Abstract
OBJECTIVE
Insulin resistance and β-cell function are major predictors of type 2 diabetes, but studies using direct methods of insulin resistance and secretion are few and relatively small. Furthermore, the strength of these associations has not been tested in different ethnic groups and various states of glucose tolerance, family history of diabetes, and obesity.
RESEARCH DESIGN AND METHODS
Predictors of incident diabetes were evaluated in Hispanic, non-Hispanic white, and African American participants in the Insulin Resistance Atherosclerosis Study (aged 40–69 years). In 557 participants with normal glucose tolerance and 269 with impaired glucose tolerance (IGT), insulin sensitivity (insulin sensitivity index [SI]) and first-phase insulin secretion (acute insulin response [AIR]) were directly measured using the frequently sampled intravenous glucose tolerance test.
RESULTS
At the 5-year follow-up examination, 128 (15.5%) individuals had developed diabetes. Both SI (odds ratio × 1 SD 0.50 [95% CI 0.37–0.68]) and AIR (0.51 [0.40–0.65]) were independent predictors of incident diabetes even after adjustment for age, sex, ethnicity, center, IGT, family history of diabetes, and BMI. The strength of the relation of SI and AIR to incident diabetes was not significantly affected by potential interactions of age, sex, ethnicity, glucose tolerance, BMI, or family history of diabetes (P ≥ 0.15).
CONCLUSIONS
Both insulin sensitivity and β-cell function predict conversion to diabetes in different ethnic groups and various states of glucose tolerance, family history of diabetes, and obesity. The prevention of type 2 diabetes should focus on interventions that improve both insulin resistance and insulin secretion.
Insulin resistance and insulin secretion are major predictors of type 2 diabetes, but much of the evidence is the result of studies that use surrogate measures of insulin resistance and β-cell function (1–4). There are few studies that have measured insulin resistance and secretion by direct methods. These studies have enrolled relatively few participants and have targeted individuals from a single ethnic group. In the study by Martin et al. (5), there were 25 incident cases of diabetes among 151 offspring of white parents who both had type 2 diabetes. In a subsequent report by Goldfine et al. (6), this cohort of individuals was compared with a cohort of 181 subjects with normal glucose tolerance (NGT) with only 6 incident cases of diabetes during a mean follow-up of 25 years (6). In the Pima Indian report, 200 subjects were studied and 38 developed type 2 diabetes (7). In a more recent study from the Netherlands, 101 white individuals with impaired glucose tolerance (IGT) were enrolled and 35 developed diabetes (8). Risk of progression to IGT and diabetes associated with direct measures of insulin sensitivity and secretion was also examined in 399 Pima Indians (9) and in 81 first-degree relatives of African Americans with type 2 diabetes (10). None of these studies adjusted their results for glucose tolerance status and adiposity. Furthermore, there are few data on how insulin resistance and secretion perform in different ethnic groups and various states of glucose tolerance, family history of diabetes, and obesity.
Because the significance of insulin resistance and secretion could differ by ethnic group, parental history of diabetes, and obesity, we examined the heterogeneity of the relation of insulin resistance and β-cell function to future development of type 2 diabetes. The Insulin Resistance Atherosclerosis Study (IRAS) is a large epidemiological study on insulin resistance and cardiovascular risk factors among individuals of three ethnic groups (African Americans, Hispanics, and non-Hispanic whites) (11). Insulin sensitivity and first-phase insulin secretion were directly measured using the frequently sampled intravenous glucose tolerance test with MINMOD analysis.
RESEARCH DESIGN AND METHODS
The IRAS is a multicenter observational epidemiological study of the relationships among insulin resistance, cardiovascular disease, and its known risk factors in different ethnic groups and various states of glucose tolerance. The design and methods of this study have been described in detail in previous publications (11). In brief, the study was conducted at four clinical centers. At centers in Oakland and Los Angeles, California, non-Hispanic whites and African Americans were recruited from Kaiser Permanente, a nonprofit HMO. Centers in San Antonio, Texas, and San Luis Valley, Colorado, recruited Hispanics from two ongoing population-based studies (the San Antonio Heart Study and the San Luis Valley Diabetes Study). A total of 1,625 individuals participated in the baseline IRAS examination (56% women), which occurred between October 1992 and April 1994. After an average of 5.2 years (range 4.5–6.6 years), follow-up examinations of this cohort were conducted using the baseline protocol. The response rate was 81%, and those who attended the follow-up examination were similar to those who did not attend in terms of ethnicity, sex, baseline glucose tolerance status, and BMI (all comparisons, P > 0.32). The IRAS protocol was approved by local institutional review committees, and all participants provided written informed consent.
