Abstract
OBJECTIVE
To determine whether continuous glucose monitoring (CGM) is effective in the management of type 1 diabetes when implemented in a manner that more closely approximates clinical practice.
RESEARCH DESIGN AND METHODS
After completion of a 6-month randomized controlled trial (RCT) evaluating CGM in children, adolescents, and adults with type 1 diabetes, CGM was initiated in the trial's control group with less intensive training and follow-up than was included in the RCT. Subjects had an outpatient training session, two follow-up phone calls, and outpatient visits at 1, 4, 13, and 26 weeks. For subjects with baseline A1C ≥7.0%, the primary outcome was change in A1C at 6 months.
RESULTS
CGM use decreased from a median of 7.0 days/week in the first month in the ≥25-year-old group, 6.3 days/week in the 15–24 year olds, and 6.8 days/week in the 8–14 year olds to 6.5, 3.3, and 3.7 days/week in the 6th month, respectively (P < 0.001 for each age-group). Among subjects with baseline A1C ≥7.0%, CGM use was associated with A1C reduction after 6 months (P = 0.02 adjusted for age-group). Severe hypoglycemia decreased from 27.7 events per 100 person-years in the 6-month control phase of the RCT to 15.0 events per 100 person-years in the 6-month follow-up CGM phase (P = 0.08).
CONCLUSIONS
Frequent use of CGM in a clinical care setting may improve A1C and reduce episodes of hypoglycemia. However, sustained frequent use of CGM is less likely in children and adolescents than in adults.
Many patients with type 1 diabetes treated with intensive insulin regimens are unable to achieve and maintain recommended A1C targets (1). Even with the availability of insulin pumps and short- and long-acting insulin analogs, patients with type 1 diabetes are frequently exposed to both excessive hyperglycemia and prolonged, dangerous hypoglycemia, particularly at night (2,3). The recent availability of real-time continuous glucose monitoring (CGM) devices has the potential to help users improve diabetes control by reducing both hyperglycemic and hypoglycemic exposures.
We conducted a 6-month randomized trial of CGM plus conventional blood glucose monitoring versus blood glucose monitoring alone in 322 intensively treated subjects with type 1 diabetes and baseline A1C ≥7.0% and demonstrated a significant reduction in A1C in the adult subjects (aged ≥25 years) (4). Although the pediatric (aged 8–14 years) and young adult (aged 15–24 years) groups did not on average show a significant drop in A1C levels, those individuals who used CGM at least 6 days a week had a reduction in A1C similar to the 0.5% mean reduction in the adults (5). In a parallel study of 129 subjects with type 1 diabetes and baseline A1C level <7.0%, CGM was shown to be effective in reducing biochemical hypoglycemia while maintaining A1C levels in the goal range (6).
Although these studies demonstrate efficacy of CGM in the controlled environment of a randomized trial with intensive subject monitoring and supervision, the generalizability of these findings to subjects receiving diabetes care in a clinical practice setting, in which clinician visits may be less frequent and of shorter duration than can be accomplished in a more rigorous randomized controlled trial (RCT), is arguably somewhat limited. Consequently, we included a 6-month single-arm crossover extension to our randomized trial, to examine whether the introduction of CGM in a manner that more closely approximates a clinical practice, would be associated with improvement in glycemic control. This report describes the results of the experience of the original control subjects after 6 months of CGM use initiated by a so-called “standard care management” approach.
RESEARCH DESIGN AND METHODS
The protocol was described in detail previously (4,6,7). Major eligibility criteria for the randomized trial included age ≥8 years, type 1 diabetes for at least 1 year, use of either an insulin pump or at least three daily insulin injections, and A1C level ≤10.0%. Subjects were randomly assigned to either a CGM group or a control group that used standard home blood glucose monitoring. This report includes 214 of the 219 subjects in the randomized trial control group who initiated CGM use for a 6-month period after completion of the randomized trial. Four subjects did not complete the randomized trial, and one subject decided not to continue after completing the trial.
