Abstract
OBJECTIVE
Recent studies suggested an impact of prandial insulin delivery on postprandial regulation of tissue blood flow. This study compared the effect of VIAject with human regular insulin and insulin lispro on postprandial oxidative stress and endothelial function in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Fourteen patients (seven men; aged 61.5 ± 1.8 years; duration of diabetes 6.6 ± 4.6 years; A1C 7.2 ± 0.5% [mean ± SEM]) received a prandial injection of VIAject, human regular insulin, and insulin lispro. At baseline and after a standardized liquid meal test (Ensure Plus), the postprandial increases in asymmetric dimethylarginine (ADMA) and nitrotyrosine levels were investigated. In addition, the postprandial effects on microvascular blood flow, skin oxygenation, and vascular elasticity were measured.
RESULTS
Treatment with VIAject resulted in a significant reduction in the peak postprandial generation of ADMA compared with human insulin and insulin lispro (VIAject −27.3 ± 22.6, human insulin 97.7 ± 24.4, and insulin lispro 66.9 ± 33.9 nmol/l; P < 0.05, respectively). The postprandial increases in nitrotyrosine levels were significantly less after VIAject than after human regular insulin (VIAject −0.22 ± 0.17 vs. human insulin 0.25 ± 0.15 μg/ml; P < 0.05), whereas nitrotyrosine after insulin lispro was in between (insulin lispro 0.09 ± 0.07 μg/ml; NS). In parallel, earlier and more pronounced increases in microvascular blood flow and skin oxygenation were obtained after VIAject compared with those after human insulin or insulin lispro (P < 0.05, respectively). All insulin formulations resulted in comparable improvements in central arterial elasticity.
CONCLUSIONS
Treatment with VIAject reduced postprandial oxidative stress and improved endothelial function compared with human regular insulin or insulin lispro.
Type 2 diabetes is closely related to atherosclerosis and the development of cardiovascular complications such as myocardial infarction or stroke. Recent studies on cardiovascular end points in patients with type 2 diabetes call into question the value of A1C-focused treatments in reducing macrovascular complications of diabetes (1–3). Other markers such as glucose excursions, hypoglycemia, or postprandial generation of oxidative stress may add important information for the judgment of cardiovascular risk in patients with type 2 diabetes (1,2). Postprandial microvascular blood flow is under dynamic regulation and is diversely affected by changes in postprandial glucose and insulin levels (4). Increasing postprandial insulin levels stimulate microvascular blood flow by inducing the endothelial release of nitric oxide via the activation of the phosphatidylinositol 3-kinase system (5,6). In contrast, increasing blood glucose levels were shown to oppose the insulin effects on endothelial cells and to impair postprandial microvascular blood flow (7). A reduced first-phase insulin release with an augmented increase in postprandial glucose levels followed by an impairment in endothelial function and postprandial microvascular blood flow is an early feature of type 2 diabetes (4,8). These findings suggest that a physiological timing of prandial insulin release fulfills an important role not only in controlling postprandial blood glucose levels but also in maintaining normal tissue perfusion and nutrition. In addition, recent studies have shown that in insulin-treated patients with type 1 and type 2 diabetes, the pharmacokinetic profile of insulin formulations affects postprandial microvascular blood flow and that treatment with fast-acting insulin analogs reduces postprandial oxidative stress and restores endothelial function more effectively than treatment with human regular insulin (9–11).
VIAject is a new, ultra–fast-acting insulin formulation shown to have more rapid insulin absorption than that for human regular insulin and insulin lispro. The aim of this study was to compare the effect of preprandial subcutaneous administration of insulin VIAject with preprandial application of human regular insulin and insulin lispro on several markers of endothelial and microvascular function after a standardized liquid meal test in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Fifteen patients with type 2 diabetes receiving stable sulfonylurea and/or metformin treatment were recruited for study participation. Patients were excluded if they had been treated with insulin, peroxisome proliferator–activated receptor-γ agonists, glinides, or glucosidase inhibitors within the last 4 weeks before screening. All other concomitant treatment was kept stable during study participation. Additional exclusion criteria were evidence of major micro- or macrovascular complications and impaired cardiovascular, respiratory, hepatic, or renal function. One patient dropped out of the study because of personal time restrictions and did not receive the last insulin treatment. Fourteen patients who received all protocol-specified insulin treatments were included in the analyses.
