Abstract
OBJECTIVE
To examine whether day napping or short night sleeping is associated with higher risk of diabetes.
RESEARCH DESIGN AND METHODS
This was a prospective study of hours of day napping and night sleeping assessed in 1996–1997 in relation to diabetes diagnosed between 2000 and 2006 (n = 10,143) among 174,542 participants in the National Institutes of Health (NIH)-AARP Diet and Health Study. Odds ratios (ORs) and 95% CI were derived from multivariate logistic regression models.
RESULTS
Longer day napping was associated with a higher risk of diabetes. After adjustment for potential confounders, ORs were 1.23 (95% CI 1.18–1.29) for those reporting <1 h and 1.55 (95% CI 1.45–1.66) for those reporting ≥1 h of napping compared with individuals who did not nap (Ptrend < 0.0001). For night sleeping, with 7–8 h as the referent, the OR was 1.46 (95% CI 1.31–1.63) for <5 h, 1.11 (1.06–1.16) for 5–6 h, and 1.11 (0.99–1.24) for ≥9 h. In both analyses, additional adjustment for BMI only modestly attenuated the associations. Further analysis showed a statistically significant interaction between hours of napping and sleeping on diabetes (Pinteraction < 0.0001). Among participants with no napping, only short night sleeping was associated with higher occurrence of diabetes, whereas among those with ≥1 h of napping, both long and short sleeping was associated with higher risk.
CONCLUSIONS
Day napping and short night sleeping are associated with higher risk of diabetes. The association between sleep duration and diabetes may be modified by napping habit.
It is recommended that adults have 7–8 h of quality sleep per night; however, national data show that short sleeping has become increasingly prevalent across all adult age and sex groups over the past decades (1). Short sleep may have deleterious health consequences, including higher risk of diabetes that was recently reported in a few prospective cohorts (2–5). It is hypothesized that obesity may in part explain this observation on short sleep duration and diabetes (6).
Day napping is common among older adults (7,8); however, the health consequences of napping are poorly understood. Recent cross-sectional analyses reported that day napping was more common among diabetic patients than among those without diabetes (8,9). These cross-sectional analyses provide little information on the direction and nature of this finding. Although it is plausible that napping is secondary to clinical diabetes, it is not unreasonable to hypothesize that napping itself may be associated with a higher risk of diabetes. A further complication is that day napping and night sleeping may not be independent of each another and may jointly affect diabetes. However, to the best of our knowledge, this possibility has not been evaluated. We therefore used data from the National Institutes of Health (NIH)-AARP (formerly known as the American Association of Retired Persons) Diet and Health cohort to prospectively evaluate the individual and joint effect of hours of day napping or night sleeping on the risk of incident diabetes.
RESEARCH DESIGN AND METHODS
The NIH-AARP Diet and Health cohort was established in 1995–1996 by the National Cancer Institute to investigate roles of diet and lifestyle in cancer etiology (10). Cohort participants included 566,402 AARP members aged 50–71 years in 1995–1996 from six states and two metropolitan areas of the U.S. All study participants completed a comprehensive dietary survey, including a 124-item food frequency questionnaire and a short survey on demographics, medications, and lifestyle (10). Six months later in 1996–1997, 334,908 participants in the original cohort further answered a second questionnaire (the risk factor survey) to provide more details on their health behaviors, including hours of day napping and nighttime sleeping. A follow-up questionnaire was mailed out to surviving participants of the original cohort in 2004–2006 to update exposures and to ascertain the occurrences of major chronic diseases, including diabetes. A total of 318,261 participants responded to the follow-up survey. The base population of the current analysis therefore included 220,934 participants who participated in both the risk factor survey in 1996–1997 and the follow up survey in 2004–2006. We excluded 481 participants with missing values on hours of day napping and night sleeping and 22,041 participants with missing values on diabetes diagnosis. Because sleeping habits were assessed in 1996–1997, to reduce the possibility that diabetes itself might have affected sleeping habits, we further excluded 23,870 participants who reported a diabetes diagnosis before 2000. The final analytic sample included 164,399 participants without diabetes and 10,143 participants with diabetic diagnosed after 2000.