Participants who were alive at the time of the follow-up visit were eligible for analysis if they were nondiabetic at the time of enrollment (n = 1,043). We excluded 217 participants (failure to return to the follow-up visit in 153 individuals and information unavailable on variables of interest in 64 individuals). Therefore, the present report includes information on 826 (79.2%) participants (332 non-Hispanic whites, 206 African Americans, and 288 Hispanics). Participants who were eligible for analysis had baseline characteristics (e.g., age, ethnicity, sex, glucose tolerance status, BMI, waist circumference, and insulin sensitivity index [SI]; all comparisons, P ≥ 0.2) similar to those of participants who were excluded except for acute insulin response (AIR) (higher in eligible participants, P = 0.005).
Clinical measurements and procedures
The IRAS protocol required two visits, 1 week apart, of ∼4 h each. Protocols were identical at the baseline and 5-year follow-up examinations. Subjects were asked before each visit to fast for 12 h, to abstain from heavy exercise and alcohol for 24 h, and to refrain from smoking on the morning of the examination. During the first baseline and follow-up visits, a 75-g oral glucose tolerance test was administered to assess glucose tolerance status. During the second baseline visit, insulin sensitivity and insulin secretion were determined using a frequently sampled intravenous glucose tolerance test (11). An injection of regular insulin was used to ensure adequate plasma insulin levels for the accurate computation of insulin sensitivity across a broad range of glucose tolerance. The SI was calculated using mathematical modeling methods (MINMOD version 3.0, 1994; Los Angeles, CA, courtesy of Richard Bergman, PhD) (12). The first-phase insulin secretion (AIR) was calculated as the mean of 2- and 4-min insulin concentrations after glucose administration.
Race/ethnicity was assessed by self-report. Family history of diabetes was defined as diabetes in parents or siblings. Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. Waist circumference was measured to the nearest 0.5 cm using a steel tape. Duplicate measures of anthropometry were made following a standardized protocol, and averages were used in the analysis. Plasma glucose concentration was determined by the glucose oxidase method with a Beckman glucose analyzer. Plasma insulin concentration was determined by the dextran-charcoal radioimmunoassay. This assay displays a high degree of cross-reactivity with proinsulin.
We used the 1999 World Health Organization criteria to define diabetes and IGT. Fasting glucose concentration ≥7.0 mmol/l and/or 2-h plasma glucose concentration ≥11.1 mmol/l was indicative of diabetes. Subjects treated with hypoglycemic medications were also considered to have diabetes. In the absence of diabetes, IGT was defined as a 2-h plasma glucose level between ≥7.8 and <11.1 mmol/l.
Statistical analyses
The analysis was performed using SAS statistical software (version 9.1; SAS Institute, Cary, NC). Baseline means ± SD or proportions were calculated for subjects by diabetes status at the follow-up examination with use of t tests or χ2 tests to assess the statistical significance of differences. The relationship among plasma glucose levels, SI, insulin secretion, and measures of obesity was examined by Spearman partial correlation analysis. Coefficients were adjusted for age, sex, ethnicity, and center. The relation of SI and AIR to risk of incident diabetes was assessed by logistic regression analysis, with diabetes status at the follow-up examination as the outcome variable. In addition, odds ratios (ORs) were expressed for binary traits or per SD increase for continuous traits. Unadjusted and adjusted ORs were calculated for demographic variables (e.g., age, sex, ethnicity, and clinical center) as well as BMI and baseline glucose tolerance status. We assessed the impact of age, sex, ethnicity, BMI, family history of diabetes, and glucose tolerance status on the relation of SI and AIR to conversion to type 2 diabetes by including interaction terms in separate logistic regression models. Log-transformed values of levels of fasting and 2-h insulin and AIR were used to improve discrimination and calibration of the models and to minimize the influence of extreme observations. We also used the log transformation of (SI + 1), given that some participants had SI = 0. These variables were then back-transformed to their units for presentation in tables.