Study procedure
This open-label study consisted of a three-arm crossover with each treatment administered in random sequence on separate study days. Regular human insulin was given at a dose of 0.10 unit/kg. To minimize risk of hypoglycemia, 90% of this dose was used for the insulin lispro administration, and 75% of the regular human insulin dose was used for VIAject administrations. Because this lower VIAject dose did not result in postprandial glucose control comparable to that with regular human insulin and lispro treatments, all subjects underwent a subsequent VIAject administration at 90% of the regular human dose after the randomized sequence. This dose administration resulted in matched postprandial glucose control and forms the basis for the between-treatment comparisons presented.
For study drug administrations, patients arrived at the study site on the morning after an overnight fast for at least 8 h. An intravenous cannula for blood sampling was inserted into a large antecubital or forearm vein. Subjects remained supine for the duration of the investigations on each investigational day. Human regular insulin was injected 15 min in advance and insulin VIAject or lispro was injected immediately before the intake of a standardized liquid meal (Ensure Plus, containing 56% carbohydrates, 29% fat, and 15% protein). No other food or drinks, with the exception of small amounts of mineral water, were allowed during the investigational period of 4 h. At baseline (before liquid meal intake) and at 10, 20, 30, 60, 120, 180, and 240 min after the liquid meal, blood was taken for the measurement of asymmetric dimethylarginine (ADMA), blood glucose, and insulin. Because of time limitations, the microvascular blood flow (laser Doppler flux [LDF]), tissue oxygenation (So2), the arterial elasticity index (augmentation index [Aix]), and nitrotyrosine measurements were only taken at baseline and at 30, 60, 120, 180, and 240 min thereafter.
Skin blood flow and oxygen saturation measurement
The technique of simultaneous micro-lightguide spectrophotometry and laser Doppler fluxmetry was used to measure microvascular skin blood flow and Hb oxygenation at the lower forearm (O2C; LEA Medizintechnik, Giessen, Germany). A skin probe (LF 2; LEA Medizintechnik) was placed on the thenar surface of the left hand in between the phalanx of the thumb and the metatarsal of the forefinger, directly adjacent to the abductor pollicis muscle. LDF and tissue oxygen tension measurements (So2) were performed at the time points given above. As shown in a previous study, this technique allows LDF measurements with a coefficient of variation of <10% and oxygen saturation measurements with a coefficient of variation of <20% (12).
Measurement of central arterial elasticity
Central arterial elasticity was measured by the technique of applanation tonometry using a highly sensitive transducer (Sphygmo Cor; AtCor Medical, West Ryde, Australia). The central arterial waveform was derived from 20 sequential waveform records obtained from the peripheral radial artery using a validated transfer function (13). Only high-quality recordings, defined as an in-device quality index with >80% reproducibility, were included in the analysis. The augmentation pressure was obtained from the difference between the second and the first systolic shoulder of the central pressure wave curve, and the Aix was calculated as the percentage of augmentation pressure from total pulse pressure.
Laboratory measurements
All laboratory measurements were analyzed at the Institute for Clinical Research and Development (ikfe, Mainz, Germany). Blood samples were centrifuged and kept at −20°C until final analysis. Plasma glucose concentrations were determined by the glucose dehydrogenase method (Super GL; RLT, Möhnesee-Delecke, Germany). ADMA was determined by an enzyme-linked immunosorbent assay according to the manufacturer's guidelines (ADMA ELISA; Immundiagnostik, Bensheim, Germany). Insulin and nitrotyrosine were measured by chemoluminescence assays (insulin: Invitron, Monmouth, U.K.; nitrotyrosine: Upstate Biotechnology, Charlottesville, VA). Insulin lispro was measured using a radioimmunoassay (Linco Laboratories, St. Charles, MO). A1C was measured by high-performance liquid chromatography (Menarini Diagnostics, Neuss, Germany).
Statistical analysis
ADMA was chosen as the primary end point because a previous study had demonstrated a significant impact of fast-acting analogs on the generation of postprandial ADMA levels in patients with type 2 diabetes (10). A sample size of 14 patients was calculated to provide 80% power, assuming a comparable effect on the postprandial excursion of plasma ADMA levels and considering a two-sided test with a significance level of 5%.
