Abstract
OBJECTIVE
We examined trends in incidence of treatment for diabetes-related end-stage renal disease (ESRD) in the U.S.
RESEARCH DESIGN AND METHODS
Using the U.S. Renal Data System, we obtained the number of individuals having diabetes listed as primary diagnosis who initiated ESRD treatment between 1990 and 2006. Incidence was calculated using the estimated U.S. population with diabetes from the National Health Interview Survey and then was age adjusted based on the 2000 U.S. standard population. Trends were analyzed using joinpoint regression.
RESULTS
The number of individuals who began diabetes-related ESRD treatment increased from 17,727 in 1990 to 48,215 in 2006. From 1990 to 1996, the age-adjusted diabetes-related ESRD incidence increased somewhat from 299.0 to 343.2 per 100,000 diabetic population (P = 0.45). However, from 1996 to 2006, the age-adjusted diabetes-related ESRD incidence decreased by 3.9% per year (P < 0.01) from 343.2 to 197.7 per 100,000 diabetic population. Among individuals with diabetes aged <45 years, diabetes-related ESRD incidence decreased by 4.3% per year (P < 0.01) from 1990 to 2006. Among older individuals, incidence increased during the 1990s but decreased in later years, by 3.9% per year (P < 0.01) among individuals aged 45–64, by 3.4% per year (P < 0.01) among individuals aged 65–74 years, and by 2.1% per year (P = 0.02) among individuals aged ≥75 years.
CONCLUSIONS
Diabetes-related ESRD incidence in the diabetic population has declined in all age-groups, probably because of a reduction in the prevalence of ESRD risk factors, improved treatment and care, and other factors.
End-stage renal disease (ESRD) (i.e., kidney failure requiring dialysis or transplantation) is a costly and disabling condition with a high mortality rate (1). In 2006, ESRD costs reached nearly $23 billion, >6% of the Medicare budget, and mortality rates were about eight times greater among individuals aged 20–64 years with ESRD treated by dialysis than among those in the general population of similar age (1). Diabetes is the leading cause of ESRD in the U.S., accounting for 44% of new cases of treated ESRD in 2006 (1). In addition, the prevalence of diabetes continues to increase (2) with nearly 24 million adults in 2007 with the disease (3). The incidence of diabetes-related ESRD in the general population, which is influenced by the increasing number of individuals with diabetes, also continues to increase. However, diabetes-related ESRD incidence among individuals with diabetes has decreased from 1997 to 2002 despite the increase in the population living with diabetes (4). This analysis used data from the U.S. Renal Data System (USRDS) to examine recent trends in incidence of treatment for ESRD in the population with diabetes, which takes into account the growing number of individuals with diabetes, and to determine whether previously reported declining trends in this population have continued.
RESEARCH DESIGN AND METHODS
USRDS collects, analyzes, and distributes information from clinical and claims data reports to the Centers for Medicare and Medicaid Services (CMS) regarding patients being treated for ESRD, with oversight from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Through the ESRD entitlement program, CMS reimburses most of the costs of ESRD treatment in the U.S. (5). USRDS includes demographic and clinical data for almost all individuals with ESRD in the U.S., including the date patients were first treated and the primary diagnosis (5). Renal care providers are required to complete the CMS Medical Evidence Report for each new patient with ESRD regardless of Medicare eligibility status. However, before 1995, the Medical Evidence Report was required only for Medicare-eligible patients (5).
