35S-labeled methionine (PerkinElmer, Waltham, MA), purified by size-exclusion chromatography on NAP-5 columns (GE Healthcare BioSciences, Uppsala, Sweden), and the recovered radioactivity was measured on a TopCount beta counter (PerkinElmer). For immunoprecipitation 20,000 cpm of recombinant radiolabeled ZnT8-NH2, when testing for ZnT8As-NH2, or a mixture of 10,000 cpm each of ZnT8-COOH R325 and W325 antigens, when testing for ZnT8As-COOH, were added in 25 μl of Tris-buffered saline (pH 7.4)-0.1% Tween 20 (TBST) to 2 μl of human serum for each test sample in duplicate wells of a polystyrene 96–deep well plate (Beckman Coulter, Fullerton, CA) and incubated overnight at 4°C. Immune complexes were recovered by the addition of 4 μl of resuspended CL4B protein A–Sepharose (GE Healthcare BioSciences) in 50 μl of TBST and incubated with agitation at 4°C for 1 h. Protein A–Sepharose beads were then washed five times by adding 750 μl of TBST followed by centrifugation at 700g for 3 min and buffer removal by aspiration. After the last wash, protein A–Sepharose beads were resuspended and transferred to the wells of a 96-well OptiPlate (PerkinElmer), added with 150 μl of MicroScint 40 scintillation fluid (PerkinElmer), and the recovered radioactivity was measured by counting each well for 5 min on a TopCount β-counter. Results are expressed in arbitrary units derived by a standard curve made of serial dilutions of a positive serum included in each assay run. The threshold for positivity was placed at the 99th percentile of 100 nondiabetic control subjects. The assay for ZnT8As-COOH showed an interassay coefficient of variation (CV) of 8.4% and an intra-assay CV of 5.5%, whereas the assay for ZnT8As-NH2 showed an interassay CV of 9.5% and an intra-assay CV of 6.3%. In the second international workshop on ZnT8As held in 2009 by the Diabetes Autoantibody Standardization Program, the assay for ZnT8As-COOH showed a laboratory-reported sensitivity of 68%, a specificity of 99%, an area under the receiver operating characteristic curve of 0.8848, and an adjusted sensitivity of 95% and specificity of 74%. The assay for ZnT8As-NH2 in the first international workshop on ZnT8As held in 2007 showed an 11% sensitivity and 99% specificity.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (version 13; SPSS, Chicago, IL). Data are expressed as frequencies, as means ± SD, or as median (interquartile range). Frequency differences were compared using the χ2 test (with the Yates continuity correction) or a Fisher exact test when appropriate. The exploration of statistical differences between groups for quantitative variables was investigated using multiple linear regression. Comparisons were adjusted for age of recruitment, duration of disease, sex, and therapy. The nonparametric Mann-Whitney test was used to investigate the relation between TPO titers (units) and number of antibodies. Data for triglycerides, HDL, and ZnT8A titers (units) were transformed using log base 10 to normalize their distributions. HLA DQB1 and DRB1 allele frequencies were in Hardy-Weinberg equilibrium (i.e., observed and expected genotype frequencies did not differ significantly). HLA class II alleles were evaluated as described previously (11).
RESULTS
Prevalence of ZnT8As
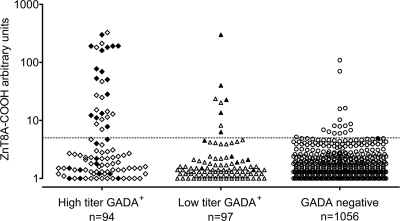
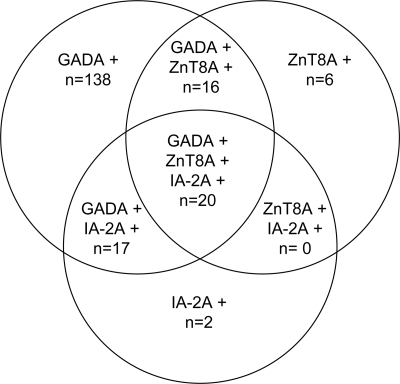
As reported previously, of the 193 patients with autoimmune diabetes identified within the NIRAD study (4.5% prevalence of adult-onset diabetes), 191 had GADAs and 39 had IA-2As; of these, 154 had GADAs only, 2 had IA-2As only, and 37 had both (9). ZnT8As-COOH were detected in 36 of 193 (18.6%) autoimmune (all 36 with GADAs and 20 with both GADAs and IA-2As) and 16 of 1,056 (1.4%) patients with type 2 diabetes (Fig. 1); ZnT8As-NH2 were rare and were found only in 4 of 193 (2.1%) patients with autoimmune diabetes (3 also having ZnT8As-COOH) and 1 of 348 (0.3%) patients of the subset of matched patients with type 2 diabetes. Therefore, ZnT8As hereafter are intended to be ZnT8As-COOH if not otherwise specified. The prevalence of ZnT8As within autoimmune patients was higher in younger patients and declined with age: in subjects aged 0–49 years, n = 22 (12.2%); in subjects aged 49.1–58.8 years, n = 12 (6.6%); and in subjects aged >58.9 years, n = 8 (4.4%) (Ptrend = 0.0058). Within the originally defined autoimmune diabetic group, 20 patients were positive for three autoantibodies, 33 for two autoantibodies (17 with GADAs and IA-2As, 16 with GADAs and ZnT8As), and 140 for one antibody only (138 with GADAs and 2 with IA-2As) (Fig. 2).