This study was designed as a pilot study, without confirmatory sample size consideration. All measurements are presented as means ± SEM. Statistical comparison between fasting and postprandial values and between groups was performed using the Student t test (paired and unpaired as appropriate); P < 0.05 (two-tailed) was considered statistically significant. Spearman correlation coefficients were calculated for each pair of variables. Peak postprandial ADMA and nitrotyrosine response was defined as the maximum increase from baseline within 120 min after the uptake of the liquid meal.
RESULTS
Seven female and seven male patients with type 2 diabetes were included in the per-protocol analysis of the study. Mean ± SD age was 61.5 ± 6.7 years, duration of diabetes was 6. 6 ± 4.6 years, A1C was 7.2 ± 0.5%, and BMI was 31.0 ± 3.4 kg/m2.
As shown in Table 1, absolute plasma glucose levels and the time course of plasma glucose levels were comparable during all three kinds of insulin treatment. Because the active ingredient in VIAject is recombinant human regular insulin, human regular insulin and VIAject insulin were measured using the same insulin assay, whereas insulin lispro was measured with a lispro-specific assay, minimizing detection of endogenous insulin. Comparing VIAject with human insulin, plasma insulin levels showed a steeper increase within the first 60 min and a faster decrease from 60 min onwards. Because of the different assays used, quantitative comparisons among the insulin levels after insulin lispro and the human insulin formulations are not useful.
Table 1.
Change from baseline in glucose, insulin, and ADMA at 10, 20, 30, 60, 120, 180, and 240 min postprandial
| Treatment | Time (min) | Glucose (mg/dl) | Insulin (μU/ml) | ADMA (nmol/l) |
|---|---|---|---|---|
| HI | 10 | 5.4 ± 2.5 | 13.7 ± 3.6 | −3.3 ± 21.9 |
| LI | 10 | 6.4 ± 4.7 | 6.8 ± 4.2 | −9.1 ± 18.8 |
| VJ | 10 | 21.1 ± 4.9*† | 20.5 ± 3.2 | 9.3 ± 30.3 |
| HI | 20 | 19.7 ± 5.1 | 26.1 ± 5.2 | 97.7 ± 24.4 |
| LI | 20 | 27.2 ± 9.4 | 17.1 ± 6.0 | 66.9 ± 33.9 |
| VJ | 20 | 35.6 ± 4.7 | 42.7 ± 5.9* | −27.3 ± 22.6*† |
| HI | 30 | 33.6 ± 6.4 | 34.1 ± 5.7 | 27.1 ± 29.3 |
| LI | 30 | 39.9 ± 10.5 | 21.8 ± 6.3 | −2.0 ± 35.8 |
| VJ | 30 | 42.4 ± 7.3 | 45.1 ± 4.6 | 35.9 ± 37.6 |
| HI | 60 | 60.8 ± 12.8 | 37.9 ± 6.1 | 14.1 ± 28.4 |
| LI | 60 | 49.9 ± 7.4 | 24.8 ± 6.2 | 8.6 ± 38.3 |
| VJ | 60 | 52.4 ± 10.2 | 47.2 ± 6.9 | 43.4 ± 37.1 |
| HI | 120 | 34.6 ± 9.3 | 32.6 ± 2.8 | 3.1 ± 26.1 |
| LI | 120 | 31.2 ± 11.6 | 16.8 ± 3.9 | −27.2 ± 34.4 |
| VJ | 120 | 19.5 ± 8.8 | 31.3 ± 3.7 | −28.8 ± 33.9 |
| HI | 180 | −7.3 ± 9.1 | 17.8 ± 2.4 | −18.2 ± 33.9 |
| LI | 180 | −19.5 ± 15.3 | 1.4 ± 1.9 | −46.2 ± 27.8 |
| VJ | 180 | −7.1 ± 9.7 | 16.0 ± 2.7 | −9.9 ± 24.6 |
| HI | 240 | −37.9 ± 7.0 | 9.2 ± 1.3 | 48.2 ± 40.7 |
| LI | 240 | −46.1 ± 10.8 | −4.5 ± 0.9 | −11.3 ± 33.7 |
| VJ | 240 | −3.9 ± 6.8 | 4.8 ± 1.2* | −94.3 ± 25.2* |
Data are means ±SEM.