The 1990–2006 USRDS data were used to determine the overall number of individuals in the U.S. with diabetes listed as the primary diagnosis who began treatment (i.e., dialysis or kidney transplantation) for ESRD. Thus, the term “incidence” will be used to refer to initiation of treatment for diabetes-related ESRD. The primary diagnosis (i.e., primary cause of renal failure) is taken from the CMS Medical Evidence Report and is based on the physician's assessment of the patient (5). Data were analyzed for whites, blacks, and Hispanics. The racial groups include individuals of both Hispanic and non-Hispanic origin; Hispanics may be of any race. The annual number of U.S. residents in whom diabetes is diagnosed was obtained from the weighted sample of the civilian noninstitutionalized population in the National Health Interview Survey (NHIS) using SUDAAN software (6) to account for the complex NHIS sample survey design. Incidence rates were calculated using the number of cases of diabetes-related ESRD and the estimated population with diabetes and then were age adjusted using the 2000 U.S. standard population. Standard errors for incidence, assuming no variance for the number of cases of diabetes-related ESRD, were calculated using Taylor series linearization methods (7) in SAS (8). The NHIS estimate of the number of U.S. residents with diabetes was unusually low in 1996, and the survey methodology was changed in 1997 (9). The low estimate in 1996 and the survey redesign in 1997 resulted in a large increase in the number of individuals with diagnosed diabetes between 1996 and 1997. Hispanic ethnicity data were not available in NHIS before 1997 (9). Finally, joinpoint regression analysis, which uses permutation tests to identify the points (i.e., “joinpoints”) where linear trends change significantly in direction or magnitude, was used to assess trends over time (e.g., 0 joinpoints indicates a simple straight line on the log scale) (10,11). The rate of change for each trend was tested to determine whether it was significantly different from 0 (e.g., no change), and each trend in the final model was described by an annual percentage change (APC). Results were considered significant if P < 0.05. The figures plot the predicted diabetes-related ESRD incidence values based on the joinpoint regression models.
RESULTS
The total number of individuals who began treatment for ESRD increased from 49,868 in 1990 to 108,928 in 2006. Of these cases of ESRD, 17,727 (36%) were diabetes related in 1990 and 48,215 (44%) were diabetes related in 2006. Throughout this period, the number of cases of diabetes-related ESRD consistently increased for all age, sex-age, and race-age groups; it also increased between 1997 and 2006 among Hispanics in all age-groups.
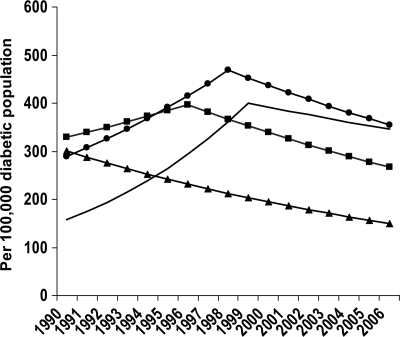
From 1990 to 1996, the crude diabetes-related ESRD incidence increased significantly, from 285.4 to 421.9 per 100,000 diabetic population (Table 1). Age-adjusted incidence increased nonsignificantly from 299.0 to 343.2 per 100,000 diabetic population. From 1996 to 2006, both crude and age-adjusted diabetes-related ESRD incidence decreased, with the age-adjusted incidence dropping from 343.2 to 197.7 per 100,000 diabetic population, a decline of 3.9% per year. Among individuals with diabetes aged <45 years, diabetes-related ESRD incidence decreased from 1990 to 2006, a 4.3% decrease per year (Table 1, Fig. 1). From 1990 to 1996, among individuals aged 45–64 years, incidence increased from 340.6 to 451.8 per 100,000 diabetic population and then declined 3.9% per year to 273.8 in 2006. Among individuals aged 65–74 years, incidence increased from 263.8 per 100,000 diabetic population in 1990 to 448.2 in 1998 and then declined 3.4% per year to 368.6 in 2006. Among individuals aged ≥75 years, incidence increased from 172.5 per 100,000 diabetic population in 1990 to 380.0 in 1999 and declined from 1999 to 2006 by 2.1% per year to 328.5 per 100,000 diabetic population.
Table 1.
