Abstract
OBJECTIVE
Evidence that the metabolic syndrome is a risk factor for poor cognition is mixed and is focused mainly on the elderly population; rarely is an adjustment made for socioeconomic factors. We examined this association in late midlife, with particular focus on cumulative effects and the role of socioeconomic circumstances.
RESEARCH DESIGN AND METHODS
Analyses were performed for 4,150 white participants from the Whitehall II study. Metabolic syndrome, using the National Cholesterol Education Program Adult Treatment Panel III criteria, was assessed three times over the 10-year follow-up (1991–2001). Cognitive function was assessed using a battery of six tests at the end of the follow-up.
RESULTS
After adjustment for demographic variables, health behaviors, and health status, participants with persistent metabolic syndrome (at least two of the three screenings) over the 10-year follow-up had lower cognitive performance than participants who never had metabolic syndrome. No significant differences in cognitive function were observed between participants with nonpersistent metabolic syndrome (one of the three screenings) and those who never had metabolic syndrome during the follow-up. Adjustment for adult occupational position attenuated this association by between 41 and 86%, depending on the measure of cognitive function. Adjustment for education had little effect.
CONCLUSIONS
Only persistent metabolic syndrome was associated with lower cognitive performance in late midlife. Adult occupational position but not education had a substantial impact on this association; these results highlight the importance of adult socioeconomic circumstances in identifying and targeting risk factors for cognitive aging.
Cardiovascular risk factors have increasingly been recognized as important contributors to cognitive outcomes such as dementia (1). The metabolic syndrome comprises five cardiovascular risk factors including abdominal obesity, hypertriglyceridemia, low HDL cholesterol, hypertension, and hyperglycemia (2). Numerous studies have shown several of the individual components of the metabolic syndrome to be linked to the risk of cognitive decline and dementia (3). However, the nature of the association between metabolic syndrome and cognition remains unclear. There are only a few studies on the metabolic syndrome as a whole, and most of them have been limited to elderly or older populations (4–12). Furthermore, the findings are mixed: although some reports suggest that metabolic syndrome predicts cognitive deficit (4), cognitive decline (5,11,12), and dementia (8,10), at least two studies showed metabolic syndrome to be associated with better cognitive performance (6) and decelerated cognitive decline (9). A further study found no significant relationship between metabolic syndrome and dementia (7).
Several limitations in previous studies that are possible to overcome may have contributed to inconsistencies in the evidence. First, as subclinical manifestations of dementia are believed to be present many years before the diagnosis, examining the role of risk factors, such as the metabolic syndrome, before old age would provide insight into their impact on cognitive function (13,14). Second, no previous study has examined the effects of persistent metabolic syndrome, assessed repeatedly rather than at a single time point, on cognition. Third, existing research has not taken full account of the potential for confounding by socioeconomic position (SEP).
In this field of research, SEP may play a particularly important role as, on the one hand, it contributes to cognitive reserve (15), and, on the other hand, it is associated with vascular and other risk factors for cognitive aging (16). Although studies on the association between metabolic syndrome and cognition usually adjust for education, they do not take into account the effects of later life measures of SEP. Education reflects early SEP but may not capture changes in socioeconomic circumstances in adult life. Other measures, such as household income or occupational position, may better reflect adult socioeconomic circumstances.
We use data from a large prospective middle-aged cohort (the Whitehall II study) to examine the association between metabolic syndrome and cognitive function in mid-life. Our focus is on investigating the effect of cumulative exposure to metabolic syndrome over 10 years and the influence of SEP as indicated by education and occupational position.
RESEARCH DESIGN AND METHODS
The target population for the Whitehall II study was all London-based office staff (n = 10,308), aged 35–55 years, working in 20 civil service departments (17). After the first medical examination (phase 1, 1985–1988), screenings by trained research staff were repeated three times over a 19-year period: phase 3 (1991–1993), phase 5 (1997–1999), and phase 7 (2003–2004). Of the 10,308 participants in phase 1, 584 had died before phase 7, 1,182 had withdrawn from the study, and 1,575 did not respond at this phase of the follow-up. The remaining 6,967 participants responded at phase 7 and, of these, 88.0% had data on cognitive function at phase 7 (n = 6,130), 71.0% (n = 4,949) had data on metabolic syndrome at phases 3, 5, and 7, and 64.0% (n = 4,461) had data on all other covariates. The present analyses are based on all white participants with complete data (n = 4,150). Ethical approval for the Whitehall II study was obtained from the University College London Medical School committee on the ethics of human research.
