Abstract
OBJECTIVE
To investigate the relationship among A1C, fasting plasma glucose (FPG), and 2-h postload plasma glucose in the Dutch general population and to evaluate the results of using A1C for screening and diagnosis of diabetes.
RESEARCH DESIGN AND METHODS
In 2006–2007, 2,753 participants of the New Hoorn Study, aged 40–65 years, who were randomly selected from the population of Hoorn, the Netherlands, underwent an oral glucose tolerance test (OGTT). Glucose status (normal glucose metabolism [NGM], intermediate hyperglycemia, newly diagnosed diabetes, and known diabetes) was defined by the 2006 World Health Organization criteria. Spearman correlations were used to investigate the agreement between markers of hyperglycemia, and a receiver operating characteristic (ROC) curve was calculated to evaluate the use of A1C to identify newly diagnosed diabetes.
RESULTS
In the total population, the correlations between fasting plasma glucose and A1C and between 2-h postload plasma glucose and A1C were 0.46 and 0.33, respectively. In patients with known diabetes, these correlations were 0.71 and 0.79. An A1C level of ≥5.8%, representing 12% of the population, had the highest combination of sensitivity (72%) and specificity (91%) for identifying newly diagnosed diabetes. This cutoff point would identify 72% of the patients with newly diagnosed diabetes and include 30% of the individuals with intermediate hyperglycemia.
CONCLUSIONS
In patients with known diabetes, correlations between glucose and A1C are strong; however, moderate correlations were found in the general population. In addition, based on the diagnostic properties of A1C defined by ROC curve analysis, the advantage of A1C compared with OGTT for the diagnosis of diabetes is limited.
Fasting glucose levels and glucose levels 2 h after a glucose tolerance test (postload glucose levels) are used for diagnosis and management of diabetes (1). In addition, the A1C level is used to monitor glycemia in patients with diabetes because it has less day-to-day variability than glucose levels and is thought to reflect chronic glycemia (2).
In 2007, a consensus statement reported on the worldwide standardization of the A1C measurement (3). One of the conclusions was that glycemic goals in clinical practice should be expressed in three types of units, one of which is the International Federation of Clinical Chemistry and Laboratory Medicine standardized method. Use of this method implies that the unit of measurement of A1C will change from a percentage to millimoles per mole. The introduction of this new unit of measurement may be confusing for patients and health care providers. Therefore, the A1C-Derived Average Glucose (ADAG) Study Group investigated whether A1C can be translated into average blood glucose levels in patients with diabetes (4). A major advantage of using the average glucose level for chronic glycemia is that it has the same unit of measurement (millimoles per liter) as that for acute glycemia. The ADAG Study Group concluded that the average glucose level was strongly correlated with A1C and that the translation of A1C into average glucose levels was therefore possible (4). Moreover, Saudek et al. (5) recommended 1) the use of an A1C level of ≥6.0% as a screening standard for the detection of individuals at high risk of developing diabetes, 2) an A1C level of ≥6.5% confirmed by a glucose test (fasting or oral glucose tolerance test [OGTT]) for the diagnosis of diabetes, and 3) A1C levels of ≥7.0% measured twice or confirmed by a glucose test for the diagnosis of diabetes. During the review process for the present article, a consensus statement from an International Expert Committee that recommended the use of A1C levels ≥6.5% for the diagnosis of diabetes instead of glucose measures was published. A1C levels between 6.0 and 6.5% are proposed to identify individuals at high risk of developing diabetes (6). It may, however, be questioned whether A1C is a good indicator of glucose in individuals with normal or moderately elevated glucose levels and whether it can therefore be used to identify those with intermediate hyperglycemia or undiagnosed diabetes. Therefore, our aim was to investigate the relationship between glucose and A1C in the general population and to evaluate the use of A1C for the screening and diagnosis of diabetes.