Exposure assessment
At the risk factor survey in 1996–1997, participants were asked the number of hours spent on day napping and night sleeping during a typical 24-h period over the past 12 months. Five choices were allowed for the day napping question: none, <1 h, 1–2 h, 3–4 h, or ≥5 h. For night sleeping, the answer included four categories: <5 h, 5–6 h, 7–8 h, and ≥9 h. The risk factor questionnaire also asked participants to recall how often they participated in light physical activities (such as bowling, slow walking, or slow dancing) or moderate to vigorous activities (such as tennis, biking, or swimming) in the past 10 years with six possible answers: none, rarely, weekly but <1 h/week, 1–3 h/week, 4–7 h/week, and >7 h/week. Finally, the risk factor questionnaire asked participants whether blood relatives of their immediate family (father, mother, brother, or sister) had diabetes.
The amount of coffee or alcohol consumption and total calorie intake were derived from the dietary survey in 1995–1996. In addition, the survey collected basic demographic and lifestyle information such as date of birth, sex, race, education level, marital status, and smoking habit. Further, participants were asked to self-evaluate their health status as excellent, very good, good, fair, or poor. Finally, participants also reported weight in pounds (0.45 kg) and height in inches (2.54 cm). BMI was calculated as weight in kilograms divided by the square of height in meters.
Ascertainment of diabetes
As part of the 1995–1996 dietary survey, participants were asked whether they had ever been told by a doctor that they had diabetes. A similar question was also asked on the follow-up questionnaire in 2004–2006 with categorical choices of the year of first diagnosis: before 1985, 1985–1994, 1995–1999, and 2000 to present. These questions did not differentiate type 2 from type 1 diabetes. However, in adults, ∼90–95% of all diagnosed diabetes is type 2 diabetes (11). Because the current study included only older adults and adults with incident cases of diabetes diagnosed after 2000, we believe that most of the participants in the current analysis should have type 2 diabetes.
Statistical analysis
Multivariate odd ratios (ORs) and 95% CIs were derived from logistic regression models. In the napping analysis, participants reporting no napping were used as the reference group and those with ≥1 h of napping were grouped together because only 0.2% of the participants reported >2 h of napping. For the sleeping analysis, we used 7–8 h of sleep as the referent because it was reported by most participants (60.1%) and is considered optimal sleep length for adults. Potential confounders included age in 5-year groups for the risk factor survey, sex, race (white vs. nonwhite), education level (<8 years, 8–11 years, 12 years or completed high school, post–high school or some college, and college and postgraduate), marital status (married or living as married, widowed, divorced, separated, or never married), smoking status (never smokers; past smokers: ≥35, 30–34, 20–29, 10–19, or 1–9 years since last smoking; and current smokers: 1–10, 11–20, or >20 cigarettes/day), coffee consumption (0, <1, 1, 2–3, or >3 cups/day), alcohol consumption (0, <1, 1–1.9, 2–2.9, or ≥3 drinks/day), general health status (excellent, very good, good, fair, or poor), family history of diabetes (yes vs. no), and total energy intake (quintiles). Because obesity and lack of physical activity may be in the pathway between napping and sleeping habits and diabetes, we first fitted the regression models without these variables and then added them individually or simultaneously. In the napping analysis, statistical significance for a linear trend was tested by assigning a value to each category of the napping variable (0 for no napping, 0.5 for <1 h, and 1.5 for ≥1 h) and including it as a continuous variable in the regression model. No such tests were conducted for the sleeping analysis as its relation to diabetes was not linear.
We conducted two additional analyses to examine the robustness of our findings. First, we limited the analysis to participants who met the all of the following criteria: self-evaluated excellent or very good health, never smoked or stopped smoking >10 years ago, nonobese (BMI <30 kg/m2), and >1 h of moderate to vigorous physical activities per week. Presumably, this population represented a fairly healthy population at baseline and therefore association identified or confirmed in this group would be less likely to be attributed to poor health status or reverse causality. The second were subgroup analyses according to age (<60, 60–64, and ≥65 years), sex, education level (below high school vs. high school or more), general health status (“excellent or very good,” “good,” and “fair or poor”), BMI (12.0–24.9, 25.0–29.9, or ≥30.0 kg/m2), smoking status (never vs. ever), and family history of diabetes (yes vs. no). When possible, detailed values of the stratifying variables were adjusted along with all other confounders to minimize the possibility of residual confounding.