During the first 6 months of the randomized trial, the control group had six follow-up visits with a phone call between each visit to mirror the schedule in the CGM group and received written instructions on how to use blood glucose meter data to make real-time insulin dose adjustments and on using computer software (for those with a home computer) to retrospectively review the glucose data to alter future insulin dosing (7,8). After completion of the 6-month outcome of the randomized trial, each control group subject was provided with one of the following CGM devices: the DexCom SEVEN (DexCom, San Diego, CA), the MiniMed Paradigm REAL-Time Insulin Pump and Continuous Glucose Monitoring System (Medtronic MiniMed, Northridge, CA), or the FreeStyle Navigator (Abbott Diabetes Care, Alameda, CA). The CGM initially was used in a blinded fashion for 1 week; these data served as a baseline for evaluating change during follow-up. After completion of the blinded use, subjects were instructed to use the device on a daily basis and to verify the accuracy of glucose measurements with a home blood glucose meter (provided by the study) before making management decisions (as per the regulatory labeling of the devices). Target glucose values were premeal 70 to 130 mg/dl, peak postmeal <180 mg/dl, and bedtime/overnight 100–150 mg/dl. Instructions for insulin dosing included determination of premeal bolus doses based on the glucose level, the carbohydrate content of the upcoming meal, rate and direction of glucose change, and guidelines for correcting glucose levels outside the target range at other times.
Follow-up visits during the clinical care CGM phase occurred after 1, 4, 13, and 26 weeks, with phone contacts 3 days after CGM initiation and 7 days after the 1-week visit. At each visit, A1C was measured with a point-of-care device (DCA 2000 at seven sites, G7-Tosoh at two sites, and Bio-Rad at one site), and at baseline and 6 months a central laboratory–measured A1C level was obtained at the University of Minnesota using the Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer method (9).
Statistical methods
Baseline was considered the visit when real-time CGM was initiated. The final outcome was 6 months later. From the downloads of the CGM devices performed at each visit, the amount of CGM use was determined. CGM was considered to be used on a day when there was at least one glucose value; on 83% of days with at least one glucose value, there were at least 12 h of glucose values. Change in CGM use over the 6-month period was assessed with a repeated-measures regression model based on rank values transformed to have a normal distribution (van der Waerden scores). van der Waerden scores were also used in the analysis of sensor glucose data because of skewed distributions. The association of CGM use ≥6 days/week during month 6 with baseline factors and with CGM use during the 1st month was assessed in logistic regression models. Analysis of A1C was limited to subjects with a value ≥7.0% at baseline. Change in A1C from baseline to 6 months was evaluated with a paired t test. The association between change in A1C and CGM use in month 6 was assessed with least squares regression models adjusting for baseline A1C. The incidence of severe hypoglycemia (defined as an event that required assistance from another person to administer carbohydrate, glucagon, or other resuscitative actions [10]) during the 6-month randomized trial (as the control group) versus the incidence during the subsequent CGM use phase was compared using a signed-rank test. The association of severe hypoglycemic events with baseline A1C was assessed with a Spearman correlation coefficient. CGM glucose data during the 6th month were compared with blinded CGM data obtained immediately before the initiation of unblinded CGM use using a signed-rank test. Changes in glucose variability from baseline to month 6 were evaluated with and without adjustment for mean glucose using a repeated-measures regression model based on the rank scores.
Analyses included only subjects completing the 6-month visit. A1C results are from the central laboratory unless otherwise stated.
RESULTS
The 214 subjects included 80 who were aged ≥25 years at the time of initiation of CGM use, 73 who were 15–24 years old and 61 who were 8–14 years old; 199 (93%) were Caucasian, 3 (1%) were African American, 5 (2%) were Hispanic, and 7 (3%) were other races. Mean ± SD A1C levels at the time of initiation of CGM use were 7.4 ± 0.7% (range 5.8–10.1%), with 156 (73%) ≥7.0% and 58 (27%) <7.0%. An insulin pump was being used by 171 (80%), the others being treated with multiple daily injections of insulin. The study was completed by 212 (99%) of the 214 subjects.