RESEARCH DESIGN AND METHODS
From July 2006 to November 2007, a population-based study on glucose tolerance was performed in the city of Hoorn, the Netherlands (the New Hoorn Study). A random sample of 6,180 men and women aged 40–65 years was drawn from the municipal population registry of Hoorn. Following Dutch privacy legislation, all potential participants received a letter on behalf of the municipality, with a description of the study, and the request to return a form with their name, address, and telephone number. When the form was not returned within 2 weeks, a reminder was sent. To increase the participation rate, a local media campaign was started, and participants had the ability to visit the Diabetes Research Center on Saturdays. The study was approved by the medical ethics committee of the VU University Medical Center Amsterdam.
Data collection
Before their visit, participants received a questionnaire containing information on demographics, lifestyle, medication, and (family) history of disease. Participants were requested to refrain from eating and drinking (except water) from 8:00 p.m. the night before the visit and from drinking alcohol from 5:00 p.m. the day before the visit. They were instructed to follow their usual diet the day before each visit and to be consistent in their diet (both in content and in approximate timing of evening meals and snacks) and physical activities on the previsit days. In addition, participants were requested not to smoke on the morning of the visit and not to come by bicycle. Participants who had not been following these instructions were asked to reschedule the visit. Upon arrival at the Diabetes Research Center, written informed consent was obtained.
Height and weight were measured without shoes and heavy clothes. BMI was calculated as weight in kilograms divided by the square of height in meters. The waist circumference was measured between the lower rib margin and the spina iliaca anterior superior, and the hip circumference was measured over the maximum of the buttocks. The waist-to-hip ratio (waist circumference divided by hip circumference) was calculated. Blood pressure was measured three times on the right arm after a 10-min rest period, using a Colin Press Mate BP 8800p noninvasive blood pressure monitor (Colin Medical Technology). Final blood pressure was calculated as the mean of the last two measurements. Fasting whole blood glucose from a capillary vein in the finger was determined on the spot using a HemoCue β-glucose analyzer. In participants with a fasting whole blood glucose level <10 mmol/l, a standard 75-g OGTT was performed. Venous blood samples were drawn before and 120 min after glucose ingestion.
Laboratory assays
All analyses were performed at the clinical chemistry laboratory of the VU University Medical Center Amsterdam. Glucose was measured in venous plasma by the glucose oxidase method (Gluco-quant/hexokinase/G6P-DH; Boehringer-Mannheim, Mannheim, Germany). A1C was assessed using Diabetes Control and Complications Trial (DCCT) standardized reverse-phase cation exchange chromatography (HA 8160 analyzer; Menarini, Florence, Italy) The intra-assay coefficient of variation was 0.65% at a mean of 4.89%, and the interassay coefficient of variation was 1.55% at a mean of 5.52%. Triglycerides, total cholesterol, and HDL cholesterol were determined from fasting plasma samples by enzymatic techniques (Boehringer-Mannheim). LDL cholesterol was estimated with the Friedewald formula, except in individuals with triglycerides >4.5 mmol/l.
Statistical analyses
Based on the results of the OGTT, participants were categorized into three groups using the 2006 World Health Organization criteria (1): normal glucose metabolism (NGM), intermediate hyperglycemia, or newly detected diabetes. In addition, known diabetes was defined by the use of insulin or oral hypoglycemic agents and self-reported known diabetes. Differences between the subgroups of glucose tolerance were tested using one-way ANOVA.
Scatter plots with estimated linear or curvilinear relationships were plotted to describe the relation between glucose and A1C in the total population. In addition, the correlations among A1C, fasting plasma glucose (FPG), and 2-h postload plasma glucose were determined using Spearman correlations. After exclusion of participants with known diabetes, the diagnostic property of A1C for the diagnosis of diabetes was evaluated by calculating a receiver operating characteristic (ROC) curve. The area under the ROC curve (AUC) with 95% CI was calculated. To describe the effect of different cutoff points of A1C, sensitivity and specificity were used. In addition, the positive predictive value (PPV) and negative predictive value (NPV) for every cutoff point were calculated. All analyses were performed using the Statistical Package for the Social Sciences (SPSS for Windows 15.0). The P value for statistical significance was set at ≤0.05.