Figure 1.
ZnT8As directed against the COOH domain are more frequent in patients with high-titer GADA positivity. Filled symbols indicate IA-2A positivity also. Dotted line indicates the threshold for positivity.
Figure 2.
Venn diagram of autoantibody combinations in autoantibody-positive patients with adult-onset diabetes.
ZnT8As, other autoantibodies, and clinical phenotype
Within the original group of autoimmune patients (with either GADAs and/or IA-2As), those having ZnT8As more frequently had associated high GADA titers (>32 arbitrary units, equivalent to 300 World Health Organization units) (11) and IA-2As (both P < 0.01 vs. ZnT8A-negative). However, within the group with high GADA titers, no statistically significant differences in clinical features were observed between ZnT8A-positive and -negative patients.
The clinical phenotype analyzed according to the number of islet autoantibodies showed a trend toward a younger age at diagnosis, more prominent features of insulin deficiency (higher fasting glucose and A1C and lower BMI, waist circumference, total cholesterol, triglycerides, and uric acid), and higher prevalence of associated TPO antibodies proportional to the number of autoantibodies (all Ptrend < 0.001). All of these traits, although attenuated and with the exception of total cholesterol and triglycerides, remained significantly different in patients with a single autoantibody compared with those with classic type 2 diabetes (all P ≤ 0.04). Accordingly, the titers of GADAs, IA-2As, and ZnT8As and the prevalence and titers of associated TPO antibodies, as well as the association with high-risk HLA genotypes followed the same trend (all P < 0.001) (Table 1).
Table 1.
Clinical characteristics of adult-onset diabetes according to the number of autoantibodies
| 3 Abs | 2 Abs | 1 Ab only | None (type 2 diabetes) | P value | P value 1 Ab vs. none | P value excluding type 2 diabetes | |
|---|---|---|---|---|---|---|---|
| n | 20 | 33 | 140 | 342 | |||
| Sex (male/female) | 10/10 | 20/13 | 71/69 | 174/168 | |||
| Age of diagnosis (years) | 43.6 ± 16.4 | 45 ± 12.6 | 52.4 ± 11.2 | 51.6 ± 10.6 | <0.0001 | 0.04 | 0.003 |
| A1C (%) | 8.2 ± 2.7 | 8.1 ± 2.2 | 7 ± 1.9 | 6.5 ± 1.4 | <0.0001 | 0.01 | 0.03 |
| BMI (kg/m2) | 24.2 ± 4.5 | 25.5 ± 4.4 | 27.6 ± 4.8 | 29.4 ± 5.1 | <0.0001 | 0.004 | 0.002 |
| Waist circumference (cm) | 84.8 ± 8.17 | 91.3 ± 13.3 | 96.2 ± 13.2 | 98 ± 13.1 | <0.0001 | 0.03 | 0.003 |
| Fasting glucose (mg/dl) | 184 ± 65 | 163 ± 41 | 166 ± 55 | 144 ± 48.9 | <0.0001 | 0.001 | NS |
| Triglycerides (mg/dl) | 83 (54–148) | 110 (76–182) | 128 (83–199) | 135 (101–196) | 0.0008 | NS | 0.03 |
| HDL cholesterol (mg/dl) | 46.5 (39–56) | 50.5 (44–59) | 48 (40–56) | 45 (38–56) | NS | NS | NS |
| Total cholesterol (mg/dl) | 168 ± 49 | 195 ± 43 | 205 ± 44 | 206 ± 43 | 0.006 | NS | 0.04 |
| Uric acid (mg/dl) | 3.8 ± 1.2 | 4.7 ± 1.79 | 4.8 ± 1.4 | 5.16 ± 1.4 | 0.003 | 0.01 | 0.04 |
| TPO Ab+ number (%) | 9 (45) | 6 (18.2) | 37 (26.4) | 36 (10.5) | <0.0001 | <0.0001 | NS |
| DRB1*03-DQB1*0201 | 7 (35) | 15 (45.4) | 52 (37.1) | 58 (17) | <0.0001 | <0.0001 | NS |
| DRB1*04-DQB1*0302 | 5 (25) | 8 (24.2) | 34 (24.3) | 35 (10.2) | 0.0001 | 0.0001 | NS |
| High-risk HLA genotypes* | 2 (10) | 2 (6) | 12 (8.6) | 4 (1.1) | <0.0001 | 0.0002 | NS |
| Moderate-risk HLA genotypes† | 5 (25) | 8 (24.2) | 33 (23.6) | 38 (11.1) | <0.0001 | 0.0009 | 0.03 |
| Low-risk HLA genotypes‡ | 13 (65) | 21 (63.6) | 95 (67.8) | 300 (87.7) | <0.0001 | 0.0001 | NS |
| ZnT8A titer | 99 (13–191) | 33 (6–110) | 1.2 (1–1.5) | 1.82 (1–2.28) | <0.01 | NS | <0.0001 |
| GADA titer | 89 (20–130) | 90 (25–98) | 14 (8–90) | <0.01§ | |||
| IA-2A titer | 0.12 (0.04–0.25) | 0.18 (0.01–0.31) | −0.04 (−0.08 to 0.16) | −0.001 (−0.01 to 0.08) | <0.01‖ | NS | |
| TPO Ab+ titer | 1,781 (244–3,120) | 2,850 (780–3,250) | 2,000 (600–4,650) | 84 (65–432) | P < 0.01¶ | NS | NS |
Data are means ± SD, median (interquartile range) for antibody titers, or n (%). All P values are for trend.