RESULTS
During the 5-year follow-up period, 128 (15.5%) of 826 IRAS participants developed type 2 diabetes: 44 (7.9%) of 557 participants with NGT at baseline and 84 (31.2%) of 269 participants with IGT at baseline. Those converting to type 2 diabetes did not differ from nonconverters in terms of sex or ethnicity (Table 1). Older age and family history of diabetes were more common among converters. Converters had also higher BMI and waist circumference measurements, higher fasting and 2-h glucose and insulin values, and lower SI and AIR.
Table 1.
Baseline demographic, anthropometric, and metabolic characteristics of participants without diabetes at baseline by conversion to diabetes at 5-year follow-up
| Converters | Nonconverters | P | |
|---|---|---|---|
| n | 128 | 698 | |
| Female sex (%) | 59.4 (50.7–67.5) | 55.4 (51.7–59.1) | 0.410 |
| Ethnicity (%) | 0.647 | ||
| Non-Hispanic whites | 39.1 (31.0–47.8) | 40.4 (36.8–44.1) | |
| African Americans | 22.7 (16.2–30.7) | 25.4 (22.3–28.7) | |
| Hispanics | 38.3 (30.3–47.0) | 34.2 (30.8–37.8) | |
| IGT (%) | 65.6 (57.0–73.3) | 26.5 (23.4–29.9) | <0.001 |
| Family history of diabetes (%) | 50.0 (41.4–58.6) | 38.5 (35.0–42.2) | 0.016 |
| Age (years) | 56.5 ± 8.1 | 54.4 ± 8.5 | 0.009 |
| BMI (kg/m2) | 30.8 ± 6.1 | 27.8 ± 5.3 | <0.001 |
| Waist circumference (cm) | 95.2 ± 12.0 | 89.3 ± 12.3 | <0.001 |
| Fasting glucose (mmol/l) | 5.83 ± 0.55 | 5.35 ± 0.54 | <0.001 |
| 2-h glucose (mmol/l) | 8.42 ± 1.69 | 6.60 ± 1.73 | <0.001 |
| Fasting insulin (pmol/l)* | 105.3 ± 98.6 | 70.9 ± 59.8 | <0.001 |
| 2-h insulin (pmol/l)* | 611.6 ± 603.9 | 390.7 ± 515.4 | <0.001 |
| SI (× 10−4 min−1 · μU−1 · ml−1)* | 1.06 ± 1.10 | 1.92 ± 2.00 | <0.001 |
| AIR (μU/ml)* | 37.6 ± 43.8 | 53.0 ± 62.4 | <0.001 |
Data are n, % (95% CI), or means ± SD. P values were derived from χ2 or t tests, as appropriate.
*Log-transformed variables. These variables were then back-transformed to their units for presentation in the table.
In Spearman partial correlation analysis, SI was negatively related to fasting glucose (r = −0.33), 2-h glucose (r = −0.49), BMI (r = −0.53), and waist circumference (r = −0.56) after adjustment for age, sex, race/ethnicity, and center (all P < 0.001). AIR was also negatively associated with SI (r = −0.33, P < 0.001), fasting glucose (r = −0.19, P < 0.001), and 2-h glucose (r = −0.10, P = 0.004) but was positively associated with BMI (r = 0.22, P < 0.001) and waist circumference (r = 0.23, P < 0.001).