Assessment of the metabolic syndrome
The metabolic syndrome was defined at phases 3, 5, and 7, using the National Cholesterol Education Program criteria (2), based on the presence of three or more of the following: waist circumference for men >102 cm and for women >88 cm; serum triglycerides ≥1.7 mmol/l; HDL cholesterol for men <1.04 mmol/l and for women <1.29 mmol/l; blood pressure ≥130/85 mmHg; and fasting glucose ≥6.1 mmol/l. Waist circumference was taken as the smallest circumference below the costal margin. Resting blood pressure was measured using the Hawksley random zero sphygmomanometer (phases 3 and 5) and the OMRON HEM 907 (phase 7). Serum triglycerides, HDL cholesterol, and fasting blood glucose were analyzed as described previously (18).
Assessment of cognitive function
Cognitive function was measured at phase 7 and consisted of six standard tasks chosen to evaluate comprehensively cognitive functioning in middle-aged white-collar workers. The tests were chosen using the following criteria: assessment of multiple cognitive domains, ability to capture effects of age and other risk factors in a middle-aged population, and no ceiling or floor effects. More details on the tests, described in brief below, can be found elsewhere (19).
Short-term verbal memory was assessed with a 20-word free recall test. Participants were presented a list of 20 words (one or two syllables long) at 2-s intervals and were then asked to recall in writing as many of the words as they could in any order.
The Alice Heim 4-I (AH4-I) is composed of a series of 65 verbal and mathematical reasoning items of increasing difficulty. It tests inductive reasoning, measuring the ability to identify patterns and infer principles and rules.
Vocabulary was assessed with the Mill Hill Vocabulary test, in its multiple-choice format, consisting of a list of 33 stimulus words ordered by increasing difficulty and six response choices.
Phonemic and semantic verbal fluency was assessed via “S” words for phonemic fluency and via “animal” words for the semantic fluency. Participants were asked to recall in writing as many words beginning with “S” and as many animal names as they could in a given period of time.
The 30-item Mini-Mental State Examination (MMSE) was used to assess global cognitive status.
Assessment of covariates
All covariates (apart from educational attainment) were measured at phase 7, concurrent with the measures of cognition. Sociodemographic variables consisted of age, sex, and marital status (married or cohabiting, single, divorced, and widowed). SEP was assessed using measures of education, for early SEP, and occupational position, for mid-life SEP. Highest educational attainment was measured at phase 5 and grouped into five levels (no academic qualification, lower secondary education, higher secondary education, university degree, and higher university degree). Current (or last for retired participants) British civil service employment grade, defined on the basis of salary, was used as a measure of occupational position and was grouped into three categories: high (senior administrators), intermediate (executives, professionals, and technical staff) and low (clerical and office support staff) grades. As of August 1992 the salary range among high-grade employees was £25,330–£87,620 and among low-grade employees was £7,387-£11,917.
Health behaviors measured were smoking (current, former, and nonsmoker), frequency of alcohol consumption (less than once per week, at least once per week, and at least once per day), and intensity of physical activity (based on frequency and duration categorized as “high,” “medium,” and “low”) (20). Health measures considered in these analyses were depressive symptoms and coronary heart disease (CHD). Depressive symptoms were measured using the 4-item depression subscale (range 0–12) of the 30-item General Health Questionnaire (21). Participants scoring ≥4 on this depression subscale were defined as having depressive symptoms (18). Prevalent CHD was identified using clinically verified events, including nonfatal myocardial infarction and definite angina as described previously (22).