RESULTS
Population characteristics
Of the 6,180 people who were invited, 2,807 agreed to participate (45.4%). Of the nonattendees, 47% provided a reason for not participating, of which the most common were no time to participate (43%) and already having regular health checks (24.5%). After exclusion of 54 participants with missing glucose values, 2,753 participants were included for the analysis. Table 1 presents the population characteristics in subgroups with different glucose tolerance status. The prevalences of NGM, intermediate hyperglycemia, newly diagnosed diabetes, and known diabetes were 77, 16, 4, and 3%, respectively. Compared with the other categories, participants with known diabetes were more often male, were older, and had higher mean blood glucose, A1C, BMI, and waist-to-hip ratio. Their blood pressure and lipid levels were lower than those in patients with newly diagnosed diabetes, probably because of the treatment regimen for diabetic patients used by general practitioners in the Netherlands (7).
Table 1.
Population characteristics and Spearman correlations between markers of hyperglycemia in the total population and in participants with normal glucose tolerance, intermediate hyperglycemia, newly diagnosed diabetes, or known diabetes
| Total population | NGM | IH | NDM | KDM | |
|---|---|---|---|---|---|
| n (%) | 2,753 | 2,122 (77) | 439 (16) | 107 (4) | 85 (3) |
| Male sex (%) | 46.9 | 44.2 | 56.4* | 47.2 | 64.6*‡ |
| Age (years) | 53.5 ± 6.7 | 52.8 ± 6.8 | 55.2 ± 6.2* | 55.8 ± 6.3* | 56.9 ± 6.0* |
| Not of Dutch origin (%) | 10.8 | 9.8 | 13.2* | 17.6* | 13.9 |
| Diabetes duration (years) | — | — | — | — | 7.3 (8.14) |
| Systolic blood pressure (mmHg) | 133.3 ± 18.1 | 130.8 ± 17.4 | 140.9 ± 18.2* | 146 ± 20.5*† | 138.6 ± 17.2*‡ |
| Diastolic blood pressure (mmHg) | 76.6 ± 10.6 | 75.5 ± 10.4 | 80.2 ± 10.4* | 82.6 ± 10.5* | 78.5 ± 9.2 ‡ |
| Weight (kg) | 79.0 ± 14.6 | 77.2 ± 13.5 | 84.1 ± 15.9* | 86.1 ± 15.4* | 91.0 ± 17.2*† |
| BMI (kg/m2) | 26.3 ± 4.0 | 25.6 ± 3.7 | 27.9 ± 4.2* | 28.8 ± 4.3* | 30.0 ± 5.1*† |
| Waist (cm) | 89.8 ± 11.8 | 87.7 ± 10.7 | 95.3 ± 11.7* | 99.1 ± 11.7*† | 103.1 ± 15.5*† |
| Waist-to-hip ratio | 0.89 ± 0.08 | 0.88 ± 0.08 | 0.93 ± 0.08* | 0.95 ± 0.07* | 0.97 ± 0.07*† |
| Total cholesterol (mmol/l) | 5.5 ± 1.0 | 5.4 ± 0.96 | 5.7 ± 1.07 | 5.7 ± 1.08 | 5.0 ± 1.41*†‡ |
| HDL cholesterol (mmol/l) | 1.51 ± 0.42 | 1.56 ± 0.42 | 1.38 ± 0.39* | 1.28 ± 0.38* | 1.29 ± 0.38* |
| Triglycerides (mmol/l)§ | 1.2 (0.9–1.7) | 1.1 (0.8–1.6) | 1.6 (1.2–2.2)* | 2.0 (1.4–2.7)*† | 1.7 (1.1–2.6)* |
| FPG (mmol/l) | 5.6 ± 0.95 | 5.30 ± 0.38 | 6.0 ± 0.51* | 7.7 ± 2.24*† | 7.9 ± 2.12*† |
| 2-h postload plasma glucose (mmol/l) | 5.9 ± 2.4 | 5.09 ± 1.13 | 7.5 ± 1.78* | 11.50 ± 3.24*† | 13.2 ± 5.07*†‡ |
| A1C (%) | 5.5 ± 0.52 | 5.3 ± 0.26 | 5.6 ± 0.33* | 6.4 ± 1.25*† | 6.7 ± 1.16*†‡ |
| A1C vs. fasting plasma glucose‖ | 0.46¶ | 0.26¶ | 0.25¶ | 0.53¶ | 0.71¶ |
| A1C vs. 2-h postload plasma glucose‖ | 0.33¶ | 0.14¶ | −0.050 | 0.43¶ | 0.79¶ |
| FPG vs. 2-h postload plasma glucose‖ | 0.40¶ | 0.20¶ | −0.50¶ | 0.13 | 0.66¶ |
Values are means ± SD, percentage, or median (25th–75th percentile).