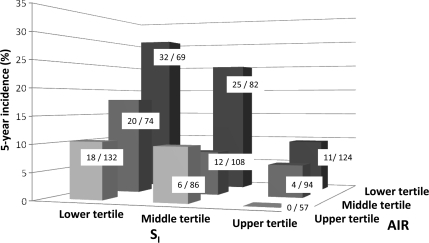
Participants were stratified by tertiles of SI and AIR. Both SI (Ptrend < 0.001) and AIR (Ptrend < 0.001) predicted future development of diabetes even after adjustment for age, sex, ethnicity, center, IGT, family history of diabetes, and BMI (Fig. 1). Risk of diabetes was highest in individuals in the lowest tertile of both SI and AIR. No one in the highest tertile of both SI and AIR developed type 2 diabetes. Similar results were obtained in a multivariate logistic regression model with SI and AIR as continuous traits (Table 2). Replacing fasting and 2-h glucose for IGT demonstrated that fasting and 2-h glucose, SI, and AIR were independently predictors of incident diabetes. Replacing waist circumference for BMI rendered similar results for both SI (OR × 1 SD 0.64 [95% CI 0.46–0.88], P = 0.006) and AIR (0.66 [0.51–0.86], P = 0.002). Neither BMI (1.16 [0.92–1.48]) nor waist circumference (1.07 [0.83–1.39)] was an independent risk factor in fully adjusted models.
Figure 1.
Five-year incidence of diabetes by tertiles of SI and AIR. Results were adjusted for age, sex, ethnicity, center, IGT, family history of diabetes, and BMI. Cut points for tertiles of SI (× 10−4 minute per microunit per milliliter): lower, ≤1.16; middle, 1.17–2.38; and upper, ≥2.39 (Ptrend < 0.001). Cut points for tertiles of AIR (microunits per milliliter): lower, ≤37.5; middle, 38.0–75.0; and upper, ≥75.5 (Ptrend < 0.001). Numerators and denominators for each cell are number of participants who converted to diabetes and total number of participants at risk, respectively.
Table 2.
Predictors of conversion to type 2 diabetes by multiple logistic regression analysis
| OR (95% CI) | P | |
|---|---|---|
| Model 1* | ||
| Age (× 1 SD) | 1.09 (0.87–1.36) | 0.468 |
| Female vs. male | 1.04 (0.66–1.63) | 0.868 |
| Family history of diabetes (yes vs. no) | 1.27 (0.82–1.97) | 0.283 |
| IGT vs. NGT | 2.39 (1.48–3.84) | <0.001 |
| BMI (× 1 SD) | 1.29 (1.03–1.61) | 0.024 |
| SI (× 1 SD)† | 0.50 (0.37–0.68) | <0.001 |
| AIR (× 1 SD)† | 0.51 (0.40–0.65) | <0.001 |
| Model 2‡ | ||
| Age (× 1 SD) | 1.10 (0.87–1.38) | 0.426 |
| Female vs. male | 1.17 (0.73–1.86) | 0.522 |
| Family history of diabetes (yes vs. no) | 1.25 (0.80–1.96) | 0.325 |
| Fasting glucose (× 1 SD) | 1.51 (1.16–1.96) | 0.002 |
| 2-h glucose (× 1 SD) | 1.93 (1.46–2.56) | <0.001 |
| BMI (× 1 SD) | 1.16 (0.92–1.48) | 0.211 |
| SI (× 1 SD)† | 0.66 (0.48–0.91) | 0.010 |
| AIR (× 1 SD)† | 0.65 (0.50–0.85) | 0.001 |
ORs are expressed for binary traits or per 1-SD unit change for continuous traits.
*Results in model 1 were adjusted also for ethnicity (P = 0.343) and clinical center (P = 0.064).
†Log-transformed variables.
‡Results in model 2 were adjusted also for ethnicity (P = 0.393) and clinical center (P = 0.051).