Finally, we conducted a joint analysis by combining categories of day napping with those of night sleeping, using participants with no napping and 7–8 h of night sleeping as the referent. We further examined the statistical interaction between these two variables by including a multiplicative interaction term in the regression model. All statistical analysis was performed by using SAS (release 9.1; SAS Institute, Cary, NC) and the significance tests were two-tailed with α = 0.05.
RESULTS
Table 1 shows population characteristics according to napping or sleeping duration. Compared with individuals who reported no napping, nappers were more likely to be older men, nonwhites, and current smokers and to report a family history of diabetes and higher calorie intake, but they were less likely to drink coffee or alcohol and to report excellent or very good health status or 7–8 h of night sleep. As expected, nappers had higher BMI and were less physically active. For night sleeping, participants with 7–8 h of sleep seemed to have the demographic and lifestyle characteristics that were often associated with favorable health outcomes. Compared with this group, individuals with <5 h of sleeping were more likely to be women, nonwhites, and current smokers and to report a higher BMI or ≥1 h of day napping, a family history of diabetes, and higher calorie intake. They were, however, less likely to be high school graduates, married or living as married, or past smokers or to report regular drinking of coffee or alcohol, excellent or very good health status, or regular physical activities.
Table 1.
Population characteristic according to hours of day napping and night sleeping
| Day napping |
Night sleeping |
||||||
|---|---|---|---|---|---|---|---|
| None | <1 h | ≥1 h | <5 h | 5–6 h | 7–8 h | ≥9 h | |
| n* | 94,165 | 67,520 | 12,335 | 3,963 | 53,030 | 111,731 | 5,620 |
| Age (years) | 61.6 ± 5.3 | 63.4 ± 5.2 | 63.5 ± 5.3 | 62.2 ± 5.4 | 62.1 ± 5.4 | 62.6 ± 5.3 | 63.3 ± 5.2 |
| Men (%) | 51.3 | 63.2 | 64.4 | 47.8 | 55.1 | 58.0 | 55.9 |
| Whites (%) | 95.3 | 94.7 | 90.3 | 87.6 | 92.0 | 96.1 | 96.6 |
| High school or more (%) | 81.3 | 78.8 | 74.2 | 67.1 | 77.1 | 81.5 | 80.3 |
| Married or couples (%) | 68.3 | 72.8 | 68.2 | 57.3 | 66.3 | 72.3 | 70.5 |
| Past smokers (%) | 49.9 | 51.8 | 51.8 | 45.7 | 49.7 | 51.4 | 52.9 |
| Current smokers (%) | 8.9 | 9.3 | 15.9 | 13.1 | 10.8 | 8.9 | 9.0 |
| ≥2 cups of coffee/day (%) | 57.6 | 56.8 | 55.4 | 51.7 | 56.8 | 57.7 | 53.0 |
| ≥1 drink of alcohol/day (%) | 26.3 | 23.6 | 21.4 | 18.2 | 21.4 | 26.3 | 34.2 |
| Health status (%) | |||||||
| Excellent or very good | 66.6 | 58.2 | 45.3 | 42.9 | 58.1 | 64.5 | 57.3 |
| Good | 28.3 | 33.9 | 39.3 | 36.9 | 33.8 | 29.8 | 31.5 |
| Fair or poor | 5.1 | 7.9 | 15.4 | 20.2 | 8.1 | 5.7 | 11.2 |
| BMI (kg/m2) | 26.1 ± 4.5 | 26.8 ± 4.7 | 27.6 ± 5.3 | 27.8 ± 5.7 | 26.8 ± 5.1 | 26.2 ± 4.5 | 26.5 ± 4.8 |
| ≥ 4 h/week light activities (%) | 62.4 | 62.7 | 58.6 | 58.3 | 60.1 | 63.4 | 62.3 |
| ≥4 h/week moderate to vigorous activities (%) | 53.2 | 52.7 | 45.1 | 48.2 | 51.0 | 53.4 | 48.2 |
| Calorie intake (kcal) | 1,770 ± 789 | 1,872 ± 861 | 2,022 ± 1,025 | 1,981 ± 1,253 | 1,833 ± 891 | 1,814 ± 783 | 1,943 ± 1,005 |
| Family history of diabetes (%) | 24.4 | 26.2 | 27.9 | 29.7 | 26.8 | 24.6 | 24.3 |
| Napping (%) | |||||||
| 0 | 44.5 | 50.3 | 56.3 | 53.9 | |||
| <1 h | 38.6 | 40.8 | 38.2 | 32.7 | |||
| ≥1 h | 16.9 | 8.8 | 5.6 | 13.4 | |||
| Sleeping (%) | |||||||
| <5 h | 1.9 | 2.3 | 5.4 | ||||
| 5–6 h | 28.3 | 32.0 | 38.0 | ||||
| 7–8 h | 66.6 | 63.0 | 50.5 | ||||
| ≥9 h | 3.2 | 2.7 | 6.1 | ||||
Data are means ± SD for continuous variables and proportions for categorical variables. N = 174,344. Hours of day napping and night sleeping, age, physical activity, and family history of diabetes were from risk factor survey in 1996–1997; all other covariates were collected at the dietary survey in 1995–1996.