*P < 0.05 vs. human regular insulin.
†P < 0.05 vs. insulin lispro. (Because they are measured with different assays, lispro vs. human insulin comparisons are not presented). HI, human insulin; LI, insulin lispro; VJ, VIAject.
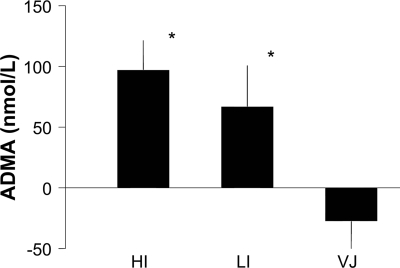
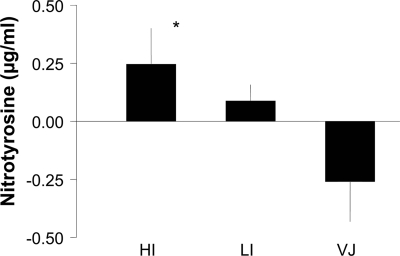
Postprandial plasma ADMA levels increased during treatment with human insulin and during treatment with insulin lispro (Table 1). The maximal increase in plasma ADMA levels was found 20 min after the meal during human insulin and insulin lispro treatment, whereas no postprandial increase in ADMA levels was observed during VIAject treatment. Figure 1 shows the peak postprandial ADMA response (20 min postprandial) according to the three treatment conditions. The maximal change in postprandial ADMA levels was significantly lower when VIAject was compared with human regular insulin or insulin lispro. As shown in Table 2, postprandial nitrotyrosine levels increased during treatment with human regular insulin and to a lesser extent with insulin lispro. The maximal increase in plasma nitrotyrosine levels was found 30 min after the meal during human insulin and during insulin lispro treatment, whereas no postprandial increase in nitrotyrosine levels was observed during VIAject treatment. Figure 2 shows the peak postprandial nitrotyrosine response (30 min postprandial) according to the three treatment conditions. The maximal increase in postprandial nitrotyrosine levels was significantly lower when VIAject was compared with human insulin but not with insulin lispro. In contrast, microvascular blood flow consistently increased during the first 60 min after the injection of VIAject (Table 2). A significantly larger increase in microvascular blood flow was found after VIAject compared with that after human regular insulin or insulin lispro during the first 60 min after injection. In parallel with microvascular skin blood flow, skin oxygenation temporarily declined after the injection of human regular insulin and insulin lispro, whereas a consistent increase in skin oxygenation could be observed after VIAject during 60 min after the test meal. Changes in skin microvascular blood flow were correlated with changes in skin oxygenation (r = 0.58, P < 0.0001).
Figure 1.
Peak postprandial change in plasma ADMA levels. *P < 0.05 vs. VIAject. HI, human insulin; LI, insulin lispro; VJ, VIAject.
Table 2.