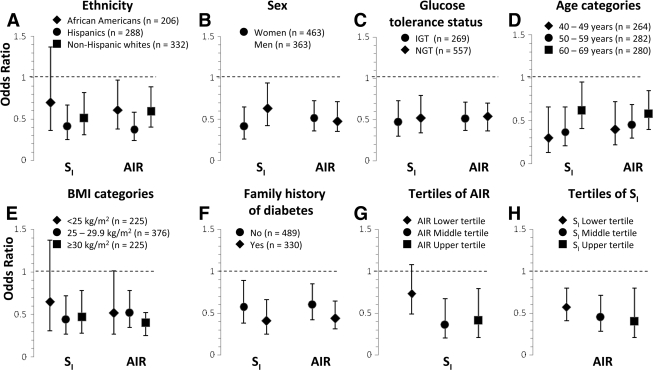
In separate logistic regression models, we included appropriate interaction terms to test the impact of age, sex, race/ethnicity, BMI, family history of diabetes, and glucose tolerance status on the relation of SI and AIR to conversion to type 2 diabetes. Age, sex, race/ethnicity, center, BMI, glucose tolerance status, family history of diabetes, SI, and AIR were all included as independent variables in all models. None of the variables had a significant interaction on the relationship between SI and conversion to diabetes (P ≥ 0.15) or between AIR and conversion to diabetes (P ≥ 0.20). The strength of the relation of SI and AIR to conversion to diabetes did not differ among categories of age, sex, race/ethnicity, glucose tolerance status, BMI, and family history of diabetes (Fig. 2A–F). In addition, the interaction term SI × AIR was not statistically significant (P ≥ 0.42). AIR was an independent risk factor across different degrees of insulin sensitivity and so was SI across various degrees of insulin secretion (Fig. 2G and H).
Figure 2.
Risk of developing diabetes associated with SI and AIR by ethnicity, sex, glucose tolerance status, BMI and age categories, and family history of diabetes. Estimates are expressed for a 1-SD unit change. Age, sex, ethnicity, center, BMI, IGT, family history of diabetes, SI, and AIR were all included as independent variables in all eight models. Log-transformed values of SI and AIR were used to improve discrimination and calibration of the models and to minimize the influence of extreme observations.
CONCLUSIONS
In three ethnic groups, two of which are at high risk for type 2 diabetes (African Americans, n = 206; non-Hispanic whites, n = 288), both SI and AIR predicted the development of future diabetes. The observation that insulin resistance measured directly predicts type 2 diabetes has been described in offspring of white parents who both had type 2 diabetes (5). A secretory defect of insulin along with insulin resistance has also been described in the high-risk Pima Indians (7,9), Dutch white individuals with IGT (9), and first-degree relatives of African Americans with type 2 diabetes (9). However, none of these studies adjusted for obesity or glucose tolerance, which are associated with both insulin resistance and secretion at baseline. Our results indicate that both insulin resistance and β-cell dysfunction predict diabetes even after adjustment for both obesity and glucose tolerance. Thus, our study validates previous studies and extends the findings to Hispanic and African American ethnic groups.
Some investigators have reported that a large proportion of individuals with type 2 diabetes among African Americans present only with a defect in insulin secretion (13). In IRAS, the proportion of insulin-sensitive diabetic subjects is small in all racial/ethnic groups (14). Differences in insulin resistance according to race/ethnicity cannot be fully explained by obesity, but nondiabetic African Americans tend to have lower SI and higher AIR than their non-Hispanic white counterparts (15,16). Hispanics have also lower SI and higher AIR than non-Hispanic whites (15). Nevertheless, the role of race/ethnicity (more specifically the role of genetic determinants) on SI and AIR remains to be elucidated (17,18). In the current study, we were unable to detect differences according to race/ethnicity in the relation of SI and AIR to incident diabetes. Therefore, both insulin resistance and secretion are important risk factors for development of diabetes across racial/ethnic groups.
Goldfine et al. (6) reported that SI was a poor predictor of type 2 diabetes in individuals with no family history of the disease. In this study, however, the incidence of diabetes was low with only six incident cases among 181 individuals during a mean follow-up period of 25 years. Older work from Pima Indians also suggested, on the basis of 2-h insulin, that insulin resistance was more important in predicting the conversion to diabetes from IGT but not from NGT (19). However, our results indicate that both SI and AIR are independent determinants of diabetes regardless of family history of diabetes or glucose tolerance status.
In a report from the UK Prospective Diabetes Study, leaner and younger diabetic subjects had higher rates of treatment failure than their obese and older counterparts (20). Because this finding suggests that the primary defect in normal-weight individuals with diabetes is a secretory defect, some treatment guidelines have recommended using metformin as a first-line treatment in overweight patients and either sulfonylurea or metformin in normal-weight patients (21). A secretory defect, however, may not be the only relevant anomaly in most lean and young individuals who develop diabetes. Ong et al. (22) have shown that obesity does not modify the glycemic response to metformin in type 2 diabetes. Our results also suggest that the relation of insulin resistance and insulin secretion to conversion to diabetes is similar across categories of BMI and age.
