Abstract
Although high circulating levels of glucocorticoids are associated with impaired cognitive performance in adults, less is known about this relationship in infancy. Furthermore, because studies have relied on acute cortisol measures in blood plasma or saliva, interpretation of the results may be difficult as acute measures may in part reflect emotional responses to testing procedures. In this study we examined whether hair cortisol, an integrated measure of HPA axis functioning, predicted performance of nursery-reared (NR) infant rhesus monkeys (N=32) on Piagetian object permanence tasks. Testing of NR infants began at 19.8±2.2 (mean±SE) days of age and continued for the next several months. Hair cortisol concentrations from the 32 NR monkeys were compared to those of 20 mother-peer-reared (MPR) infants. Hair was shaved at day 14, allowed to re-grow, and obtained again at month 6, thus representing integrated cortisol over a 5.5-month period of time. NR and MPR infants did not differ in month 6 hair cortisol values (t(50)=0.02, p=0.98). Linear regression revealed that hair cortisol predicted object permanence performance in the NR infants. Infants with higher hair cortisol reached criterion at later ages on the well (p<0.01), screen (p<0.05), and A-not-B (p<0.05) tasks and required more test sessions to complete the well (p<0.01) and screen tasks (p<0.05). These data are the first to implicate hair cortisol as a reliable predictor of early cognitive performance in infant macaque monkeys.
Keywords: Rhesus macaque, infant, cortisol, cognition, object permanence
INTRODUCTION
Chronic hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis results in long-term exposure to elevated circulating glucocorticoids, which exert long-lasting and often detrimental effects on an organism’s growth and development (Burguera et al., 1990; Magiakou et al., 1994), immune functioning (Gold, Goodwin, and Chrousos, 1988; Smith et al., 2002), reproduction (MacConnie et al., 1986; Chrousos, Torpy, and Gold, 1998), mood and addictive tendencies (Kaye et al., 1987; Wand and Dobs, 1991; Charmandari et al., 2003), and cognitive functioning. With respect to cognitive functioning, numerous studies have demonstrated a relationship between high levels of glucocorticoids (namely, cortisol) and impaired performance in several different populations. For example, in healthy adults both the administration of glucocorticoids and the implementation of a brief stressor increase salivary cortisol levels and adversely affect non-emotional memory processes (Kirschbaum et al., 1996; Lupien et al., 1999). Higher blood cortisol levels predict poorer cognitive performance in healthy aged subjects (Lupien et al., 1994; Lupien et al., 1998). Neglected children exhibit higher salivary and blood cortisol levels and lower intelligence test scores and reading abilities as adults (Glazer, 2000; Perez and Widom, 1994).
While much research exists for the adult human population, less is known about the relationship between cortisol and cognitive functioning in human infants. To our knowledge, only two studies have examined this relationship. Haley, Weinberg, and Grunau (2005) found that small but significant increases in salivary cortisol across sampling periods on a single day of assessment were related to better memory on a contingency learning task (kicking/mobile movement). In contrast, Thompson and Trevathan (2008) subsequently reported that decreases in salivary cortisol within a testing session were associated with better learning and association on a preferential looking task. These dissimilar results leave open the question of whether cortisol is positively or negatively related to cognitive function in infants.
Complimenting and expanding on the human literature, several nonhuman primate studies have examined the relationship between HPA axis functioning and cognitive performance. Glucocorticoid administration impairs inhibitory control on detour reach tasks which test prefrontal cortex functioning (Lyons et al., 2000). Conversely, rhesus monkeys exhibiting self-injurious behavior (SIB), a condition that is characterized by blunted cortisol responses to mild stress, performed better than controls on visible and invisible displacement tasks (Kelly, Novak, and Meyer, 2007). Interestingly, squirrel monkeys inoculated to stress by a brief intermittent exposure to maternal separation during infancy later exhibited lower plasma cortisol responses to a subsequent stressors. As juveniles they performed better on response inhibition tasks than controls, suggesting that stress inoculation may be beneficial to cognitive processes (Parker et al., 2005; Lyons and Parker, 2007). Taken together, these findings indicate that high levels of circulating glucocorticoids play a role in several different kinds of learning tasks. However, similar to the human literature, information is lacking on the relationship between glucocorticoids and cognitive functioning in infant nonhuman primates. Obtaining such information is important in understanding the maturation of both the HPA axis and learning-related neural circuitry (including the prefrontal cortex and hippocampus) during this developmental period and beyond.