CGM use
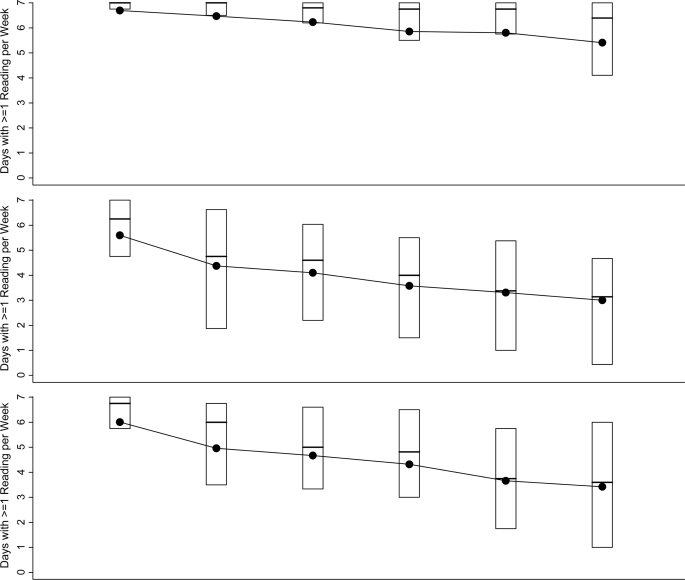
As seen in Fig. 1, CGM use tended to decrease over time (P < 0.001) in all age-groups, although less so in the adults. In the 1st month, median use was 7.0 days/week in the ≥25 year olds, 6.3 days/week in the 15–24 year olds, and 6.8 days/week in the 8–14 year olds, which decreased in month 6 to 6.5, 3.3, and 3.7 days/week, respectively. In month 6, the percentage of ≥25 year olds using CGM at least 6 days per week was significantly greater than the percentages in the two younger age-groups (64, 19, and 25%, respectively, P < 0.001). Four (5%), 15 (21%), and 12 (20%) subjects in the three age-groups, respectively, were no longer using CGM in month 6.
Figure 1.
CGM use over 6 months according to age-group. Box plots indicating sensor use in each 4-week time period for the 212 subjects who completed the study (two subjects who did not complete the study are not included) are shown. The top and bottom of the boxes denote the 25th and 75th percentiles, the line represents the median, and the dot represents the mean. The numbers of subjects who did not use CGM during a 4-week period are indicated below each box plot (these data are included in each box plot).
The only factor other than age that was significantly associated with lower CGM use in month 6 was the occurrence of a severe hypoglycemic event during the preceding 6 months (as the control group in the randomized trial; P = 0.008 adjusted for age) (Supplementary Table 1, available at http://care.diabetesjournals.org/cgi/content/full/dc09-1502/DC1). Of the 10% of patients who experienced a severe hypoglycemic event in the previous 6 months, only 14% used CGM >6 days at the end compared with 40% of those who did not have a severe hypoglycemic event. CGM use in month 6 did not vary with the baseline A1C level (P = 0.53 adjusted for age). There was a trend toward CGM use being higher in pump users than in multiple daily injection users (41 vs. 23%, P = 0.07 adjusted for age).
Sensor use in month 6 was strongly associated with use in the first 4 weeks (P < 0.001 adjusted for age) (Supplementary Table 2, available in an online appendix). Sensor use was ≥6 days/week in month 6 in 69 (57%) of 122 subjects who used the sensor at least 27 of 28 days in the first 4 weeks, in 8 (25%) of the 32 subjects who used the sensor 24–26 days, and in 2 (3%) of the 58 subjects who used the sensor <24 days. A similar association was seen in all three age-groups (Supplementary Table 3, available in an online appendix).
A1C outcome
As shown in Table 1, among the 154 subjects with baseline A1C ≥7.0% who completed the 6-month visit, change in A1C from baseline to 6 months varied with age-group (P = 0.002). There was a significant decrease in ≥25 year olds (n = 51, mean ± SD change −0.4 ± 0.5%, P < 0.001) but not in the 15–24 year olds (n = 56, +0.01 ± 0.7%, P = 0.95) or in the eight to 14 year olds (n = 47, +0.02 ± 0.7%, P = 0.85). This association of change in A1C and age-group was related to the amount of CGM use. Greater CGM use was associated with a greater A1C decrease (P = 0.01 adjusted for age-group) (Table 1), and after adjustment for CGM use, the relationship between age-group and change in A1C was weaker (P = 0.07). In subjects with baseline A1C <7.0%, mean A1C was 6.6% at baseline and 6.8% at 6 months
Table 1.