*The final number of participants for individual variables varies because of missing values.
Duration of day napping in 1996–1997 was associated with higher risk of diabetes after 2000 in a dose-response manner (Table 2). Compared with participants who reported no napping, for those with <1 h of napping the multivariate OR was 1.23 (95% CI 1.18–1.29) and for those with ≥1 h of napping it was 1.55 (1.45–1.66) (Ptrend < 0.0001). The association was essentially unchanged after further adjustment for physical activities. It was moderately attenuated after adjustment for BMI alone or simultaneously with physical activities. The OR between ≥1 h versus no napping decreased from 1.55 to 1.37 after further adjustment for BMI and to 1.36 with simultaneous adjustment of BMI and physical activities. Nevertheless, in both models, the association between napping and diabetes remained statistically significant.
Table 2.
ORs (95% CI) of diabetes diagnosed after 2000 according to hours of day napping or night sleeping
| Day napping* |
Night sleeping |
||||||
|---|---|---|---|---|---|---|---|
| None | <1 h | ≥1 h | <5 h | 5–6 h | 7–8 h | ≥9 h | |
| n of cases | 4,465 | 4,463 | 1,172 | 390 | 3,427 | 5,965 | 348 |
| Basic model† | 1.0 | 1.23 (1.18–1.29) | 1.55 (1.45–1.66) | 1.46 (1.31–1.63) | 1.11 (1.06–1.16) | 1.0 | 1.11 (0.99–1.24) |
| + physical activities | 1.0 | 1.23 (1.18–1.29) | 1.53 (1.42–1.64) | 1.47 (1.31–1.64) | 1.11 (1.06–1.16) | 1.0 | 1.09 (0.97–1.22) |
| + BMI | 1.0 | 1.16 (1.11–1.21) | 1.37 (1.28–1.47) | 1.33 (1.19–1.49) | 1.06 (1.01–1.11) | 1.0 | 1.10 (0.98–1.23) |
| + physical activities and BMI | 1.0 | 1.16 (1.11–1.21) | 1.36 (1.27–1.46) | 1.34 (1.20–1.50) | 1.06 (1.01–1.11) | 1.0 | 1.09 (0.97–1.22) |
| n‡ | 991 | 842 | 126 | 37 | 601 | 1,262 | 64 |
| OR (95% CI) | 1.0 | 1.16 (1.06–1.28) | 1.34 (1.10–1.63) | 1.37 (0.97–1.93) | 1.15 (1.04–1.27) | 1.0 | 1.18 (0.91–1.53) |
Data are ORs (95% CI).
*All Ptrend < 0.0001 in the day napping and diabetes analysis with the exception of sensitivity analysis (Ptrend = 0.0002). No trend test was conducted for hours of night sleeping because the relationship was not linear.
†The basic model included the following covariates: age, sex, race, education, marital status, smoking, coffee and alcohol consumption, calorie intake, family history of diabetes, and general health status.
‡The sensitivity analysis was based on the full model and was limited to participants who met all of the following criteria: excellent or very good health status, never smokers or stopped >10 years ago, nonobese (BMI <30 kg/m2), and >1 h of moderate to vigorous physical activities per week in the past 10 years.
The association between hours of night sleeping and diabetes appeared to be nonlinear (Table 2). With participants of 7–8 h of night sleep as the referent, the multivariate OR was 1.46 (95% CI 1.31–1.63) for those reporting <5 h of night sleep, 1.11 (1.06–1.16) for those reporting 5–6 h, and 1.11 (0.99–1.24) for those reporting ≥9 h. As for the napping analysis, although the adjustment for physical activity variables made little difference, additional controls for BMI alone or with physical activities moderately attenuated this association.