The studies described above rely on acute measures of HPA activity, which may make results difficult to interpret as salivary or plasma cortisol levels may be confounded by emotional responses to testing procedures. Hair cortisol has lately received considerable attention as an integrated, chronic measure of HPA activity. Our laboratory recently developed and validated a reliable method for measuring cortisol in the hair of adult rhesus macaques (Davenport et al., 2006), which reflects cortisol concentrations over several months and, more importantly, can be used to study responses to major life stressors in these subjects (e.g., housing relocation; Davenport et al., 2006, 2008). Moreover, recent human studies have identified hair cortisol as a biomarker of chronic stress in neonates (Yamada et al., 2007) and a valid reflection of increased circulating cortisol concentrations during the last trimester of pregnancy (Kirschbaum et al., 2009). However, no studies have examined the relationship between hair cortisol levels and cognitive abilities in infant human or nonhuman primates.
The present experiment was designed to assess the relationship between hair cortisol and cognitive development in infant rhesus monkeys. Object permanence tasks were used to assess performance as they have been used extensively as a measure of emerging cognition in infant monkeys (Wise, Wise, & Zimmerman, 1974; Walker et al, 1992; Schneider, 1992; Dettmer et al., 2003; Sackett et al., 2006). We specifically sought to determine whether hair cortisol predicts object permanence performance. Based on an extension of the existing adult literature, we hypothesized that elevated HPA activity during infancy would be negatively related to cognitive performance in infant rhesus monkeys.
METHODS
Subjects
Subjects were 32 infant rhesus macaques (Macaca mulatta) born at the Laboratory for Comparative Ethology (LCE) of the National Institutes of Health in Poolesville, MD. All infants that were nursery-reared (NR) were part of a larger, long-term LCE research program focused on early rearing experiences and gene by environment interactions. Nursery infants were reared according to methods previously described in detail (Ruppenthal, 1979; Ruppenthal and Sackett, 1992; Shannon, Champoux, and Suomi, 1998).
Object Permanence Testing
The standard protocol dictated that each infant began object permanence testing at 14 days of age. If day 14 fell on a weekend, testing began on the following Monday. However, five subjects actually began testing after 25 days of age (range: 27–59 d). This variation was taken into account in the statistical analysis. The mean±SE start age for object permanence testing for this study was 19.8±2.2 d. We followed testing procedures described in detail by Ruppenthal and Sackett (1992) and Sackett et al. (2006), which involves testing the animals three out of five nonconsecutive days per week. Object permanence testing consisted of four stages: plain reach, screen, well, and A-not-B (see Sackett et al., 2006, for detailed descriptions of each testing stage and testing apparatus). Briefly, the plain reach stage tested each infant’s ability to pick up a brightly colored toy covered with a fruit reward. Once infants passed the plain reach phase (3/5 full pick-ups of the toy in one test session), they moved onto the screen and well tasks, each of which consisted of three conditions: no hide, partial hide, and full hide. The screen task involved covering the toy by sliding a screen across a track in front of the toy, and the well task involved covering the toy by placing the toy into a well and moving a lid over the top of the well. For the screen and well tasks, each infant was given 5 trials of each condition for a total of 15 trials per testing day, and screen and well testing days were alternated. Criterion for screen and well tasks was 8/10 full pick-ups in the full-hide condition across two consecutive test sessions. If criterion was achieved on either task before the other it was still tested until criterion was achieved on both. The final task assessed the A-not-B error, and consisted of 5 full-hide trials per day alternating between 2 possible wells. Criterion was 80% correct across two test sessions.
Hair Cortisol
Hair was shaved from the posterior vertex region of the neck at day 14 during a routine neonatal assessment to provide a baseline time point and to eliminate the possibility that the hair sample collected later contained cortisol deposited prenatally (which would likely include maternal sources of the hormone). Hair was allowed to re-grow until 6 months of age at which time it was shaved from the same location again. Shaving at 6 months was necessary to allow the infants enough time to re-grow the 250mg of hair required for cortisol analysis (personal observation; Davenport et al., 2006). Upon shaving, hair was processed and analyzed via enzyme immunoassay for cortisol concentrations following a reliable procedure developed and validated in our laboratory (Davenport et al., 2006; Davenport et al., 2008). Hair cortisol concentrations from the NR infants were compared to those of 20 mother-peer-reared (MPR) infants at the same age to test the hypothesis that nursery-rearing resulted in elevated chronic cortisol levels in the first several months of life. The necessity of separating infants from their mothers and peers for cognitive testing precluded our ability to conduct object permanence testing of the MPR infants (see Discussion).