Statistical methods
Cognitive test scores were standardized using T scores (mean 50, SD 10) to allow comparisons among tests. We examined the association between cumulative exposure to metabolic syndrome, measured three times over the 10-year follow-up and cognitive functioning at phase 7. For these analyses we categorized the three measures of metabolic syndrome over the 10-years of follow-up as “never” having metabolic syndrome, “nonpersistent” (1 of 3 screenings), and “persistent” (≥2 of 3 screenings). ANCOVA was performed to calculate adjusted mean differences in cognitive T scores across the cumulative measure of metabolic syndrome, with “never having metabolic syndrome” as the reference.
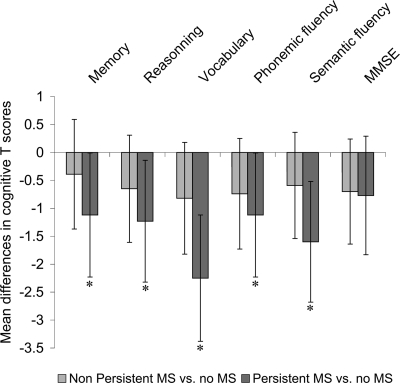
ANCOVA was used to assess mean differences in cognitive test scores as a function of metabolic syndrome. For these analyses, the adjustment for covariates was performed in three steps. First, a basic model adjusted for age and sex; then a second model further adjusted for marital status, health behaviors, and measures of health status. Finally, education or occupational position was added sequentially to the basic model (Fig. 2) and to the second model (supplementary Tables A and B, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1218/DC1). Interactions among covariates and frequency of metabolic syndrome across the phases were tested and found to be nonsignificant. The level of statistical significance was set at P ≤ 0.05; marginal significance was defined as 0.05 < P ≤ 0.10. All analyses were conducted using SAS software (version 9; SAS Institute, Cary, NC).
Figure 2.
Mean differences (95% CIs) in cognitive T scores between participants with persistent metabolic syndrome and those with no metabolic syndrome after sequential adjustment for education and occupational position. White bars, model 1: analyses adjusted for sex and age; light gray bars, model 1 additionally adjusted for education; dark gray bars, model 1 additionally adjusted for occupational position.
RESULTS
Compared with all 6,967 respondents at phase 7, participants included in the current analyses (n = 4,150) were more likely to be men (73.9 vs. 64.8%), less likely to be in low occupational positions (7.2 vs. 17.0%), and less likely to have no academic qualification (8.1 vs. 10.0%). Participants excluded from the present analyses had lower mean scores on all cognitive tests (P < 10−4) and a higher prevalence of metabolic syndrome at phase 7 (20.0 vs. 10.9%, P < 10−4) compared with those included in the analysis.
An increase in the proportion of participants with metabolic syndrome was observed over the 10-year follow-up: 8.3% at phase 3, 8.9% at phase 5, and 11.0% at phase 7. Across the phases, 10.1% of participants showed nonpersistent metabolic syndrome (1 of 3 screenings), whereas 7.7% showed persistent metabolic syndrome (≥2 of 3 screenings). Characteristics of the participants as a function of cumulative exposure to the metabolic syndrome are presented in Table 1. Participants with metabolic syndrome (persistent or not) were more likely to be older and more likely to be men. Smoking, low physical activity, and high prevalence of CHD were more common in participants with the metabolic syndrome. Although education was not associated with the metabolic syndrome, a higher proportion of participants in the low occupational position group had metabolic syndrome during follow-up.
Table 1.