*High: DRB1*03-DQB1*0201/DRB1*04-DQB1*0302 genotype (DRB1*04 different from 0403, 06, 11).
†Moderate: DRB1*04-DQB1*0302/DRB1*04-DQB1*0302, DRB1*03-DQB1*0201/DRB1*03-DQB1*0201, DRB1*04-DQB1*0302/X, and DRB1*03/X (X different from DRB1*03, DRB1*04-DQB1*0302 [DRB1*04 not 0403, 06, 11], or DQB1*0602/03) genotypes.
‡Low: other genotypes.
§Ptrend for 1 vs. 2 or vs. 3 antibodies.
‖P value for 3 or 2 vs. 1 or vs. none antibodies.
¶Ptrend for trend 3 or 2 or 1 vs. none antibodies. Ab, antibody; NS, not significant.
CONCLUSIONS
This study shows that ZnT8As, recently identified as autoantibodies associated with juvenile-onset type 1 diabetes, are also a marker of adult-onset autoimmune diabetes. Adult diabetes-associated antibodies largely recognized the COOH terminal of the antigen (amino acids 268–369), whereas antibodies against the NH2-terminal moiety (amino acids 1–74) were rare. Therefore, in adult diabetes ZnT8As essentially correspond to ZnT8As-COOH. In the cohort of the NIRAD study, ZnT8As were detected in 18.6% of patients previously identified by GADAs and/or IA-2As; the overall prevalence was similar to that of IA-2As: higher in younger patients and declining with age. In the original report, it was shown that the prevalence of ZnT8As was low in younger individuals but increased dramatically from 3 years onward, peaked at 80% in late adolescence, and tended to decline thereafter (1). The present findings are consistent with the evidence of a further decrease of ZnT8A prevalence by increasing age, being observed in ∼12% of adult patients <50 years of age and becoming very rare after age 60. In patients with adult-onset diabetes previously identified as nonautoimmune based on GADA and IA-2A screening, testing for ZnT8As identified an additional 1.4% of subjects as autoantibody positive. This number represents a marginal increase over the expected 1% of positive subjects based on the threshold adopted in our assay and, if extrapolated to the whole NIRAD cohort, would bring the potential prevalence of autoantibody-positive subjects with adult-onset diabetes up from the original 4.5% (11) to 5.9%. It is debatable, however, whether positivity for a single low-titer islet autoantibody other than GADA is a reliable indicator of autoimmune disease.
To further extend our analysis, we correlated the clinical phenotype to the number of islet autoantibodies, a characteristic that has been demonstrated to reflect the intensity of autoimmune response and predict future insulin insufficiency (5,7,10,14). The availability of ZnT8As as a marker in addition to GADAs and IA-2As allowed stratification across the intensity of islet autoimmune response, which is clearly reflected by the clinical phenotype of patients with adult diabetes, with features of more severe insulin insufficiency proportional to the number and, accordingly, titers of islet autoantibodies. Indeed, when compared with antibody-negative type 2 diabetes, patients with multiple antibodies exhibited characteristics more similar to those of type 1 diabetes, with younger age at disease onset, higher blood glucose and A1C, lower BMI, waist circumference, cholesterol, triglycerides, and uric acid, and higher prevalence of associated TPO antibodies; most of these distinctive traits, although attenuated, were still present in patients with a single autoantibody, indicating that even a single marker of autoimmunity is able to distinguish a degree of insulin insufficiency more severe than that of classic type 2 diabetes.