Emotionality
Emotionality scores were recorded for each subject on each day of testing. Scores ranged from 1 to 3 with 1 being least reactive (calm and relaxed, quiet, does not need consolation), 2 being mildly reactive (somewhat agitated, some vocalizations, easy to console), and 3 being most reactive (very agitated, screeching, body-jerking, difficult to console). Emotionality scores were averaged for each subject, as well as for the entire group of test subjects, for each of the four testing stages.
Statistical Analysis
Hair cortisol was first tested for normal distribution using the Shapiro-Wilk (W) test. Because hair cortisol was not normally distributed (W=0.874; p<0.001), it was log-transformed (Tukey, 1977) and log10hair was used for analysis with object permanence performance (W=0.957; p=0.06). Independent-sample t-tests were used to examine rearing (MPR vs. NR) and start group (normal vs. late) differences in month 6 hair cortisol concentrations.
In order to test the hypothesis that elevated hair cortisol in five outliers (see Results) was a function of emotionality, one-sample t-tests were employed to examine differences in average emotionality scores for each task between hair cortisol outliers and the entire testing group.
Linear regression was performed to determine whether month 6 hair cortisol concentrations significantly predicted object permanence performance (dependent variables: postnatal day of age at criterion and number of test sessions to criterion for each task). To account for the late testing start ages in five of the subjects, start group (normal vs. late) was first entered into the model. Start group was a significant predictor for age at criterion for the plain reach, screen, and A-not-B tasks (Table 2) so it was retained in these models. Month 6 hair cortisol was then entered as a second predictor and the ΔR2 test was employed to determine whether the addition of month 6 hair cortisol added significant predictive power to the model after start age had been accounted for. Because start group did not significantly predict number of test sessions to criterion for any of the four tasks, only hair cortisol was entered as a predictor.
Table 2.
Hair cortisol at month 6 as a predictor of object permanence performance.
| Test Stage | Criteria | Model | Intercept | Predictors | F | R2 | ΔR2 | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Start Age |
Log10 Month 6 Hair |
||||||||
| Plain Reach | Age | 1 | 24.11 | 29.29 | - | 37.94 | 0.56 | - | <0.001 |
| 2 | 7.45 | 28.70 | 7.56 | 38.38 | 0.56 | 0.007 | NS | ||
| Sessions | 1 | 0.42 | - | 1.76 | 0.20 | 0.08 | - | NS | |
| Well | Age | 1 | −145.79 | - | 108.63 | 9.01 | 0.23 | - | <0.01 |
| Sessions | 1 | −29.83 | - | 19.68 | 8.22 | 0.46 | - | <0.01 | |
| Screen | Age | 1 | 78.37 | 54.43 | - | 13.42 | 0.31 | - | 0.001 |
| 2 | −76.76 | 48.95 | 70.36 | 17.93 | 0.40 | 0.09 | <0.05 | ||
| Sessions | 1 | −20.74 | - | 14.78 | 5.86 | 0.40 | - | <0.05 | |
| A-not-B | Age | 1 | 101.70 | 38.90 | - | 5.71 | 0.16 | - | <0.05 |
| 2 | −66.58 | 32.95 | 76.32 | 10.17 | 0.27 | 0.11 | <0.05 | ||
| Sessions | 1 | −2.61 | - | −2.61 | 0.71 | 0.15 | - | NS | |
An alpha level of p< 0.05 was considered statistically significant, and SPSS was used for all analyses.