Incidence rates of diabetes-related ESRD and APC, by age, sex, and race or ethnicity, United States, 1990–2006
| Incidence rate* |
Trend |
||||
|---|---|---|---|---|---|
| 1990 | 2006 | Period | APC (95% CI) | P | |
| Total | 285.4 | 278.4 | 1990–1996 | 5.8 (3.5 to 8.0) | <0.01 |
| 1996–2006 | −2.9 (−3.6 to −2.2) | <0.01 | |||
| Total† | 299.0 | 197.7 | 1990–1996 | 1.1 (−1.9 to 4.1) | 0.45 |
| 1996–2006 | −3.9 (−4.7 to −3.1) | <0.01 | |||
| Age (years) | |||||
| <45 | 300.1 | 142.3 | 1990–2006 | −4.3 (−5.1 to −3.4) | <0.01 |
| 45–64 | 340.6 | 273.8 | 1990–1996 | 3.2 (0.6 to 5.9) | 0.02 |
| 1996–2006 | −3.9 (−4.6 to −3.2) | <0.01 | |||
| 65–74 | 263.8 | 368.6 | 1990–1998 | 6.2 (3.2 to 9.2) | <0.01 |
| 1998–2006 | −3.4 (−5.4 to −1.4) | <0.01 | |||
| ≥75 | 172.5 | 328.5 | 1990–1999 | 10.9 (8.9 to 12.9) | <0.01 |
| 1999–2006 | −2.1 (−3.7 to −0.3) | 0.02 | |||
| Sex† | |||||
| Male | 363.7 | 230.5 | 1990–2006 | −2.8 (−3.6 to −1.9) | <0.01 |
| Female | 250.6 | 168.6 | 1990–1996 | 2.4 (−1.5 to 6.4) | 0.20 |
| 1996–2006 | −4.3 (−5.3 to −3.3) | <0.01 | |||
| Race†‡ | |||||
| White | 266.2 | 164.7 | 1990–1996 | 1.3 (−3.0 to 5.7) | 0.53 |
| 1996–2006 | −5.0 (−6.0 to −4.0) | <0.01 | |||
| Black | 408.9 | 327.7 | 1990–2006 | −1.7 (−2.9 to −0.6) | <0.01 |
| Ethnicity†§ | |||||
| Hispanic‖ | 306.7¶ | 254.3 | 1997–2006 | −1.5 (−3.3 to 0.3) | 0.09 |
*Per 100,000 diabetic population.
†Age-adjusted based on the 2000 U.S. standard population.
‡The racial groups include people of both Hispanic and non-Hispanic origin.
§NHIS began collecting Hispanic ethnicity data in 1997.
‖Hispanics may be of any race.
¶Data for 1997. APC, annual percent change.
Figure 1.
Modeled age-specific incidence of diabetes-related ESRD in the population with diabetes, U.S., 1990–2006. Predicted values: ▲——▲, <45 years; ■——■, 45–64 years; ●——●, 65–74 years; ——, ≥75 years. Predicted values were modeled using joinpoint regression analysis.
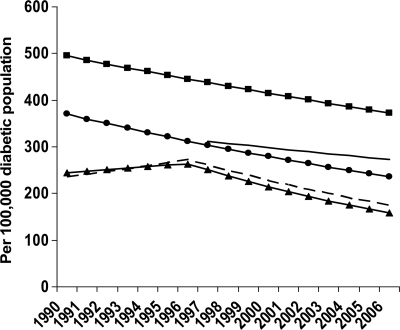
From 1990 to 2006, the age-adjusted diabetes-related ESRD incidence was higher among men than among women (Fig. 2). Throughout this period, the age-adjusted diabetes-related ESRD incidence among men decreased at an average of 2.8% per year, from 363.7 per 100,000 diabetic population in 1990 to 230.5 in 2006 (Table 1). The decline in incidence among women did not begin until 1996, with the age-adjusted incidence decreasing at an average of 4.3% per year, from 299.3 per 100,000 diabetic population in 1996 to 168.6 in 2006 (Table 1). Before 1996, the age-adjusted diabetes-related ESRD incidence among women displayed a nonsignificant increase from 250.6 per 100,000 diabetic population in 1990 to 299.3 in 1996 (Table 1).