Change from baseline in nitrotyrosine, skin blood flow (LDF), skin oxygen tension (So2), and pulse wave index (Aix) at 30, 60, 120, 180, and 240 min postprandial
| Treatment | Time (min) | Nitrotyrosine (μg/ml) | LDF (AU) | So2 (%) | Aix (%) |
|---|---|---|---|---|---|
| HI | 30 | 0.25 ± 0.15 | −2.2 ± 2.5 | −2.1 ± 1.1 | −3.9 ± 1.4 |
| LI | 30 | 0.09 ± 0.07 | −5.0 ± 4.5 | −2.4 ± 1.6 | −1.1 ± 1.6 |
| VJ | 30 | −0.26 ± 0.17* | 4.1 ± 2.4*† | 2.5 ± 2.3*† | −3.0 ± 2.2 |
| HI | 60 | 0.22 ± 0.17 | 1.2 ± 3.1 | −1.9 ± 1.6 | −5.0 ± 1.1 |
| LI | 60 | −0.05 ± 0.16 | 1.9 ± 7.6 | −2.5 ± 1.9 | −3.5 ± 1.4 |
| VJ | 60 | −0.03 ± 0.20 | 7.1 ± 5.7 | 2.7 ± 2.4*† | −3.5 ± 1.8 |
| HI | 120 | 0.22 ± 0.23 | 5.4 ± 2.2 | 0.4 ± 1.2 | −2.6 ± 1.8 |
| LI | 120 | 0.16 ± 0.18 | 0.5 ± 3.5 | 1.4 ± 1.7 | −0.3 ± 1.7 |
| VJ | 120 | 0.00 ± 0.22 | 3.1 ± 5.0 | 0.6 ± 2.4 | −2.8 ± 1.5 |
| HI | 180 | 0.06 ± −0.24 | 4.8 ± 2.9 | 0.8 ± 1.5 | −0.9 ± 1.3 |
| LI | 180 | −0.06 ± 0.22 | −1.2 ± 7.3 | −0.6 ± 2.1 | 2.6 ± 1.7 |
| VJ | 180 | −0.16 ± 0.17 | 4.5 ± 7.6 | −0.8 ± 2.2 | 1.6 ± 2.4 |
| HI | 240 | 0.16 ± 0.24 | 1.1 ± 3.3 | 1.2 ± 1.2 | −0.4 ± 1.2 |
| LI | 240 | −0.07 ± 0.23 | 1.4 ± 6.7 | −1.2 ± 1.4 | 1.1 ± 1.2 |
| VJ | 240 | −0.26 ± 0.18 | 9.1 ± 9.4 | −0.8 ± 1.8 | 0.8 ± 2.2 |
Data are means ± SEM.
*P < 0.05 vs. human regular insulin.
†P < 0.05 vs. insulin lispro. AU, arbitrary units; HI, human insulin; LI, insulin lispro; VJ, VIAject.
Figure 2.
Peak postprandial change in plasma nitrotyrosine levels *P < 0.05 vs. VIAject. HI, human insulin; LI, insulin lispro; VJ, VIAject.
All insulin formulations improved arterial elasticity as given by a decrease in the Aix with no differences observed among the insulin formulations.
CONCLUSIONS
Type 2 diabetes is associated with an increase in micro- and macrovascular complications. There is increasing evidence that postprandial metabolism including the generation of postprandial oxidative stress might predict vascular risk to a greater extent than A1C or fasting glucose values (14,15). Besides its role in the regulation of glucose metabolism, insulin has been shown to mediate important vascular effects by stimulating the endothelial secretion of nitric oxide (5,16). Recent studies suggest that the pharmacokinetic profile of subcutaneous insulin absorption might have an impact on the generation of postprandial oxidative stress and the development of endothelial dysfunction (9–11,17). Insulin may directly affect the generation or degradation of ADMA, and insulin resistance is associated with increased ADMA levels (18,19). ADMA has been identified as the major endogenous inhibitor of the physiological nitric oxide synthase system (20). Elevated ADMA levels cause endothelial nitric oxide synthase uncoupling, a mechanism that leads to decreased nitric oxide availability. A study by Fard et al. (21) showed that plasma levels of ADMA were accentuated after high-fat meals. This was accompanied by a decline in endothelial function, as indicated by a reduction in the flow-mediated vasodilation of the brachial artery. In a recent study, we were able to demonstrate that the time course of subcutaneous insulin absorption has an impact on the generation of postprandial ADMA levels, supporting the hypothesis that postprandial metabolism significantly influences atherogenic potency in patients with type 2 diabetes (10).
VIAject is a newly developed human insulin formulation that has been shown to provide more rapid subcutaneous absorption than human regular insulin and fast-acting insulin analogs in lean nondiabetic subjects (22). In agreement with this observation, our investigation confirmed more rapid absorption of subcutaneously administered VIAject compared with that of human regular insulin or insulin lispro in patients with type 2 diabetes. Non–insulin-treated patients with type 2 diabetes received a single dose of each insulin formulation, which was calculated according to the patient's body weight and not adjusted for postprandial glucose excursions. Even though postprandial glucose excursions were well matched between the regular human insulin and 90% lispro and VIAject treatments, the treatments could be differentiated in that VIAject treatments were associated with a reduction in postprandial ADMA levels and a diminished generation of oxidative stress.