Studies that use surrogate measures of insulin resistance and β-cell function indicate that insulin resistance and insulin secretion are major determinants of type 2 diabetes (1–4). Lyssenko et al. (4) have shown that longitudinal changes in insulin resistance and insulin secretion in converters as measured by homeostasis model assessment of insulin resistance and secretion are greater than changes in nonconverters. Furthermore, the key element for the onset of diabetes is the inability of the β-cells to compensate for the change in insulin resistance (4,23). Our study is novel because it highlights the relevance of both insulin resistance and β-cell dysfunction in different ethnic groups and various states of glucose tolerance, family history of diabetes, and obesity. Our results also indicate that insulin secretion is an independent risk factor across different degrees of insulin resistance and so is insulin resistance across various degrees of insulin secretion.
Our study has several limitations. We expressed insulin secretion as the mean of 2- and 4-min insulin concentrations after glucose administration, reflecting first-phase insulin secretion. Thus, we have no information on alternative aspects of β-cell function such as second-phase insulin secretion, potentiation of insulin release by glucose, or the pulsatility and oscillation of insulin secretion.
In summary, consistent with the multifactorial nature of diabetes suggested by extant genetic studies (17,18,24), our results indicate that both insulin sensitivity and β-cell function are important determinants of future diabetes in different ethnic groups and various states of glucose tolerance, family history of diabetes, and obesity. Therefore, our findings indicate that the prevention of type 2 diabetes should focus on interventions that improve both insulin resistance and insulin secretion. Prevention approaches should not differ in non-Hispanic whites, African Americans, and Hispanics. These conclusions are reinforced by the results of the Diabetes Prevention Program study (25), which showed similar benefit across ethnic groups. Therapeutic approaches may be also useful in individuals with a broad range of established risk factors including age, sex, obesity, glucose tolerance status, and family history of diabetes.
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute Grants HL-47887, HL-47889, HL-47890, HL-47892, and HL-47902 and the General Clinical Research Centers program, National Center for Research Resources Grants M01-RR-431 and M01-RR-01346.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Charles MA, Bennett PH: A two-step model for development of non-insulin-dependent diabetes. Am J Med 1991; 90: 229– 235 [PubMed] [Google Scholar]
- 2. Kahn SE: The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003; 46: 3– 19 [DOI] [PubMed] [Google Scholar]
- 3. Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, Andres R, Saudek C, Edelstein SL, Arakaki R, Murphy MB, Shamoon H: the Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes 2005; 54: 2404– 2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissén M, Isomaa B, Forsen B, Homström N, Saloranta C, Taskinen MR, Groop L, Tuomi T: the Botnia Study Group. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005; 54: 166– 174 [DOI] [PubMed] [Google Scholar]
- 5. Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR: Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 1992; 340: 925– 929 [DOI] [PubMed] [Google Scholar]
- 6. Goldfine AB, Bouche C, Parker RA, Kim C, Kerivan A, Soeldner JS, Martin BC, Warram JH, Kahn CR: Insulin resistance is a poor predictor of type 2 diabetes in individuals with no family history of disease. Proc Natl Acad Sci USA 2003; 100: 2724– 2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C: Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med 1993; 329: 1988– 1992 [DOI] [PubMed] [Google Scholar]
- 8. Nijpels G, Boorsma W, Dekker JM, Hoeksema F, Kostense PJ, Bouter LM, Heine RJ: Absence of an acute insulin response predicts onset of type 2 diabetes in a Caucasian population with impaired glucose tolerance. J Clin Endocrinol Metab 2008; 93: 2633– 2638 [DOI] [PubMed] [Google Scholar]