Figure 2.
Modeled age-adjusted incidence of diabetes-related ESRD in the population with diabetes, by sex and race or ethnicity, U.S., 1990–2006. Predicted values: – – –, women; ●——●, men; ▲——▲, whites; ■——■, blacks; ——, Hispanics. Predicted values were modeled using joinpoint regression analysis. The racial groups include individuals of both Hispanic and non-Hispanic origin; Hispanics may be of any race. NHIS began collecting Hispanic ethnicity data in 1997.
From 1990 to 2006, the age-adjusted diabetes-related ESRD incidence by race or ethnicity was highest among blacks and lowest among whites (Fig. 2). During this period, the age-adjusted diabetes-related ESRD incidence among blacks decreased significantly by 1.7% per year from 408.9 per 100,000 diabetic population to 327.7 (Table 1). However, among whites, diabetes-related ESRD incidence did not begin to decline until 1996, from 296.7 per 100,000 diabetic population to 164.7 in 2006, a decline of 5.0% per year (Table 1). Among Hispanics, the age-adjusted diabetes-related ESRD incidence decreased between 1997 and 2006 but not significantly (Table 1).
CONCLUSIONS
We found that diabetes-related ESRD incidence in the population with diabetes has continued to decline, which is consistent with a previous report (4) and suggests that current efforts in prevention of ESRD may be successful. The decreasing trends in diabetes-related ESRD incidence are now being seen in all age-groups, men, women, whites, and blacks, unlike previously reported data showing declining incidence only among individuals aged <65 years, women, and whites (4). However, from 1997 to 2006, diabetes-related ESRD incidence among Hispanics did not decrease as it did among the other populations, so additional strategies are needed to improve this trend.
Diabetes-related ESRD incidence declined throughout the study period among individuals with diabetes aged <45 years. Most individuals with diabetes in this age-group are likely to have type 1 diabetes, and intensive insulin therapy has been shown to reduce the risk of kidney disease in individuals with type 1 diabetes (12). Between 1999 and 2002 and 2003 and 2006, the proportion of individuals with diabetes aged 20–39 years achieving glycemic control target levels increased significantly (13), suggesting that improved glycemic control may have contributed to the declining trends. In the older age-groups, diabetes-related ESRD incidence began to decline in 1996 for those aged 45–64 years, in 1998 for those aged 65–74 years, and in 1999 for those aged ≥75 years. Reasons to explain the decreasing trends cannot be gleaned from surveillance data but may include early detection and management of kidney disease, improved treatment and care, better control of ESRD risk factors (i.e., diabetes and hypertension), or other factors (14–16). Furthermore, new pharmacological agents, such as ACE inhibitors and angiotensin-receptor blockers, also have been determined to be renoprotective, independent of their ability to reduce blood pressure (17). Between 70 and 75% of patients aged ≥65 years with diabetes and chronic kidney disease (CKD) use ACE inhibitors or angiotensin receptor blockers, and the percentage is nearly 80% among those aged 20–64 years (1).
An alternative explanation for the decline in diabetes-related ESRD incidence is that the large and sustained increase of new cases of diabetes that has occurred since the 1990s (2) may have led to a large number of individuals who have not had diabetes long enough to develop ESRD. Furthermore, in 1997, the American Diabetes Association revised the diagnostic criteria for diabetes, lowering the threshold of the fasting glucose value from 140 to 126 mg/dl (18). This change may have resulted in a greater number of individuals with milder disease, detected earlier in the disease process. Once these patients with new-onset, milder disease have had diabetes long enough, it is possible that the encouraging trends in diabetes-related ESRD incidence may reverse. In addition, it is possible that improved survival among individuals with diabetes could lead to longer diabetes durations and, thus, greater opportunity to develop ESRD.