In addition to the laboratory markers for postprandial endothelial function and oxidative stress, our study revealed that VIAject increases microvascular blood flow in the skin comparable to the postprandial increase in microvascular blood flow in nondiabetic control subjects (4). In accordance with the increase in microvascular skin blood flow, postprandial skin oxygenation increased after the injection of VIAject.
Systemic arterial stiffness or reduced compliance is an independent predictor of coronary artery disease and cardiovascular mortality. In a recent study, an increase in arterial compliance was observed after the intake of a carbohydrate-rich meal, which was predicted by the magnitude of glucose and insulin response (23). Euglycemic clamp studies revealed that insulin rather than glucose is the main determinant of arterial stiffness in the postprandial state (24), and the kinetics of the postprandial insulin release might have an impact on the modulation of arterial stiffness. In contrast to the more distinct effects on microvascular blood flow in skin, all insulin formulations in our study improved vascular elasticity comparably. Although insulin has proven to exert its vasodilatory effect on peripheral resistance vessels by activation of phosphatidylinositol 3-kinase and subsequent nitric oxide release from the endothelial cell, insulin effects on large arteries and the mechanism by which insulin modulates arterial elasticity is less clear (23,25). The distinct vascular effects of insulin observed in microvascular blood flow and in central arterial compartments observed in our study imply that there are differential effects on microvascular circulation and central arteries.
In summary, our study confirms important implications of prandial insulin kinetics for the regulation of endothelial integrity and microvascular function in patients with type 2 diabetes. The ultra-rapid absorption of VIAject insulin was associated with a reduction in postprandial oxidative stress and improvements in microvascular function after a liquid test meal more so than that after human regular insulin or a fast-acting insulin analog.
This study has important limitations. It was an exploratory study to evaluate the vascular effects of the new ultra-rapid insulin VIAject and needs to be confirmed by larger confirmatory studies. It is not known whether single-dose administration reflects the effects that would be seen with chronic dosing. Further studies with larger patient populations and longer observational periods are necessary to ascertain the clinical impact of the results obtained in this short-term mechanistic study. The study did not include a healthy control group. Therefore, no conclusion can be made as to what degree the insulin treatments were able to normalize postprandial oxidative stress and endothelial function in type 2 diabetic patients.
Acknowledgments
This study was supported by Biodel, Danbury, Connecticut. T.F. and A.P. received unrestricted research funds from Biodel and are members of the scientific advisory board and consultants for Biodel. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
Footnotes
Clinical trial registry no. NCT00849576, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545– 2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560– 2572 [DOI] [PubMed] [Google Scholar]
- 3. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD: the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129– 139 [DOI] [PubMed] [Google Scholar]
- 4. Forst T, Kunt T, Pohlmann T, Goitom K, Löbig M, Engelbach M, Beyer J, Pfutzner A: Microvascular skin blood flow following the ingestion of 75g glucose in healthy individuals. Exp Clin Endocrinol Diabetes 1998; 106: 454– 459 [DOI] [PubMed] [Google Scholar]
- 5. Forst T, Hohberg C, Pfützner A: Cardiovascular effects of disturbed insulin activity in metabolic syndrome and in type 2 diabetic patients. Horm Metab Res 2009; 41: 123– 131 [DOI] [PubMed] [Google Scholar]
- 6. Liu Z: Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab 2007; 293: E1250– E1255 [DOI] [PubMed] [Google Scholar]
- 7. Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA: Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 1998; 97: 1695– 1701 [DOI] [PubMed] [Google Scholar]
- 8. Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Esposito K, Giugliano D: Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes 2004; 53: 701– 710 [DOI] [PubMed] [Google Scholar]