*Significantly different from normal glucose tolerance.
†Significantly different from intermediate hyperglycemia.
‡Significantly different from newly diagnosed diabetes.
§Log-transformed before testing.
‖Spearman correlations.
¶Statistically significant (P ≤ 0.05) correlation. IH, intermediate hyperglycemia; NDM, newly diagnosed diabetes; KDM, known diabetes.
Correlations among A1C, FPG, and 2-h postload plasma glucose
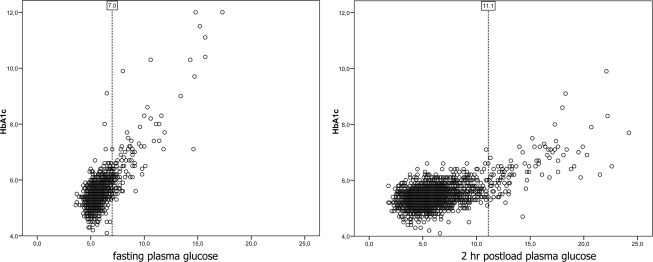
Figure 1 presents the scatter plots for the association between FPG and A1C and between 2-h postload plasma glucose and A1C. Using a linear association model, R2 values, a measure of explained variance, were 0.57 for the association between FPG and A1C and 0.35 for the association between 2-h postload plasma glucose and A1C (both P < 0.01). Use of exponential or quadratic models did not improve the fit of the model (data not shown) nor could a threshold be identified. In Table 1, the Spearman correlations among A1C, FPG, and 2-h postload plasma glucose are presented. In the total population, the correlations between A1C and FPG were stronger than those between A1C and 2-h postload plasma glucose (0.46 and 0.33, respectively). Because earlier research provided correlations in subgroups of glucose tolerance status, we also provide correlations within these subgroups (Table 1, bottom rows). Correlations between A1C and glucose in subgroups with NGM, intermediate hyperglycemia, and newly diagnosed diabetes ranged between −0.050 and 0.53. In patients with known diabetes, the correlations were stronger and reached levels between 0.71 and 0.79.
Figure 1.
Scatter plots of FPG and 2-h postload plasma glucose in relation to A1C in the total population. Diabetic patients are indicated by dotted reference lines at FPG levels of 7.0 mmol/l and 2-h postload plasma glucose levels of 11.1 mmol/l.
Diagnostic properties of A1C
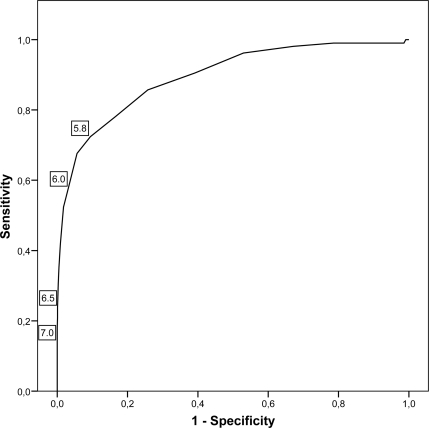
Figure 2 shows the results of the analyses of A1C as a possible tool for the screening and diagnosis of diabetes, based on a ROC curve. The AUC was 0.895 (95% CI 0.861–0.930). In Table 2, the diagnostic properties of different A1C cutoff points are described. The cutoff point for identifying newly diagnosed diabetes with the highest sum of sensitivity (72%) and specificity (91%) was an A1C level of 5.8%. Of all individuals with an A1C level >5.8%, 24% had diabetic glucose levels. When the recently proposed cutoff point of 6.0% for the screening of individuals at high-risk of diabetes (5,6) was used, the sensitivity and specificity were 56 and 97%, respectively. Less than half of the individuals with A1C levels >6.0% had diabetic glucose levels (PPV of 42%) and 15% of the participants with intermediate hyperglycemia had A1C levels >6.0%. For the diagnosis of diabetes, A1C levels >6.5 and 7.0% were proposed (5,6). Of the participants with A1C >6.5 and 7.0%, 93 and 100% had diabetic glucose levels, respectively. However, of all patients with diabetes, 24% had A1C levels >6.5 and 12% had A1C levels >7.0%.