Unlike GAD and IA-2, ZnT8 is highly β-cell specific; therefore, the presence of ZnT8As in patients from the NIRAD study demonstrates that antigens exclusively expressed in pancreatic β-cells are targets of the autoimmune process in adult-onset diabetes also and supports the usefulness of ZnT8A measurement in adult patients already identified with single GADA positivity to define patients with a more severe and islet-specific autoimmunity.
In summary, ZnT8As are detected in a significant proportion of patients with adult-onset autoimmune diabetes and seem to be a valuable marker to differentiate clinical phenotypes within this patient population.
Acknowledgments
V.L. and Ez.B. have received support from the European Union (FP7-HEALTH-2007, Diabetes Type 1 Prediction, Early Pathogenesis and Prevention N202013 Grant).
This study was sponsored by the Research Foundation of Società Italiana di Diabetologia, funded by an unconditioned grant from Novo Nordisk, Italy. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC: The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007; 104: 17040– 17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL: Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 1983; 222: 1337– 1339 [DOI] [PubMed] [Google Scholar]
- 3. Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P, Camilli PD: Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 1990; 347: 151– 156 [DOI] [PubMed] [Google Scholar]
- 4. Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E: Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J Immunol 1995; 155: 5419– 5426 [PubMed] [Google Scholar]
- 5. Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, Shattock M, Bottazzo GF, Holman R: UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet 1997; 350: 1288– 1293 [DOI] [PubMed] [Google Scholar]
- 6. Tuomi T, Carlsson A, Li H, Isomaa B, Miettinen A, Nilsson A, Nissén M, Ehrnström BO, Forsén B, Snickars B, Lahti K, Forsblom C, Saloranta C, Taskinen MR, Groop LC: Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes 1999; 48: 150– 157 [DOI] [PubMed] [Google Scholar]
- 7. Bottazzo GF, Bosi E, Cull CA, Bonifacio E, Locatelli M, Zimmet P, Mackay IR, Holman RR: IA-2 antibody prevalence and risk assessment of early insulin requirement in subjects presenting with type 2 diabetes (UKPDS 71). Diabetologia 2005; 48: 703– 708 [DOI] [PubMed] [Google Scholar]
- 8. Vardi P, Ziegler AG, Mathews JH, Dib S, Keller RJ, Ricker AT, Wolfsdorf JI, Herskowitz RD, Rabizadeh A, Eisenbarth GS: Concentration of insulin autoantibodies at onset of type I diabetes. Inverse log-linear correlation with age. Diabetes Care 1988; 11: 736– 739 [DOI] [PubMed] [Google Scholar]
- 9. Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, Bingley PJ, Bonifacio E, Palmer JP, Eisenbarth GS, Wolfsdorf J, Skyler JS: the TrialNet Natural History Committee, the Type 1 Diabetes TrialNet Study Group. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009; 10: 97– 104 [DOI] [PubMed] [Google Scholar]
- 10. Krischer JP, Cuthbertson DD, Yu L, Orban T, Maclaren N, Jackson R, Winter WE, Schatz DA, Palmer JP, Eisenbarth GS: Screening strategies for the identification of multiple antibody-positive relatives of individuals with type 1 diabetes. J Clin Endocrinol Metab 2003; 88: 103– 108 [DOI] [PubMed] [Google Scholar]
- 11. Buzzetti R, Di Pietro S, Giaccari A, Petrone A, Locatelli M, Suraci C, Capizzi M, Arpi ML, Bazzigaluppi E, Dotta F, Bosi E: the Non Insulin Requiring Autoimmune Diabetes Study Group. High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care 2007; 30: 932– 938 [DOI] [PubMed] [Google Scholar]
- 12. Petrone A, Suraci C, Capizzi M, Giaccari A, Bosi E, Tiberti C, Cossu E, Pozzilli P, Falorni A, Buzzetti R: the NIRAD Study Group. The protein tyrosine phosphatase nonreceptor 22 (PTPN22) is associated with high GAD antibody titer in latent autoimmune diabetes in adults: Non Insulin Requiring Autoimmune Diabetes (NIRAD) Study 3. Diabetes Care 2008; 31: 534– 538 [DOI] [PubMed] [Google Scholar]
- 13. Buzzetti R, Petrone A, Capizzi M, Bosi E: the Non Insulin Requiring Autoimmune Diabetes (NIRAD) Study Group. High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes: response to Kobayashi et al. Diabetes Care 2007; 30: e127. [DOI] [PubMed] [Google Scholar]
- 14. Achenbach P, Warncke K, Reiter J, Naserke HE, Williams AJ, Bingley PJ, Bonifacio E, Ziegler AG: Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 2004; 53: 384– 392 [DOI] [PubMed] [Google Scholar]