Change in A1C from baseline* to month 6 by amount of CGM use in month 6 in subjects with A1C ≥7.0% at the time of initiation of CGM
| All | CGM use in month 6 |
||||
|---|---|---|---|---|---|
| 0 days/week | >0–<4 days/week | 4–<6 days/week | ≥6 days/week | ||
| Overall | |||||
| n | 154 | 26 | 45 | 23 | 60 |
| Baseline A1C (%)† | 7.8 | 8.0 | 7.7 | 7.8 | 7.6 |
| Change in A1C from baseline to month 6 | |||||
| Mean change (%) | −0.1 ± 0.6 | +0.2 ± 0.9 | 0.0 ± 0.6 | −0.4 ± 0.7 | −0.2 ± 0.4 |
| Improved ≥0.5% | 49 (32) | 8 (31) | 8 (18) | 13 (57) | 20 (33) |
| Worsened ≥0.5% | 27 (18) | 8 (31) | 13 (29) | 2 (9) | 4 (7) |
| A1C <7.0% | 29 (19) | 2 (8) | 5 (11) | 9 (39) | 13 (22) |
| Mean change from 0–6 months in prior RCT | 0.0 ± 0.6 | −0.1 ± 0.5 | +0.1 ± 0.6 | 0.0 ± 0.7 | +0.1 ± 0.5 |
| Age-group ≥25 years | |||||
| n | 51 | 4 | 4 | 6 | 37 |
| Baseline A1C (%) | 7.6 | 8.0 | 7.6 | 7.5 | 7.6 |
| Change in A1C from baseline to month 6 | |||||
| Mean change | −0.4 ± 0.5 | +0.1 ± 0.9 | −0.4 ± 0.7 | −0.5 ± 0.3 | −0.4 ± 0.4 |
| Improved ≥0.5% | 23 (45) | 1 (25) | 2 (50) | 4 (67) | 16 (43) |
| Worsened ≥0.5% | 3 (6) | 1 (25) | 1 (25) | 0 | 1 (3) |
| A1C <7.0% | 15 (29) | 0 | 2 (50) | 3 (50) | 10 (27) |
| Mean change from 0–6 months in prior RCT | +0.2 ± 0.5 | +0.4 ± 0.5 | +0.3 ± 0.6 | +0.3 ± 0.5 | +0.1 ± 0.4 |
| Age-group 15–24 years | |||||
| n | 56 | 11 | 26 | 7 | 12 |
| Baseline A1C (%) | 7.9 | 8.1 | 7.9 | 8.1 | 7.7 |
| Change in A1C from baseline to month 6 | |||||
| Mean change (%) | 0.0 ± 0.7 | +0.4 ± 1.2 | 0.0 ± 0.5 | −0.6 ± 0.3 | 0.0 ± 0.3 |
| Improved ≥0.5% | 14 (25) | 4 (36) | 4 (15) | 5 (71) | 1 (8) |
| Worsened ≥0.5% | 10 (18) | 4 (36) | 5 (19) | 0 | 1 (8) |
| A1C <7.0% | 6 (11) | 0 | 2 (8) | 3 (43) | 1 (8) |
| Mean change from 0–6 months in prior RCT (%) | +0.1 ± 0.7 | −0.1 ± 0.5 | +0.1 ± 0.6 | −0.1 ± 0.8 | +0.2 ± 0.7 |
| Age-group 8–14 years | |||||
| n | 47 | 11 | 15 | 10 | 11 |
| Baseline A1C (%) | 7.8 | 7.8 | 7.6 | 7.9 | 7.8 |
| Change in A1C from baseline to month 6 | |||||
| Mean change (%) | 0.0 ± 0.7 | −0.1 ± 0.6 | +0.2 ± 0.6 | −0.2 ± 0.9 | 0.0 ± 0.6 |
| Improved ≥0.5% | 12 (26) | 3 (27) | 2 (13) | 4 (40) | 3 (27) |
| Worsened ≥0.5% | 14 (30) | 3 (27) | 7 (47) | 2 (20) | 2 (18) |
| A1C <7.0% | 8 (17) | 2 (18) | 1 (7) | 3 (30) | 2 (18) |
| Mean change from 0–6 months in prior RCT (%) | −0.2 ± 0.6 | −0.2 ± 0.4 | −0.1 ± 0.7 | −0.2 ± 0.7 | −0.1 ± 0.5 |
Data are means, means ± SD, or n (%).