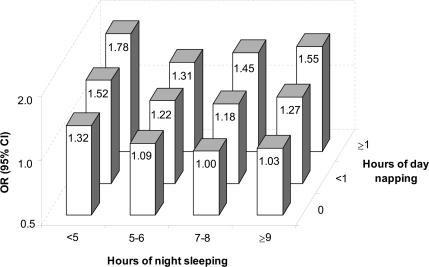
The associations between hours of day napping or night sleeping and diabetes were both confirmed among participants who were presumably healthy at the baseline risk factor survey (Table 2) and in various subgroup analyses (supplementary Table, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1143/DC1). There was a statistically significant interaction between hours of day napping and night sleeping on diabetes (Pinteraction < 0.0001) (Fig. 1). Day napping was associated with higher diabetes risk in a dose-response manner within each subgroup of night sleeping duration (all Ptrend < 0.05). However, the relationship between night sleeping and diabetes depended on the hours of day napping. Among participants who reported no napping, only short sleepers had a higher risk of diabetes. In contrast, among participants with ≥1 h of napping, both short and long hours of night sleep were associated with higher diabetes risk. Overall, participants with no napping and 7–8 h of night sleep had the lowest risk of diabetes, whereas individuals who napped ≥1 h during the day but slept <5 h at night had the highest risk.
Figure 1.
Joint analysis on day napping and night sleeping in relation to the risk of diabetes after 2000. Multivariate ORs for diabetes were adjusted for age, sex, race, education, marital status, smoking status, coffee and alcohol consumptions, calorie intake, family history of diabetes, general health status, light physical activity level, moderate to vigorous physical activity level, and BMI. The subgroup Ptrend in the napping analyses was 0.02 for participants with <5 h of night sleep, 0.002 for participants with 5–6 h of night sleep, <0.001 for participants with 7–8 h of night sleep, and 0.006 for participants with ≥9 h of night sleep.
CONCLUSIONS
In this large prospective study, we confirmed the previously reported association between short sleeping and higher risk of incident diabetes. More importantly, we observed a higher diabetes risk among day nappers and evaluated the combined effects of day napping and night sleeping on the risk of diabetes. The napping-diabetes relationship could not be explained by short night sleep as it presented across all subgroups of sleeping duration, including 7–8 h or even ≥9 h of sleep per night. The associations between napping or sleeping duration and diabetes may in part be confounded by obesity or mediated by weight gain. However, reverse causality due to clinically evident diabetes was a less likely explanation because the analyses only included incident cases diagnosed at least 3 years after the exposure assessment; furthermore, both associations were barely changed in sensitivity analyses among apparently healthy participants at baseline.
Several prospective studies have evaluated sleep duration in relation to diabetes. In most of these studies, short sleep was associated with a higher risk of diabetes compared with 7–8 h of sleep per night (2,5,6,12). Several biological mechanisms have been proposed, including increased sympathetic nerve activity, increased orexin activity, higher levels of inflammatory markers, and alterations in hormones and neuroendocrine control of appetite, which in turn induce insulin resistance, obesity, and diabetes (6). However, on the other hand, sleep duration is associated with various socioeconomic factors and is affected by individual health status (13), which may in part contribute to the observed relationship (14). Compared with previous studies, the current study is substantially larger and conducted thorough sensitivity analyses among healthy individuals and subgroup analyses. The results support an independent relationship between short sleep and risk of diabetes. Adjustment for BMI modestly attenuated but did not eliminate this relationship.
Most prospective studies also showed higher diabetes risk among longer sleepers; however, the interpretation of this finding appears to be more complicated (2,5,6,12). This finding has been hypothesized to be a result of unmeasured confounding (e.g., from poor sleep quality) or of reverse causality due to chronic inflammation with undiagnosed diabetes (2,5). In our analysis with the first 3 years of follow-up excluded, long sleepers had a marginally higher risk of diabetes and this risk elevation seems to be limited to participants who napped ≥1 h/day. Our joint analyses showed that both short and long sleep were associated with increased diabetes risk among long nappers (≥1 h/day), whereas among participants who did not nap, only short sleep was associated with higher risk. This preliminary finding on a potential interaction between napping and sleeping durations on diabetes needs to be confirmed in future studies.