Characteristics of the population at phase 7 as a function of persistence of the metabolic syndrome over the 10-year follow-up
| Cumulative exposure to the metabolic syndrome |
||||
|---|---|---|---|---|
| Never | Nonpersistent | Persistent | P* | |
| n | 3,414 | 418 | 318 | |
| Female sex | 27.0 | 21.8 | 22.3 | 0.02 |
| Age (years) | 60.5 ± 5.9 | 61.4 ± 5.9 | 61.4 ± 6.1 | 0.001 |
| Marital status, married or cohabited | 77.5 | 74.6 | 72.3 | 0.15 |
| Occupational position, lowest position | 6.8 | 7.2 | 11.6 | 0.01 |
| Education, no academic qualification | 7.8 | 10.3 | 8.5 | 0.24 |
| Smoking habits, current smokers | 10.7 | 12.4 | 11.0 | <10−4 |
| Alcohol consumption, >1 drink/day | 49.8 | 46.6 | 44.0 | <10−4 |
| Physical activity, low | 13.7 | 17.7 | 20.7 | 0.0004 |
| CHD prevalence | 5.3 | 8.1 | 12.3 | <10−4 |
| Depressive symptoms | 11.0 | 9.31 | 14.1 | 0.11 |
| Central obesity criterion of metabolic syndrome† | 12.7 | 57.8 | 70.3 | <10−4 |
| High triglyceride criterion of metabolic syndrome† | 14.4 | 53.6 | 75.8 | <10−4 |
| Low HDL cholesterol criterion of metabolic syndrome† | 3.7 | 24.6 | 45.0 | <10−4 |
| Hypertension criterion of metabolic syndrome† | 35.3 | 63.2 | 68.2 | <10−4 |
| High fasting glucose criterion of metabolic syndrome† | 6.6 | 29.7 | 52.0 | <10−4 |
Data are % or means ± SD. n = 4,150. Nonpersistent metabolic syndrome was defined as having it once during the three screenings over the 10 years of follow-up. Persistent metabolic syndrome was defined as having it at least twice during the three screenings.
*Results of the χ2 tests for heterogeneity.
†Each criterion of the metabolic syndrome was defined using the National Cholesterol Education Program criteria (2). For central obesity, there were eight missing values; for high triglyceride, low HDL cholesterol, and hypertension, there was one missing value; and for high fasting glucose criteria, there were five missing values.
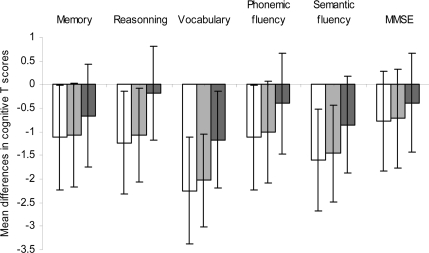
Figure 1 shows the sex- and age-adjusted mean differences in cognitive T scores at phase seven for cumulative exposure to the metabolic syndrome over the 10-year follow-up. Compared with participants who never had the metabolic syndrome, those with persistent exposure to the metabolic syndrome had significantly lower cognitive scores on all tests except for the MMSE. However, no significant differences were observed between participants who never had metabolic syndrome and those with nonpersistent metabolic syndrome.
Figure 1.
Mean differences (95% CIs) in cognitive T scores across the cumulative exposure to the metabolic syndrome over the 10-year follow-up (n = 4,150), adjusted for sex and age. *Mean difference in cognitive T-scores statistically significant (P ≤ 0.05). MS, metabolic syndrome.
The effect of further adjustments, first for education and then for occupational position, on the score differences between participants with persistent metabolic syndrome and participants who never had metabolic syndrome over the follow-up, is shown in Fig. 2. After controlling for education, participants with persistent metabolic syndrome had lower scores on memory, reasoning, vocabulary, and semantic fluency. In contrast, adjustment for occupational position attenuated this difference by 41% for memory, 86% for reasoning, 48% for vocabulary, 65 and 47% for phonemic and semantic fluency, and 49% for the MMSE. After adjustment for occupational position, no association remained significant between persistent metabolic syndrome and cognitive test scores, except for that with the vocabulary test.
The analysis of the distribution of all covariates, metabolic syndrome, and the tests of cognition as a function of occupational position showed (supplemental Table A), on the one hand, that the lower the occupational position, the lower the cognitive scores and the higher the prevalence of persistent metabolic syndrome. On the other hand, occupational position was associated with all potential confounders of the metabolic syndrome–cognitive function relationship considered in this analyses.
Analyses presented in Fig. 2 were repeated after taking into account the other demographic factors, health behaviors, and health status (supplemental Table B). In the model including education, lower cognitive T scores were observed in participants with persistent metabolic syndrome over the 10-year follow-up compared with those who never had metabolic syndrome. The associations were statistically significant for vocabulary (adjusted mean difference in T scores, δm = −1.89 [95% CI −2.87 to −0.90], P = 0.0002) and semantic fluency (δm = −1.30 [−2.37 to −0.24], P = 0.02) and marginally significant for memory (δm = −0.92 [−2.01 to 0.18], P = 0.10). However, these associations were substantially attenuated when education was replaced by occupational position.