RESULTS
Hair Cortisol
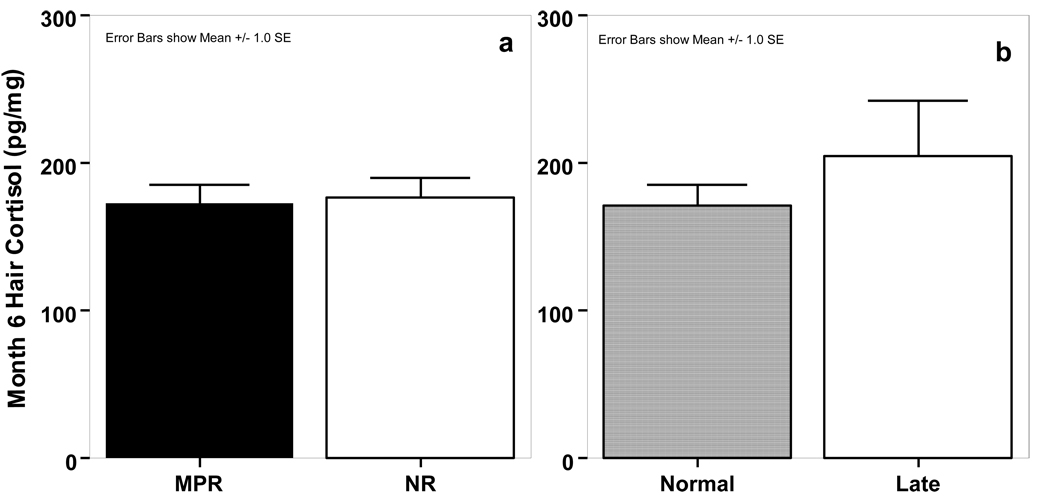
Subjects did not differ by rearing condition (t(50)=0.02, p=0.98; mean±SE: MPR=172.94±12.36 pg/mg, NR=176.88±13.08 pg/mg) or start group (t(30)=−1.01, p=0.32; normal=171.70±13.95 pg/mg, late=204.87±37.69 pg/mg) in month 6 hair cortisol concentrations (Figure 1).
Figure 1.
Month 6 hair cortisol concentrations in MPR and NR infants.
Emotionality
Five subjects were identified as outliers for month 6 hair cortisol concentrations. However, their average emotionality scores did not significantly differ from the group average on any of the four tasks (plain reach: t(4)=−0.537, p=0.62; well: t(4)=0.159, p=0.88; screen: t(4)=0.687, p=0.53; A-not-B: t(4)=−0.500, p=0.64; Table 1).
Table 1.
Average emotionality scores (±SE) for each stage of object permanence testing.
| Test Stage |
Hair Cortisol Outliers |
Remaining Subjects |
t value |
p value |
|---|---|---|---|---|
| Plain Reach | 1.39±0.25 | 1.37±0.05 | −0.537 | 0.62 |
| Well | 1.30±0.19 | 1.27±0.03 | 0.159 | 0.88 |
| Screen | 1.32±0.18 | 1.20±0.31 | 0.687 | 0.53 |
| A-not-B | 1.27±0.27 | 1.40±0.07 | −.500 | 0.64 |
Object Permanence Performance
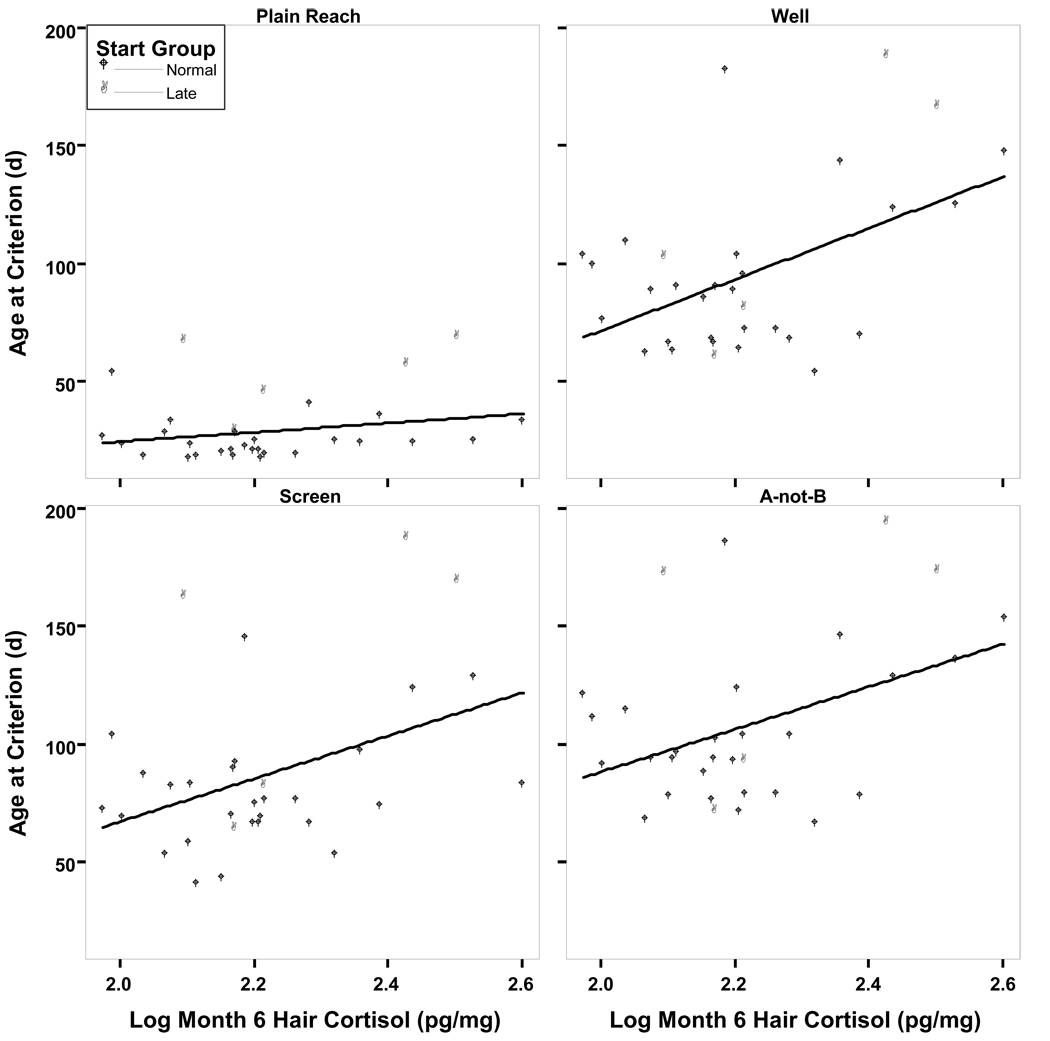
Start group (normal vs. late) significantly predicted age at criterion for the plain reach (R2=0.56, F(1,30)=37.94, p<0.001), screen (R2=0.31, F(1,30)=13.42, p=0.001), and A-not-B tasks (R2=0.16, F(1,30)=5.731, p=0.02; see Table 2). Hair cortisol added significant predictive power to the regression model for the screen (ΔR2=0.09, p<0.05) and A-not-B tasks (ΔR2=0.11, p<0.05), and hair cortisol also significantly predicted age at criterion for the well task (R2=0.23, F(1,30)=9.012, p<0.01; Table 2; Figure 2). Infants with higher hair cortisol reached criterion at later ages.
Figure 2.
Hair cortisol at month 6 predicts age at criterion for the well, screen, and A-not-B tasks. (Well: AgeCrit=−145.79 + 108.63Log10Hair; R2=0.23; p<0.01; Screen: AgeCrit=−76.76 + 48.95StartAge + 70.36Log10Hair; R2=0.40; p<0.05; A-not-B: Screen: AgeCrit=−66.58 + 32.95StartAge + 76.32Log10Hair; R2=0.27; p<0.05)