Figure 2.
ROC curve for identification of participants with previously undiagnosed diabetes, using A1C for diagnosis and an OGTT as criterion. A1C cutoff points of 5.8, 6.0, 6.5, and 7.0% are indicated on the curve.
Table 2.
Sensitivity, specificity, PPV, and NPV for diabetes using different A1C cutoff points
| A1C | % total population | % high risk (IH) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| 5.5% | 41 | 70 | 91 | 61 | 9 | 99 |
| 5.6% | 28 | 57 | 86 | 74 | 12 | 99 |
| 5.7% | 19 | 42 | 78 | 83 | 16 | 99 |
| 5.8% | 12 | 30 | 72 | 91 | 24 | 99 |
| 5.9% | 8 | 21 | 67 | 94 | 33 | 98 |
| 6.0% | 5 | 15 | 56 | 97 | 42 | 98 |
| 6.1% | 4 | 9 | 42 | 98 | 54 | 98 |
| 6.5% | 1 | 1 | 24 | 99 | 93 | 97 |
| 7.0% | 1 | 0 | 12 | 100 | 100 | 95 |
IH, intermediate hyperglycemia.
In clinical care, FPG instead of an OGTT is mostly used to diagnose diabetes. To compare the diagnostic properties of A1C with FPG, we performed similar ROC curve analyses for FPG and for A1C, using OGTT as the criterion. The AUC for FPG was 0.937 (95% CI 0.905–0.969) (data not shown).
CONCLUSIONS
In the general Dutch population, the correlations between glucose (fasting and postload) and A1C were moderate compared with these correlations in patients with known diabetes. In addition, when one is using A1C levels ≥6.0% to screen for diabetes in the general population, almost half of the people with diabetic glucose levels would be missed. Last, the A1C cutoff point of 7.0% has a specificity of 100% and is present among 12% of patients with newly diagnosed diabetes.
Nathan et al. (4) reported recently that A1C and average glucose levels measured during the day were highly associated in a population of mainly type 1 diabetic patients. This observation is consistent with our findings in patients with diagnosed type 2 diabetes and earlier research (8). Our research, however, shows that the correlations between glucose and A1C were moderate in a random sample of the general population.
The cutoff point for screening for diabetes with the highest sum of sensitivity and specificity in our data was an A1C level of 5.8%. This cutoff point would detect 72% of patients with newly diagnosed diabetes and 30% of individuals at high risk of developing diabetes (participants with intermediate hyperglycemia). Use of A1C levels of 6.0% for the screening of individuals at high risk of developing diabetes would lead to identifying 15% of the individuals with intermediate hyperglycemia. Moreover, 44% of patients with newly diagnosed diabetes had A1C levels <6.0%. This observation is consistent with findings in the National Health and Nutrition Examination Survey (NHANES), in which an A1C level of 5.8% was also found to have the highest sum of sensitivity and specificity (9,10) and in which 60% of patients with newly diagnosed diabetes had A1C levels <6.1% (11).