All the analyses presented so far used the National Cholesterol Education Program definition but were repeated using the International Diabetes Federation definition (http://www.idf.org/metabolic_syndrome) of metabolic syndrome. In general terms, the International Diabetes Federation definition leads to greater prevalence of metabolic syndrome and somewhat smaller associations with cognitive function. However, the general pattern of results was similar (results not shown but available on request).
CONCLUSIONS
In this prospective cohort study of a middle-aged population followed up for 10 years, participants with persistent metabolic syndrome had lower cognitive scores for reasoning, vocabulary, semantic fluency, and, to a lesser extent, memory, compared with participants who never had metabolic syndrome. These associations remained after adjustment for demographic variables, education, health behavior, and health status. No difference in cognitive function was observed between participants with nonpersistent metabolic syndrome and those who never had metabolic syndrome. Our study sheds light on the impact of SEP on these relationships: whereas education had little impact on the metabolic syndrome–cognition relationship, occupational position substantially attenuated the association.
There is increasing interest in the possible impact of vascular and metabolic disorders on dementia, and several studies have investigated this association in elderly individuals. Two cross-sectional studies showed that the presence of metabolic syndrome was associated with a higher prevalence of Alzheimer's disease (10) and also with poorer cognitive function (4). In four longitudinal studies, metabolic syndrome was shown to be associated with a greater cognitive decline (5,11,12) and increased risk of all-cause and vascular dementia (8). However, another study did not show evidence of the detrimental effect of metabolic syndrome on dementia (7). Finally, two further studies carried out in the oldest old individuals found the opposite: metabolic syndrome was associated with better cognition (6) and decelerated cognitive decline (9).
The potential explanations for the above inconsistencies include differences in the length of follow-up, the sensitivity of cognitive tests to detect a decline, the low statistical power of several studies, and survival bias in studies involving the oldest old. Our study provides a further explanation: the measurement of the metabolic syndrome at one point in time may not capture the impact of long-term exposure to this syndrome as there is considerable within-individual variation in metabolic syndrome status over time. Our finding suggests that long-term exposure to metabolic syndrome, persistent metabolic syndrome rather than metabolic syndrome status, at a given moment is associated with poor cognitive function.
Because poor cognition in midlife has been shown to predict cognitive decline and dementia later in life, the examination of the role of risk factors before old age is important. It allows circumvention of the problems of survival bias and reverse causality that may be common in studies in the oldest age-groups. To date, very few studies have investigated the effects of metabolic syndrome on cognition in middle-age. Kalmijn et al. (14) showed a long-term association between a cluster of seven metabolic cardiovascular risk factors measured at middle age and the risk of dementia among men in old age, but a recent cross-sectional study performed in 853 participants aged 61 years reported no association between metabolic syndrome and cognitive scores (13). Thus, our observation of an association between persistent metabolic syndrome and cognitive function in a large middle-aged population constitutes a novel finding.
Our findings emphasize the potential importance of socioeconomic circumstances in the association between metabolic syndrome and cognitive function. By analyzing the relationship between metabolic syndrome and cognition after adjustment for a measure of early SEP, here education, or alternatively a later life measure of SEP, occupational position, our study highlights the different impact of these two measures. Education, although extensively examined in relation to cognitive outcomes, has been shown in several studies, including Whitehall II, not to be associated with metabolic syndrome (5,6,13). The modest impact of education on the metabolic syndrome–cognition relationship observed in the present study is consistent with findings from other studies. On the other hand, occupational position was strongly related to sociodemographic factors, health behavior, and several health measures, and the relationship between metabolic syndrome and cognition was substantially attenuated after adjustment for occupational position. To our knowledge, no other study has taken into account the effects of later life measures of SEP, such as occupational position, making comparison with other studies difficult.