- 9. Weyer C, Tataranni PA, Bogardus C, Pratley RE: Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 2001; 24: 89– 94 [DOI] [PubMed] [Google Scholar]
- 10. Osei K, Rhinesmith S, Gaillard T, Schuster D: Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care 2004; 27: 1439– 1446 [DOI] [PubMed] [Google Scholar]
- 11. Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, Henkin L, Howard G, Savage PJ, Saad MF, Bergman RN, Hamman R: The Insulin Resistance Atherosclerosis Study (IRAS): design, objectives and recruitment results. Ann Epidemiol 1995; 5: 464– 472 [DOI] [PubMed] [Google Scholar]
- 12. Pacini G, Bergman RN: MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 1986; 23: 113– 122 [DOI] [PubMed] [Google Scholar]
- 13. Chaiken RL, Banerji MA, Pasmantier R, Huey H, Hirsch S, Lebovitz HE: Patterns of glucose and lipid abnormalities in black NIDDM subjects. Diabetes Care 1991; 14: 1036– 1042 [DOI] [PubMed] [Google Scholar]
- 14. Haffner SM, Howard G, Mayer E, Bergman RN, Savage PJ, Rewers M, Mykkänen L, Karter AJ, Hamman R, Saad MF: Insulin sensitivity and acute insulin response in African-Americans, non-Hispanic whites, and Hispanics with NIDDM: the Insulin Resistance Atherosclerosis Study. Diabetes 1997; 46: 63– 69 [DOI] [PubMed] [Google Scholar]
- 15. Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkänen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE, Bergman RN: Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Diabetes 1996; 45: 742– 748 [DOI] [PubMed] [Google Scholar]
- 16. Goedecke JH, Dave JA, Faulenbach MV, Utzschneider KM, Lambert EV, West S, Collins M, Olsson T, Walker BR, Seckl JR, Kahn SE, Levitt NS: Insulin response in relation to insulin sensitivity: an appropriate β-cell response in black South African women. Diabetes Care 2009; 32: 860– 865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rich SS, Goodarzi MO, Palmer ND, Langefeld CD, Ziegler J, Haffner SM, Bryer-Ash M, Norris JM, Taylor KD, Haritunians T, Rotter JI, Chen YD, Wagenknecht LE, Bowden DW, Bergman RN: A genome-wide association scan for acute insulin response to glucose in Hispanic-Americans: the Insulin Resistance Atherosclerosis Family Study (IRAS FS). Diabetologia 2009; 52: 1326– 1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palmer ND, Goodarzi MO, Langefeld CD, Ziegler J, Norris JM, Haffner SM, Bryer-Ash M, Bergman RN, Wagenknecht LE, Taylor KD, Rotter JI, Bowden DW: Quantitative trait analysis of type 2 diabetes susceptibility loci identified from whole genome association studies in the Insulin Resistance Atherosclerosis Family Study. Diabetes 2008; 57: 1093– 1100 [DOI] [PubMed] [Google Scholar]
- 19. Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH: Sequential changes in serum insulin concentration during development of non-insulin-dependent diabetes. Lancet 1989; 1: 1356– 1359 [DOI] [PubMed] [Google Scholar]
- 20. United Kingdom Prospective Diabetes Study 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group. Ann Intern Med 1998; 128: 165– 1675 [DOI] [PubMed] [Google Scholar]
- 21. McIntosh A, Hutchinson A, Home PD, Brown F, Bruce A, Damerell A, Davis R, Field R, Frost. Marshall S, Roddick J, Tesfaye S, Withers H, Suckling R, Smith S, Griffin S, Kaltenthaler E, Peters J, Feder G: Clinical Guidelines and Evidence Review for Type 2 Diabetes: Management of Blood Glucose. Sheffield U.K., ScHARR, University of Sheffield, 2001 [Google Scholar]
- 22. Ong CR, Molyneaux LM, Constantino MI, Twigg SM, Yue DK: Long-term efficacy of metformin therapy in nonobese individuals with type 2 diabetes. Diabetes Care 2006; 29: 2361– 2364 [DOI] [PubMed] [Google Scholar]
- 23. Festa A, Williams K, D'Agostino R, Jr, Wagenknecht LE, Haffner SM: The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes 2006; 55: 1114– 1120 [DOI] [PubMed] [Google Scholar]
- 24. Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L: Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 2008; 359: 2220– 2232 [DOI] [PubMed] [Google Scholar]
- 25. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]