Because all participants with A1C levels >7.0% had diabetic glucose levels, our data confirm that A1C levels of ≥7.0% can be used as a threshold not requiring further glucose testing for the diagnosis of diabetes. A limitation of this diagnostic criterion is the low sensitivity as shown in our study as well as in the NHANES studies (9,10). Therefore, in most of individuals, an OGTT would still be necessary to verify the diagnosis. Moreover, our data showed that FPG had a higher area under the ROC curve than A1C, indicating a higher diagnostic value of FPG than A1C for identifying newly diagnosed diabetes. The conclusion in other population-based research was that A1C had no additional diagnostic value compared with fasting glucose alone (12), except in high-risk groups (13). Despite the currently discussed limitations of A1C for the diagnosis of diabetes compared with an OGTT, A1C may be superior than OGTT in terms of cost-effectiveness and practical use in the clinical setting, because fasting measures are not necessary, and measurement of A1C is less time-consuming than an OGTT. Moreover, A1C might be a good indicator for future complications. Large-scale epidemiological studies have shown that A1C level is associated with complications such as cardiovascular disease, even in the nondiabetic range of glucose tolerance (14,15).
The moderate correlations of glucose with A1C found in this study may imply that A1C and glucose reflect different processes, especially in the nondiabetic range of glucose tolerance. The amount of glycosylation is known to vary interindividually (16,17). Possible mechanisms are genetic traits (18), aging (19), differences in erythrocyte environments (20), heterogeneity in red-cell lifespan (21), and racial differences (22). In participants with known diabetes, the correlations between glucose and A1C were stronger than those in participants with newly diagnosed diabetes. It can be speculated that longer diabetes duration is accompanied by changes in the intraerythrocyte environment. Another explanation may be the degree of glycemic control. Kilpatrick et al. (23) showed in the DCCT that for any given A1C, the plasma glucose levels of participants in different treatment groups were not the same.
The New Hoorn Study is a population study of individuals who were randomly selected. Therefore, we had the ability to study the relation between A1C and glucose in a representative sample of the Dutch population with different stadia of glucose tolerance. A limitation of the present study was that only 45.4% of the originally invited sample agreed to participate. As a result, we cannot exclude the possibility that self-selection occurred, possibly leading to overrepresentation of individuals with an interest in diabetes. Second, we did not have the availability of continuous glucose monitoring during the day and only one OGTT was performed on all participants. It can be suggested that the moderate correlations between glucose and A1C in the present study are due to the rather high variability of FPG and 2-h postload plasma glucose within individuals (24). However, de Vegt et al. (25) showed that this variability did not result in a different reproducibility for the categorization of glucose tolerance over 6 years. In addition, the correlations between glucose and A1C in patients with known diabetes found in our study are comparable to the correlations found in the study by Nathan et al. (4), in which continuous glucose monitoring was used in patients with known diabetes. Correlation coefficients are sensitive for the differences in ranges of the variables included and tend to be lower in subgroups with a smaller range. The range of glucose and A1C is large in patients with diabetes, resulting in strong correlations. However, in our study the correlations between glucose and A1C were stronger in participants with diabetes than in the total population, whereas the ranges of both glucose and A1C are larger in the total population. These findings indicate that differences in ranges are not the only explanation of the differences found in the correlations among the total population and subpopulations with diabetes.