The most important finding of this study is the monotonic relationship between hours of day napping and future risk of diabetes. To the best of our knowledge, this is the first prospective study suggesting that day napping may be an independent risk factor for diabetes. Day napping has been linked to diabetes cross-sectionally, which was interpreted by the authors as a consequence of diabetes (8,9,15). The current analysis was prospective with cases of diabetes diagnosed at least 3 years after the exposure assessment; therefore, our finding is less compatible with the possibility of reverse causation. Furthermore, this association cannot be explained by compensational napping for short night sleeping as it appeared within each subgroup of sleeping duration. Finally, this association persisted in all confounder subgroups and in the sensitivity analysis and therefore alleviated concerns about substantial influences from confounding or biases due to poor health status.
Habitual napping is prevalent among older adults. In some cultures such as the Chinese, napping is often considered as a healthy lifestyle for older adults (16). Day napping has been investigated in the context of its potential affects on night sleeping. Most previous studies showed that napping was not related to night sleeping duration or quality but rather was affected by individuals' health status (7–9,17). On the other hand, day napping itself may also have independent health consequences that have not yet been well investigated (18). Several studies have examined napping in relation to overall or cause-specific mortalities, but the results are inconsistent (16,19–21). In our study, day napping was associated with higher risk of diabetes even among individuals who appeared to be healthy at baseline. This finding underlines the needs for further investigations into the potential health consequences of napping among older adults.
The major strengths of the current study include its large sample size, prospective design, detailed epidemiologic profiles, and thorough statistical analyses. Our study also has several limitations. First, in such a large cohort, we had to rely on self-reports to identify patients with clinically diagnosed diabetes. Okura et al. (22) found that self-reported diabetes had a satisfactory agreement (κ = 0.76) with medical records, but it had a low sensitivity (66%). Further, without annual glucose tolerance screening, underdiagnosis of type 2 diabetes in our study population is also a concern. Therefore, diabetes in some participants might never have been identified or reported, and this possibility might have introduced bias if the identification of diabetes was differentially associated with napping or sleeping duration. In addition, participants with undiagnosed preclinical diabetes at baseline or in early follow-up might alter their sleeping or napping habits as a result of the underlying disease. To minimize potential bias from this source, we excluded participants with diabetes diagnosed in the first 3 years of follow-up and conducted sensitivity analysis among apparently healthy individuals.
Second, we did not collect data on the quality of night sleeping such as sleep fragmentations or on diseases such as obstructive sleep apnea or depression that themselves may be associated with napping or sleep duration and risk of diabetes (6,7,23–25). In particular, napping is associated with obstructive sleep apnea that may in turn increase the risk of type 2 diabetes (23,25). Although the napping-diabetes association persisted in the apparently healthy population as evident in the sensitivity analysis, we could not exclude the possibility that napping may be a marker of other health conditions that increase the risk of diabetes.
Third, as in other large prospective cohorts (2,5), information on hours of napping and sleeping was also self-reported and therefore misclassifications are likely. However, with the prospective design, exposure misclassification was probably nondifferential with respect to the outcome and might thus have attenuated the true relationship. Fourth, the current analyses were limited to participants of the follow-up survey in 2004–2006 and therefore included only ∼59% of eligible participants of the risk factor survey. Selection bias could have been introduced if napping or sleeping was associated with participation in the follow-up survey differentially by diabetes status. Finally, although we have controlled for and stratified by a variety of potential confounders, the study is observational in nature, and we could not exclude the possibility of residual confounding from unmeasured or inadequately measured confounders. Adjustment for BMI modestly attenuated the associations. It is possible that a more precise measurement of adiposity may further attenuate the results. However, given the strong statistical significance, it seems unlikely that obesity entirely explains these associations.
In summary, this large prospective study among U.S. older adults shows that long day napping (≥1 h) and short night sleeping (<5 h) are associated with a higher risk of diabetes. These results may in part be explained by obesity or weight gain, and we could not exclude the possibility that napping was a marker of other health conditions that increase the risk of diabetes. Further, the impact of sleeping duration on diabetes may depend on individuals' napping habits. Future prospective or mechanistic studies are needed to confirm these findings and to elucidate underlying mechanisms.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of the NIH, the National Institute of Environmental Health Sciences (Z01-ES-101986) and the National Cancer Institute (Z01-CP-010196-02).