The discrepant effect of adjustment for education or occupational position should be interpreted in light of the fact that these measures cover different dimensions of SEP. Compared with education, reached by the individual usually in early adult life, midlife occupational position more accurately reflects SEP conditions over the adult life span. SEP is an important determinant of mortality and morbidity in many countries (23), and its influence on health is believed to work in several ways. Poor SEP is seen to increase the biological vulnerability to diseases by acting directly on physiological processes but also through unhealthy behaviors (24). Our report emphasizes the importance of taking into account SEP in the metabolic syndrome–cognition relationship to understand the potential impact of metabolic syndrome on cognitive aging. Adjustment for occupational position attenuated the association between persistent metabolic syndrome and cognition by between 41 and 86%. There are three possible implications of our findings. One, studies on cognitive aging that adjust for education to control the influence of socioeconomic factors are clearly not taking the socioeconomic environment of older adults fully into account. Two, given the size of the attenuation of the association, it is possible that the observed association between metabolic syndrome and cognition is simply the result of confounding by socioeconomic factors. This would imply that the observed association is the result of the impact of adult SEP on both metabolic syndrome and cognitive function, and there is no true causal effect of metabolic syndrome on cognition. Three, given the association between the components of the metabolic syndrome and cognition evident in the literature, it is possible that “persistent” metabolic syndrome is a risk factor for cognitive function. The attenuation observed in our analysis could therefore simply be due to occupational position being a good proxy for factors mediating the association between metabolic syndrome and cognition. Further research is needed to delineate the precise mechanisms underlying the link between metabolic syndrome and cognition.
Our report has several limitations. First, the participants of the Whitehall II study are mainly office-based civil servants, not fully representative of the British population, and analysis was restricted to “white” participants, which may limit the generalizability of our findings. Second, we showed that participants with lower cognitive performances or with metabolic syndrome were less likely to be included in our analyses. This potential selection bias would probably lead to underestimation of the relationship between metabolic syndrome and cognition. Finally, the use of a single assessment of cognitive function is a limitation as it does not allow conclusions to be drawn on the direction of causality between metabolic syndrome and cognitive decline.
In summary, this seems to be the first study to explore the association between cumulative exposure to the metabolic syndrome over a 10-year follow-up and cognitive functioning in late midlife. Our results suggest that rather than metabolic syndrome status at a given moment per se, the persistence of the syndrome is the factor that has adverse effects on later cognitive performances during adult life. Furthermore, our report showed the different effects of SEP measures on the relationship, depending on whether education or occupational position was considered; education had little impact, but occupational position had a greater impact. These results highlight the importance of the type of socioeconomic variable in identifying and targeting risk factors for cognitive aging.
Supplementary Material
Acknowledgments
The Whitehall II study has been supported by grants from the British Medical Research Council (MRC); the British Heart Foundation; the British Health and Safety Executive; the British Department of Health; the National Heart, Lung, and Blood Institute (Grant HL36310); the National Institute on Aging (Grant AG13196); the Agency for Health Care Policy and Research (Grant HS06516); and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socioeconomic Status and Health. T.N.A. was supported by the Academy of Finland (Grants 117604 and 124322). M.K. is supported by the Academy of Finland. M.J.S. is supported by the British Heart Foundation. M.G.M. is supported by an MRC research professorship. J.E.F. is supported by the MRC (Grant G8802774). A.S.-M. is supported by a European Young Investigator Award from the European Science Foundation.
The sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
We thank all men and women who participated in the Whitehall II study; all participating Civil Service departments and their welfare, personnel, and establishment officers; the Occupational Health and Safety Agency; and the Council of Civil Service Unions. The Whitehall II Study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants and data entry staff, who make the study possible.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A: Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 2001; 322: 1447– 1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486– 2497 [DOI] [PubMed] [Google Scholar]
- 3. Yaffe K: Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Dis Assoc Disord 2007; 21: 167– 171 [DOI] [PubMed] [Google Scholar]
- 4. Dik MG, Jonker C, Comijs HC, Deeg DJ, Kok A, Yaffe K, Penninx BW: Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care 2007; 30: 2655– 2660 [DOI] [PubMed] [Google Scholar]
- 5. Komulainen P, Lakka TA, Kivipelto M, Hassinen M, Helkala EL, Haapala I, Nissinen A, Rauramaa R: Metabolic syndrome and cognitive function: a population-based follow-up study in elderly women. Dement Geriatr Cogn Disord 2007; 23: 29– 34 [DOI] [PubMed] [Google Scholar]
- 6. Laudisio A, Marzetti E, Pagano F, Cocchi A, Franceschi C, Bernabei R, Zuccalà G: Association of metabolic syndrome with cognitive function: the role of sex and age. Clin Nutr 2008; 27: 747– 754 [DOI] [PubMed] [Google Scholar]
- 7. Muller M, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA: Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement Geriatr Cogn Disord 2007; 24: 185– 192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raffaitin C, Gin H, Empana JP, Helmer C, Berr C, Tzourio C, Portet F, Dartigues JF, Alpérovitch A, Barberger-Gateau P: Metabolic syndrome and risk for incident Alzheimer's disease or vascular dementia: the Three-City Study. Diabetes Care 2009; 32: 169– 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Berg E, Biessels GJ, de Craen AJ, Gussekloo J, Westendorp RG: The metabolic syndrome is associated with decelerated cognitive decline in the oldest old. Neurology 2007; 69: 979– 985 [DOI] [PubMed] [Google Scholar]
- 10. Vanhanen M, Koivisto K, Moilanen L, Helkala EL, Hänninen T, Soininen H, Kervinen K, Kesäniemi YA, Laakso M, Kuusisto J: Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology 2006; 67: 843– 847 [DOI] [PubMed] [Google Scholar]
- 11. Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N: Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging study. J Am Geriatr Soc 2007; 55: 758– 762 [DOI] [PubMed] [Google Scholar]
- 12. Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB: The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004; 292: 2237– 2242 [DOI] [PubMed] [Google Scholar]
- 13. Gatto NM, Henderson VW, St John JA, McCleary C, Hodis HN, Mack WJ: Metabolic syndrome and cognitive function in healthy middle-aged and older adults without diabetes. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2008; 15: 627– 641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, Ross GW, Havlik RJ, Launer LJ: Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol 2000; 20: 2255– 2260 [DOI] [PubMed] [Google Scholar]
- 15. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R: Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994; 271: 1004– 1010 [PubMed] [Google Scholar]
- 16. Matthews KA, Räikkönen K, Gallo L, Kuller LH: Association between socioeconomic status and metabolic syndrome in women: testing the reserve capacity model. Health Psychol 2008; 27: 576– 583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marmot M, Brunner E: Cohort profile: the Whitehall II study. Int J Epidemiol 2005; 34: 251– 256 [DOI] [PubMed] [Google Scholar]
- 18. Akbaraly TN, Kivimäki M, Brunner EJ, Chandola T, Marmot MG, Singh-Manoux A, Ferrie JE: Association between metabolic syndrome and depressive symptoms in middle-aged adults: results from the Whitehall II study. Diabetes Care 2009; 32: 499– 504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh-Manoux A, Sabia S, Lajnef M, Ferrie JE, Nabi H, Britton AR, Marmot MG, Shipley MJ: History of coronary heart disease and cognitive performance in midlife: the Whitehall II study. Eur Heart J. 22 July 2008. [ E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh-Manoux A, Hillsdon M, Brunner E, Marmot M: Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II prospective cohort study. Am J Public Health 2005; 95: 2252– 2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldberg DP, Hillier VF: A scaled version of the General Health Questionnaire. Psychol Med 1979; 9: 139– 145 [DOI] [PubMed] [Google Scholar]
- 22. Singh-Manoux A, Britton A, Kivimaki M, Guéguen A, Halcox J, Marmot M: Socioeconomic status moderates the association between carotid intima-media thickness and cognition in midlife: evidence from the Whitehall II study. Atherosclerosis 2008; 197: 541– 548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mackenbach JP, Stirbu I, Roskam AJ, Schaap MM, Menvielle G, Leinsalu M, Kunst AE: European Union Working Group on Socioeconomic Inequalities in Health. Socioeconomic inequalities in health in 22 European countries. N Engl J Med 2008; 358: 2468– 2481 [DOI] [PubMed] [Google Scholar]
- 24. Singh-Manoux A, Marmot MG, Adler NE: Does subjective social status predict health and change in health status better than objective status? Psychosom Med 2005; 67: 855– 861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.