To summarize, our results support the ongoing research on the translation of A1C into A1C-derived glucose in people with known diabetes. However, based on the moderate correlations between glucose and A1C in the total sample, translation of A1C into average glucose in the general population is not recommended. Moreover, based on diagnostic properties only, the advantage of using A1C instead of an OGTT for both screening and diagnosis of diabetes is limited, and glucose measures will still be necessary in most patients to verify the outcome. However, research including cost-effectiveness, practical use, and the role of A1C in the development of future complications is needed to further explore this conclusion.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva, World Health Org., 2006 [Google Scholar]
- 2. Nathan DM, Singer DE, Hurxthal K, Goodson JD: The clinical information value of the glycosylated hemoglobin assay. N Engl J Med 1984; 310: 341– 346 [DOI] [PubMed] [Google Scholar]
- 3. Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care 2007; 30: 2399– 2400 [DOI] [PubMed] [Google Scholar]
- 4. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ: Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31: 1473– 1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB: A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 2008; 93: 2447– 2453 [DOI] [PubMed] [Google Scholar]
- 6. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327– 1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rutten GEHM, De Grauw WJC, Nijpels G, Goudswaard AN, Uitewaal PJM, Van der Does FEE, Heine RJ, Van Ballegooie E, Verduijn MM, Bouma M: NHG-standard diabetes mellitus type 2. Huisarts Wet 2006; 49: 137– 152 [in Dutch] [Google Scholar]
- 8. Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE: Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 2002; 25: 275– 278 [DOI] [PubMed] [Google Scholar]
- 9. Buell C, Kermah D, Davidson MB: Utility of A1C for diabetes screening in the 1999–2004 NHANES population. Diabetes Care 2007; 30: 2233– 2235 [DOI] [PubMed] [Google Scholar]
- 10. Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flegal KM, Eberhardt MS, Goldstein DE: Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care 2000; 23: 187– 191 [DOI] [PubMed] [Google Scholar]
- 11. Davidson MB, Schriger DL, Peters AL, Lorber B: Relationship between fasting plasma glucose and glycosylated hemoglobin: potential for false-positive diagnoses of type 2 diabetes using new diagnostic criteria. JAMA 1999; 281: 1203– 1210 [DOI] [PubMed] [Google Scholar]
- 12. Glümer C, Jørgensen T, Borch-Johnsen K: Targeted screening for undiagnosed diabetes reduces the number of diagnostic tests: Inter99(8). Diabet Med 2004; 21: 874– 880 [DOI] [PubMed] [Google Scholar]
- 13. Perry RC, Shankar RR, Fineberg N, McGill J, Baron AD: the Early Diabetes Intervention Program (EDIP). HbA1c measurement improves the detection of type 2 diabetes in high-risk individuals with nondiagnostic levels of fasting plasma glucose: the Early Diabetes Intervention Program (EDIP). Diabetes Care 2001; 24: 465– 471 [DOI] [PubMed] [Google Scholar]
- 14. Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N: Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141: 413– 420 [DOI] [PubMed] [Google Scholar]
- 15. Barr EL, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE: Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 2009; 52: 415– 424 [DOI] [PubMed] [Google Scholar]
- 16. Modan M, Meytes D, Rozeman P, Yosef SB, Sehayek E, Yosef NB, Lusky A, Halkin H: Significance of high HbA1 levels in normal glucose tolerance. Diabetes Care 1988; 11: 422– 428 [DOI] [PubMed] [Google Scholar]
- 17. Cohen RM, Smith EP: Frequency of HbA1c discordance in estimating blood glucose control. Curr Opin Clin Nutr Metab Care 2008; 11: 512– 517 [DOI] [PubMed] [Google Scholar]
- 18. Cohen RM, Snieder H, Lindsell CJ, Beyan H, Hawa MI, Blinko S, Edwards R, Spector TD, Leslie RD: Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care 2006; 29: 1739– 1743 [DOI] [PubMed] [Google Scholar]
- 19. Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, Sullivan L, D'Agostino RB, Nathan DM: Effect of aging on A1C levels in persons without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care 2008; 31: 1991– 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khera PK, Joiner CH, Carruthers A, Lindsell CJ, Smith EP, Franco RS, Holmes YR, Cohen RM: Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes 2008; 57: 2445– 2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, Palascak MB, Joiner CH: Red cell lifespan heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 2008; 112: 4284– 4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E: the Diabetes Prevention Program Research Group. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007; 30: 2453– 2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kilpatrick ES, Rigby AS, Atkin SL: Variability in the relationship between mean plasma glucose and HbA1c: implications for the assessment of glycemic control. Clin Chem 2007; 53: 897– 901 [DOI] [PubMed] [Google Scholar]
- 24. Selvin E, Crainiceanu CM, Brancati FL, Coresh J: Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 2007; 167: 1545– 1551 [DOI] [PubMed] [Google Scholar]
- 25. de Vegt F, Dekker JM, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ: Similar 9-year mortality risks and reproducibility for the World Health Organization and American Diabetes Association glucose tolerance categories: the Hoorn Study. Diabetes Care 2000; 23: 40– 44 [DOI] [PubMed] [Google Scholar]