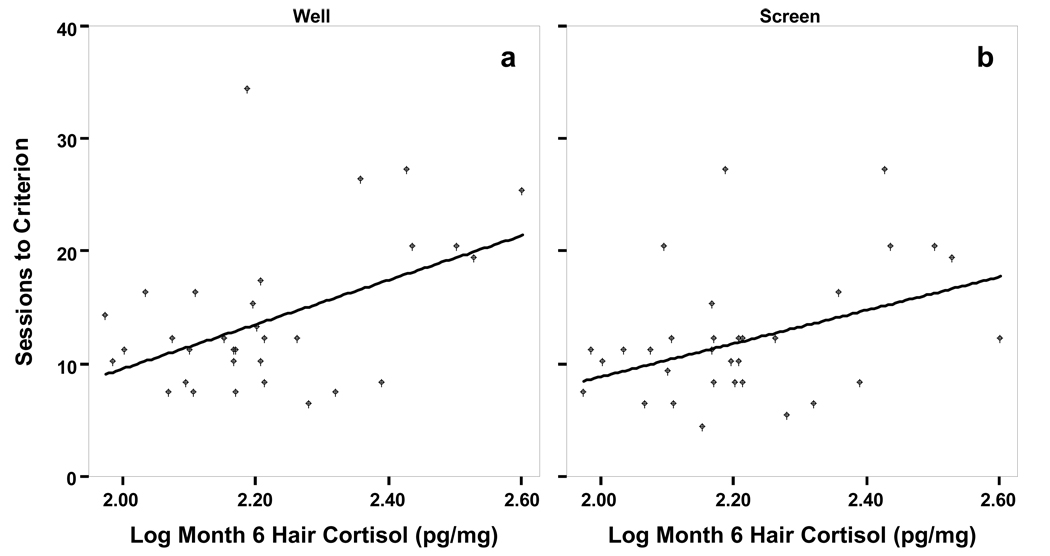
Start group did not predict the number of test sessions to criterion for any stage of testing, so it was omitted from the regression model. Hair cortisol significantly predicted the number of test sessions to criterion for the well (R2=0.22, F(1,30)=8.220, p<0.01) and screen tasks (R2=0.16, F(1,30)=5.861, p<0.05; Table 2; Figure 3). Infants with higher hair cortisol required more sessions to reach criterion on these tasks.
Figure 3.
Hair cortisol at month 6 predicts number of test sessions to criterion for the well and screen tasks. (Well: SessCrit=−29.83 + 19.68Log10Hair; R2=0.46; p<0.01; Screen: SessCrit=−20.74 + 14.78Log10Hair; R2=0.40; p<0.05)
DISCUSSION
We sought to utilize hair cortisol, representing in this case an integrated measure of HPA activity across 5.5 months, as an indicator of an infant monkey’s hypothalamic-pituitary-adrenocortical “phenotype” and further to determine whether this “phenotype” was useful in predicting early cognitive performance that occurred within the same time period. Our findings reveal that overall, higher hair cortisol concentrations predicted poorer performance on object permanence tasks. Infants with higher hair cortisol required more test sessions and completed the tasks measuring object permanence (screen and well) at later ages.
One possible explanation for these findings is that the infants with the highest hair cortisol are also the most emotionally reactive, thereby requiring more test sessions to reach criterion on the tasks (and thus completing them at later ages). However, examination of the emotionality scores given to each infant on each day of testing revealed no significant difference in average emotionality between the five infants with the highest hair cortisol levels and the test group of subjects on any task. Further, these subjects were habituated to human