- 9. Forst T, Pohlmann T, Kazda Ch, Welter K, Langer F, Forst S, Pfutzner A: The impact of insulin lispro and regular insulin on postprandial endothelial function and microvascular blood flow in type 1 diabetic patients. Diabetes 2003; 51( Suppl. 2): A292 [Google Scholar]
- 10. Hohberg C, Forst T, Larbig M, Safinowski M, Diessel S, Hehenwarter S, Weber MM, Schöndorf T, Pfützner A: Effect of insulin glulisine on microvascular blood flow and endothelial function in the postprandial state. Diabetes Care 2008; 31: 1021– 1025 [DOI] [PubMed] [Google Scholar]
- 11. Ceriello A, Cavarape A, Martinelli L, Da Ros R, Marra G, Quagliaro L, Piconi L, Assaloni R, Motz E: The post-prandial state in type 2 diabetes and endothelial dysfunction: effects of insulin aspart. Diabet Med 2004; 21: 171– 175 [DOI] [PubMed] [Google Scholar]
- 12. Forst T, Hohberg C, Tarakci E, Forst S, Kann P, Pfützner A: Reliability of lightguide spectrophotometry (O2C) for the investigation of skin tissue microvascular blood flow and tissue oxygen supply in diabetic and non-diabetic subjects. J Diabetes Sci Technol 2008; 2: 1151– 1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilhelm B, Klein J, Friedrich Ch, Forst S, Pfützner A, Kann P, Weber M, Forst T: Increased arterial augmentation and augmentation index as surrogate parameters for arteriosclerosis in subjects with diabetes mellitus and non-diabetic subjects with cardiovascular disease. J Diabetes Sci Technol 2007; 2: 260– 263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C: Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681– 1687 [DOI] [PubMed] [Google Scholar]
- 15. Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, Anfossi G, Costa G, Trovati M: Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006; 91: 813– 819 [DOI] [PubMed] [Google Scholar]
- 16. Montagnani M, Quon MJ: Insulin action in vascular endothelium: potential mechanisms linking insulin resistance with hypertension. Diabetes Obes Metab 2000; 2: 285– 292 [DOI] [PubMed] [Google Scholar]
- 17. Scognamiglio R, Negut C, de Kreutzenberg SV, Tiengo A, Avogaro A: Effects of different insulin regimes on postprandial myocardial perfusion defects in type 2 diabetic patients. Diabetes Care 2006; 29: 95– 100 [PubMed] [Google Scholar]
- 18. Marcovecchio ML, Widmer B, Dunger DB, Dalton RN: Effect of acute variations of insulin and glucose on plasma concentrations of asymmetric dimethylarginine in young people with type 1 diabetes. Clin Sci (Lond) 2008; 115: 361– 369 [DOI] [PubMed] [Google Scholar]
- 19. Pereira EC, Ferderbar S, Bertolami MC, Faludi AA, Monte O, Xavier HT, Pereira TV, Abdalla DS: Biomarkers of oxidative stress and endothelial dysfunction in glucose intolerance and diabetes mellitus. Clin Biochem 2008; 41: 1454– 1460 [DOI] [PubMed] [Google Scholar]
- 20. Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, Imaizumi T: Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation 1999; 99: 1141– 1146 [DOI] [PubMed] [Google Scholar]
- 21. Fard A, Tuck CH, Donis JA, Sciacca R, Di Tullio MR, Wu HD, Bryant TA, Chen NT, Torres-Tamayo M, Ramasamy R, Berglund L, Ginsberg HN, Homma S, Cannon PJ: Acute elevations of plasma asymmetric dimethylarginine and impaired endothelial function in response to a high-fat meal in patients with type 2 diabetes. Arterioscler Thromb Vasc Biol 2000; 20: 2039– 2044 [DOI] [PubMed] [Google Scholar]
- 22. Steiner S, Hompesch M, Pohl R, Simms P, Flacke F, Mohr T, Pfützner A, Heinemann L: A novel insulin formulation with a more rapid onset of action. Diabetologia 2008; 51: 1602– 1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenfield JR, Samaras K, Chisholm DJ, Campbell LV: Effect of postprandial insulinemia and insulin resistance on measurement of arterial stiffness (augmentation index). Int J Cardiol 2007; 114: 50– 56 [DOI] [PubMed] [Google Scholar]
- 24. Wilhelm B, Weber MM, Kreisselmeier HP, Kugler M, Ries C, Pfützner A, Kann P, Forst T: Endothelial function and arterial stiffness in uncomplicated type 1 diabetes and healthy controls and the impact of insulin on these parameters during an euglycaemic clamp. J Diabetes Sci Technol 2007; 582– 589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westerbacka J, Seppälä-Lindroos A, Yki-Järvinen H: Resistance to acute insulin induced decreases in large artery stiffness accompanies the insulin resistance syndrome. J Clin Endocrinol Metab 2001; 86: 5262– 5268 [DOI] [PubMed] [Google Scholar]