Even though diabetes-related ESRD incidence in the population with diabetes has decreased since 1996, diabetes-related ESRD incidence in the general population and the number of new cases of diabetes-related ESRD continue to increase, albeit at a slower rate (1,2). Reducing the number of new cases of diabetes-related ESRD will be difficult to achieve as our population ages and the prevalence of ESRD risk factors, such as diabetes, continues to increase (2). Nearly 24 million adults in the U.S. had diabetes in 2007 (3), and 75% of adults with diabetes have hypertension (i.e., blood pressure ≥130/80 mm of mercury or use prescription medications for hypertension) (16). Furthermore, >26 million U.S. adults were estimated to have CKD (19), placing them at risk of progressing to ESRD. For reasons that are not yet clear, individuals belonging to minority populations in the U.S. may progress rapidly from CKD to ESRD (20). Increased awareness of the risk of developing kidney disease among individuals with diabetes or hypertension (21) and interventions, such as blood glucose and blood pressure control (22,23), to prevent or delay the onset of kidney disease or reduce its progression are needed to sustain and improve trends in diabetes-related ESRD incidence.
The findings in this report are subject to several limitations. First, data were collected for individuals whose ESRD treatment was reported to CMS; those who died from ESRD before receiving treatment, those who refused treatment, and those whose treatment was not reported to CMS were not included (5). Second, because the incidence of diabetes-related ESRD was defined in terms of initiation of ESRD treatment, changes in incidence may have been due to changes in factors other than disease incidence. These factors may include better access to ESRD treatment or acceptance of treatment, changes in treatment and care practices, greater recognition of the etiologic role of diabetes in ESRD, or a combination of these. The primary diagnosis was taken from the CMS Medical Evidence Report and was based on the physician's assessment of the patient (5), which may have affected trends, especially if patients had other ESRD risk factors such as hypertension. From 1999 to 2004, three of four individuals with diabetes also had hypertension (16). Third, the estimated population with diabetes from the NHIS was based on self-report and underestimates the total population with diabetes in the U.S. Approximately 25% of individuals with diabetes have not had their diabetes diagnosed (3). Fourth, the low estimate of the population with diabetes in 1996 and the survey redesign in 1997 resulted in a large increase in the number of individuals with diagnosed diabetes between 1996 and 1997 (9). Despite the dip in the number of cases between 1996 and 1997, joinpoint regression analysis indicated that the prevalence and incidence of diabetes increased steadily (i.e., without significant changes in direction or magnitude) between 1990 and 2006 (2,24). Furthermore, exclusion of the 1996 data did not substantially affect diabetes-related ESRD incidence trends (4). More investigation is needed to determine what other factors might explain the declining diabetes-related ESRD incidence in the population with diabetes since 1996. Finally, the correlation between the length of time diabetic patients had the disease and their risk for developing diabetes-related ESRD was not assessed because of a lack of data on duration of diabetes.
Diabetes-related ESRD is a costly and disabling condition with a high mortality rate that continues to disproportionately affect U.S. minority populations (1). In 2006, the age-adjusted diabetes-related ESRD incidence among Hispanics and blacks with diabetes was 1.5 and 2.0 times greater than that for whites. Intervention programs to prevent ESRD and eliminate racial/ethnic disparities should aim to raise awareness about kidney disease and promote early diagnosis, prevent and control ESRD risk factors, and improve patient outcomes, especially among populations with a greater burden of ESRD. Continued surveillance of diabetes-related ESRD using USRDS data will help public health officials monitor and assess progress in lowering incidence and reducing racial/ethnic disparities. New initiatives at the Centers for Disease Control and Prevention are currently underway to establish a national surveillance system for CKD and its risk factors that can estimate the magnitude of disease in the U.S., monitor trends, and identify groups at risk (25).