*Baseline refers to the time of initiation of CGM use (following the 6 months in the RCT as control group).
†One subject was missing a baseline laboratory A1C, and the point-of-care A1C was imputed using a least squares regression model.
Severe hypoglycemia
The incidence rate of severe hypoglycemic events was 15.0 events per 100 person-years during the 6 months of the follow-up. This rate trended lower than the rate in these subjects in the 6 months of the randomized trial that preceded this study period (27.7 events per 100 person-years, P = 0.08). A similar trend was present in all three age-groups as seen in Table 2. The severe hypoglycemia incidence rate during the 6 months of CGM use was not significantly associated with baseline A1C (P = 0.26).
Table 2.
Rate of severe hypoglycemia 6 months before CGM use and during 6 months of CGM use
| Severe hypoglycemia | 6-month control period | 6-month CGM use period |
|---|---|---|
| Age ≥25 years | 38.5 person-years (n = 78) | 39.1 person-years (n = 78) |
| n events (n seizure or loss of consciousness) | 13 (2) | 9 (2) |
| n (%) subjects with at least 1 event | 9 (12) | 8 (10) |
| Incidence rate (per 100 person-years) | 33.7 | 23.0 |
| Age 15–24 years | 35.9 person-years (n = 73) | 36.6 person-years (n = 72) |
| n events (n seizure or loss of consciousness) | 8 (3) | 3 (2) |
| n (%) subjects with at least 1 event | 7 (10) | 3 (4) |
| Incidence rate (per 100 person-years) | 22.3 | 8.2 |
| Age 8–14 years | 30.3 person-years (n = 61) | 30.8 person-years (n = 61) |
| n events (n seizure or loss of consciousness) | 8 (1) | 4 (2) |
| n (%) subjects with at least 1 event | 6 (10) | 3 (5) |
| Incidence rate (per 100 person-years) | 26.4 | 13.0 |
CGM glucose data
In the ≥25-year-old group, there was an increase in time per day with the glucose level in the range of 71–180 mg/dl, with a decrease in the time in both the hypoglycemic range and hyperglycemic range (Table 3). In the 15–24 year olds, there was a decrease in time in the hypoglycemic range but no consistent change in hyperglycemia. In the 8–14 year olds, there was no substantial change in time spent as hypoglycemic or hyperglycemic. Results were similar for both daytime and nighttime (Supplementary Table 4, available in an online appendix) and in subgroups based on baseline A1C (<7.0% and ≥7.0%) (Supplementary Table 5, available in an online appendix).
Table 3.