No potential conflicts of interest relevant to this article were reported.
We are grateful for the continuous contribution of the NIH-AARP Diet and Health Study participants.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged >18 years who reported an average of <6 hours of sleep per 24-hour period, by sex and age group—National Health Interview Survey, United States, 1985 and 2006. MMWR Morb Mortal Wkly Rep 2008; 57: 209 [Google Scholar]
- 2. Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB: A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003; 26: 380– 384 [DOI] [PubMed] [Google Scholar]
- 3. Mallon L, Broman JE, Hetta J: High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care 2005; 28: 2762– 2767 [DOI] [PubMed] [Google Scholar]
- 4. Yaggi HK, Araujo AB, McKinlay JB: Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006; 29: 657– 661 [DOI] [PubMed] [Google Scholar]
- 5. Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D: Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007; 30: 1667– 1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tasali E, Leproult R, Spiegel K: Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis 2009; 51: 381– 391 [DOI] [PubMed] [Google Scholar]
- 7. Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK: Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry 2007; 15: 344– 350 [DOI] [PubMed] [Google Scholar]
- 8. Picarsic JL, Glynn NW, Taylor CA, Katula JA, Goldman SE, Studenski SA, Newman AB: Self-reported napping and duration and quality of sleep in the lifestyle interventions and independence for elders pilot study. J Am Geriatr Soc 2008; 56: 1674– 1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldman SE, Hall M, Boudreau R, Matthews KA, Cauley JA, Ancoli-Israel S, Stone KL, Rubin SM, Satterfield S, Simonsick EM, Newman AB: Association between nighttime sleep and napping in older adults. Sleep 2008; 31: 733– 740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, Freedman LS, Brown CC, Midthune D, Kipnis V: Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001; 154: 1119– 1125 [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008 [Google Scholar]
- 12. Beihl DA, Liese AD, Haffner SM: Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol 2009; 19: 351– 357 [DOI] [PubMed] [Google Scholar]
- 13. Krueger PM, Friedman EM: Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol 2009; 169: 1052– 1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horne J: Short sleep is a questionable risk factor for obesity and related disorders: statistical versus clinical significance. Biol Psychol 2008; 77: 266– 276 [DOI] [PubMed] [Google Scholar]
- 15. Asplund R: Daytime sleepiness and napping amongst the elderly in relation to somatic health and medical treatment. J Intern Med 1996; 239: 261– 267 [DOI] [PubMed] [Google Scholar]
- 16. Lan TY, Lan TH, Wen CP, Lin YH, Chuang YL: Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. Sleep 2007; 30: 1105– 1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dautovich ND, McCrae CS, Rowe M: Subjective and objective napping and sleep in older adults: are evening naps “bad” for nighttime sleep? J Am Geriatr Soc 2008; 56: 1681– 1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vitiello MV: We have much more to learn about the relationships between napping and health in older adults. J Am Geriatr Soc 2008; 56: 1753– 1755 [DOI] [PubMed] [Google Scholar]
- 19. Burazeri G, Gofin J, Kark JD: Siesta and mortality in a Mediterranean population: a community study in Jerusalem. Sleep 2003; 26: 578– 584 [DOI] [PubMed] [Google Scholar]
- 20. Stone KL, Ewing SK, Ancoli-Israel S, Ensrud KE, Redline S, Bauer DC, Cauley JA, Hillier TA, Cummings SR: Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc 2009; 57: 604– 611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hays JC, Blazer DG, Foley DJ: Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc 1996; 44: 693– 698 [DOI] [PubMed] [Google Scholar]
- 22. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ: Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004; 57: 1096– 1103 [DOI] [PubMed] [Google Scholar]
- 23. Masa JF, Rubio M, Pérez P, Mota M, de Cos JS, Montserrat JM: Association between habitual naps and sleep apnea. Sleep 2006; 29: 1463– 1468 [DOI] [PubMed] [Google Scholar]
- 24. Mezuk B, Eaton WW, Albrecht S, Golden SH: Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008; 31: 2383– 2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tasali E, Mokhlesi B, Van Cauter E: Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 2008; 133: 496– 506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.