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The data reported here have been supplied by the U.S. Renal Data System. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. United States Renal Data System. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 2. Centers for Disease Control and Prevention. Diabetes data & trends [article online]. Available from http://www.cdc.gov/diabetes/statistics/index.htm Accessed 28 January 2009
- 3. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008 [Google Scholar]
- 4. Burrows NR, Wang J, Geiss LS, Venkat Narayan KM, Engelgau MM: Incidence of end-stage renal disease among persons with diabetes—United States, 1990–2002. MMWR Morb Mortal Wkly Rep 2005; 54: 1097– 1100 [PubMed] [Google Scholar]
- 5. United States Renal Data System. Researcher's Guide to the USRDS Database. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007 [Google Scholar]
- 6. Research Triangle Institute. SUDAAN: Software for the Statistical Analysis of Correlated Data. Release 9.0. Research Triangle Park, NC, Research Triangle Institute, 2004 [Google Scholar]
- 7. Vonesh EF, Wang H, Majumdar D: Generalized least squares, Taylor series linearization, and Fisher's scoring in multivariate nonlinear regression. J Am Stat Assoc 2001; 96: 282– 291 [Google Scholar]
- 8. SAS Institute. SAS. Release 9.1. Cary, NC, SAS Institute, 2002 [Google Scholar]
- 9. Centers for Disease Control and Prevention. 1997 National Health Interview Survey (NHIS) public use data release: NHIS survey description [article online], 2000. Hyattsville, MD, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Available from http://ftp.cdc.gov/pub/health_statistics/nchs/dataset_documentation/nhis/1997/srvydesc.pdf Accessed 28 October 2008 [Google Scholar]
- 10. Surveillance Research Program of the U.S. National Cancer Institute. Joinpoint Regression Program. Version 3.0, 2008. Available from http://srab.cancer.gov/joinpoint/ Accessed 28 October 2008
- 11. Kim HJ, Fay MP, Feuer EJ, Midthune DN: Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335– 351 [DOI] [PubMed] [Google Scholar]
- 12. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977– 986 [DOI] [PubMed] [Google Scholar]
- 13. Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS: Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med 2009; 122: 443– 453 [DOI] [PubMed] [Google Scholar]
- 14. Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, Narayan KM: Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med 2006; 144: 465– 474 [DOI] [PubMed] [Google Scholar]
- 15. Imperatore G, Cadwell BL, Geiss L, Saadinne JB, Williams DE, Ford ES, Thompson TJ, Narayan KM, Gregg EW: Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: National Health and Nutrition Examination Surveys, 1971–2000. Am J Epidemiol 2004; 160: 531– 539 [DOI] [PubMed] [Google Scholar]
- 16. Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS: Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18: 222– 229 [DOI] [PubMed] [Google Scholar]
- 17. Parving HH, Hovind P: Microalbuminuria in type 1 and type 2 diabetes mellitus:evidence with angiotensin converting enzyme inhibitors and angiotensin II receptor blockers for treating early and preventing clinical nephropathy. Curr Hypertens Rep 2002; 4: 387– 393 [DOI] [PubMed] [Google Scholar]
- 18. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183– 1197 [DOI] [PubMed] [Google Scholar]
- 19. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038– 2047 [DOI] [PubMed] [Google Scholar]
- 20. Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 2003; 14: 2902– 2907 [DOI] [PubMed] [Google Scholar]
- 21. Plantinga LC, Boulware LE, Coresh J, Stevens LA, Miller ER, 3rd, Saran R, Messer KL, Levey AS, Powe NR: Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med 2008; 168: 2268– 2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837– 853 [PubMed] [Google Scholar]
- 23. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317: 703– 713 [PMC free article] [PubMed] [Google Scholar]
- 24. Geiss LS, Wang J, Gregg EW: Long term trends in the prevalence and incidence of diagnosed diabetes (Abstract). Diabetes 2007; 56( Suppl. 1): A33 [Google Scholar]
- 25. Centers for Disease Control and Prevention. Chronic kidney disease initiative [article online]. Available from http://www.cdc.gov/diabetes/projects/kidney.htm Accessed 28 January 2009