Glycemic indices
| Age ≥25 years (n = 74)* |
Age 15–24 years (n = 55)* |
Age 8–14 years (n = 44)* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (blinded) | Month 6 | P | Baseline (blinded) | Month 6 | P | Baseline (blinded) | Month 6 | P | |
| Glucose (mg/dl) | 157 ± 25 | 152 ± 18 | 0.08 | 165 ± 27 | 173 ± 28 | 0.02 | 172 ± 29 | 169 ± 28 | 0.50 |
| Minimum/day 71–180 mg/dl | 882 | 980 | <0.001 | 822 | 788 | 0.14 | 797 | 812 | 0.18 |
| Hypoglycemia | |||||||||
| Minimum/day (mg/dl) | |||||||||
| ≤70 | 55 | 45 | 0.02 | 93 | 55 | 0.005 | 56 | 37 | 0.61 |
| ≤60 | 20 | 17 | 0.006 | 49 | 23 | 0.001 | 19 | 11 | 0.23 |
| ≤50 | 5 | 4 | 0.02 | 19 | 4 | 0.008 | 2 | 1 | 0.61 |
| AUC† | 0.3 | 0.3 | 0.008 | 0.7 | 0.3 | 0.002 | 0.4 | 0.2 | 0.42 |
| LBGI | 0.9 | 0.9 | 0.02 | 1.5 | 0.9 | 0.004 | 1.0 | 0.8 | 0.56 |
| Hyperglycemia | |||||||||
| Minimum/day (mg/dl) | |||||||||
| >180 | 439 | 390 | 0.03 | 494 | 582 | 0.03 | 569 | 568 | 0.37 |
| >200 | 296 | 256 | 0.004 | 381 | 436 | 0.03 | 460 | 452 | 0.23 |
| >250 | 114 | 72 | <0.0001 | 166 | 210 | 0.07 | 218 | 193 | 0.58 |
| AUC‡ | 15.1 | 12.1 | <0.001 | 21.7 | 25.0 | 0.06 | 25.3 | 23.9 | 0.43 |
| HBGI | 6.8 | 5.8 | 0.002 | 8.2 | 9.4 | 0.04 | 9.6 | 9.6 | 0.45 |
| Glycemic variability§ | |||||||||
| SD (mg/dl) | 60 | 54 | <0.001 (<0.001) | 67 | 68 | 0.35 (0.38) | 66 | 66 | 0.61 (0.95) |
| MAGE (mg/dl) | 110 | 100 | 0.14 (0.35) | 126 | 124 | 0.44 (0.38) | 129 | 119 | 0.04 (0.06) |
| MARC (mg · dl−1 · min−1) | 0.65 | 0.68 | 0.13 (0.03) | 0.79 | 0.80 | 0.01 (0.16) | 0.75 | 0.77 | 0.72 (0.49) |
| Coefficient of variation (%)‖ | 38 | 36 | 0.002 (<0.001) | 41 | 41 | 0.27 (0.32) | 41 | 39 | 0.99 (0.90) |
Data are means ± SD and medians unless otherwise indicated.
*n includes subjects with at least 24 h of data at both baseline and month 6.
†Total area <70 mg/dl, reflecting both percentage and severity of glucose values in the hypoglycemic range.
‡Total area >180 mg/dl, reflecting both percentage and severity of glucose values in the hyperglycemic range.
§For variability, the P values unadjusted and adjusted for mean glucose are both given: unadjusted (adjusted).
CONCLUSIONS
A purpose of this study was to determine whether CGM is beneficial for individuals with type 1 diabetes when implemented in a manner typical of a clinical practice setting, as we had shown in the more intensive RCT setting. Unlike in the RCT, the subjects in this study had a full 6 months of intensification of treatment and optimization of metabolic control using standard glucose monitoring before starting CGM. Nevertheless, in qualitative terms the results were similar to those that we had previously observed in the RCT. Adult subjects with baseline A1C levels ≥7.0% had a significant reduction in A1C (−0.4%); no reduction in A1C was seen in the two younger age-groups. As in the RCT, after adjustment for frequency of CGM use, there was no significant relationship between age and change in A1C. Subjects in the 6-month extension study who used the devices consistently also saw a significant increase in the amount of time spent in the target glucose range and decreased exposure to hyperglycemia. The association between the amount of CGM use and reduction in A1C also was found in the GuardControl study, in which a significant improvement in A1C levels over 3 months was observed in individuals with poorly controlled type 1 diabetes who were assigned to use CGM continuously compared with a control group but not in those assigned to use CGM intermittently (11). An interesting and somewhat counterintuitive finding was that the occurrence of a severe hypoglycemic episode in the previous 6 months was a strong predictor of lack of sensor use at the conclusion of the study. It is possible (but speculative) that in this context, the occurrence of severe hypoglycemia and nonuse of CGM may both be surrogate markers for difficulties with diabetes management–related tasks and behaviors.
Despite less intensive implementation of CGM in this study, the exposure to biochemical hypoglycemia was reduced in all three age-groups, although the difference did not achieve statistical significance. During the RCT, we were able to determine the rate of severe hypoglycemic events in these subjects with optimized management with standard blood glucose monitoring (i.e., 27.7 events per 100 patient years). Although the study was not powered to detect a difference in severe hypoglycemic events, it is noteworthy that the rate of severe hypoglycemia was reduced by almost 50% when the former control subjects switched to CGM.
There were some differences in the response to CGM when it was implemented with a less intensive approach to follow-up contacts. Across all age-groups, the frequency of use of CGM was reduced compared with that in the original CGM group in the RCT. Less frequent use may have contributed to the slightly smaller fall in A1C levels in adults with baseline A1C values ≥7.0%. It would not be surprising to learn that the effectiveness of CGM as an adjunct to standard blood glucose monitoring in individuals with type 1 diabetes is enhanced with more frequent personal contact with diabetes clinicians (12). These contacts may have helped to troubleshoot device-related issues such as alarms, calibrations, and site irritation that greatly affect the frequency of home CGM use. As CGM technology continues to evolve, focus should be placed on improvements likely to increase independent use of the devices, such as reduction in sensor size, less frequent need for calibration, greater accuracy resulting in fewer false alarms, and computer or web-based training modules to aid in the interpretation and application of sensor data, so that the benefits of CGM can be more easily realized in clinical practice.
Supplementary Material
Acknowledgments
Stuart Weinzimer received research support, a speaker honorarium, and travel reimbursement from Medtronic MiniMed and a speaker honorarium from Animas Corp/Lifescan. Craig Kollman received consulting fees from Medtronic MiniMed. Lori Laffel has received research grant support from Medtronic and advisory board for Medtronic. William Tamborlane received consulting fees from Abbott Diabetes Care and Lifescan and consulting fees, a speaker honorarium, and research funding from Medtronic MiniMed. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 69th annual meeting of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group recognizes the efforts of the subjects and their families and thanks them for their participation.
APPENDIX
Lead authors of the Writing Committee are Stuart Weinzimer, MD, Kellee Miller, MPH, Roy Beck, MD, PhD, and Dongyuan Xing, MPH. Additional members of the Writing Committee (alphabetical) are Rosanna Fiallo-Scharer, MD, Lisa K. Gilliam, MD, PhD, Craig Kollman, PhD, Lori Laffel, MD, MPH, Nelly Mauras, MD, Katrina Ruedy, MSPH, William Tamborlane, MD, and Eva Tsalikian, MD.
Footnotes
The study was designed and conducted by the investigators, who collectively wrote the manuscript and vouch for the data. The investigators had complete autonomy to analyze and report the trial results. There were no agreements concerning confidentiality of the data between the Juvenile Diabetes Research Foundation and the authors or their institutions. The Jaeb Center for Health Research had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Resnick HE, Foster GL, Bardsley J, Ratner RE: Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care 2006; 29: 531– 537 [DOI] [PubMed] [Google Scholar]
- 2. Bode BW, Schwartz S, Stubbs HA, Block JE: Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care 2005; 28: 2361– 2366 [DOI] [PubMed] [Google Scholar]
- 3. Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV: Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care 2001; 24: 1858– 1862 [DOI] [PubMed] [Google Scholar]
- 4. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464– 1476 [DOI] [PubMed] [Google Scholar]
- 5. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care 2009; 32: 1947– 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009; 32: 1378– 1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. JDRF CGM Study Group. JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther 2008; 10: 310– 321 [DOI] [PubMed] [Google Scholar]
- 8. Diabetes Research In Children Network (DirecNet) Study Group. Use of the DirecNet Applied Treatment Algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the Freestyle Navigator). Pediatr Diabetes 2008; 9: 142– 147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gibb I, Parnham A, Fonfrède M, Lecock F: Multicenter evaluation of Tosoh glycohemoglobin analyzer. Clin Chem 1999; 45: 1833– 1841 [PubMed] [Google Scholar]
- 10. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977– 986 [DOI] [PubMed] [Google Scholar]
- 11. Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M: Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 2006; 29: 2730– 2732 [DOI] [PubMed] [Google Scholar]
- 12. Messer L, Ruedy K, Xing D, Coffey J, Englert K, Caswell K, Ives B: Educating families on real time continuous glucose monitoring: the DirecNet navigator pilot study experience. Diabetes Educ 2009; 35: 124– 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W: Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care 1997; 20: 1655– 1658 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.