handling and were accustomed to the testing procedure. Thus we can rule out the likelihood of emotional arousal during object permanence testing causing the elevated hair cortisol values. These data support the notion that chronic circulating levels of cortisol may exert detrimental effects on the brain areas associated with early cognition.
Before generalizing the present results to all infant rhesus monkeys, it is important to note that we were unable to test MPR infants on object permanence tasks because of the difficulty in testing without also inducing distress reactions in the infants during the periods in which they would be separated from their mothers and/or peers. Additionally, it is possible that our results were due in part to the NR paradigm producing higher levels of hair cortisol, a notion that is supported by an extensive literature demonstrating elevated acute concentrations of cortisol in NR monkeys (Champoux et al., 1989; Capitanio et al., 2005; Sanchez et al., 2005; Barrett et al., 2009). However, the literature on NR and HPA activity is not entirely consistent: initially it appeared that NR infants exhibited higher basal plasma cortisol concentrations than MR infants, but that the two did not differ in stress levels of cortisol (Champoux et al., 1989). However, later studies demonstrated the reverse, namely that NR infants exhibited similar or lower basal plasma cortisol levels (Clarke, 1993; Shannon et al., 1998; Capitanio et al., 2005) and higher or lower concentrations of plasma cortisol than MR monkeys after stress (Higley, Suomi, and Linnoila, 1992; Davenport et al., 2003). Finally, one study (Winslow et al., 2003) found that neither basal nor stress-related cerebrospinal fluid (CSF) cortisol concentrations differed between MPR and NR infants. The lack of general agreement in these studies may reflect differing NR protocols across laboratories as well as the inherent limitations in measuring cortisol in point samples such as plasma, saliva, or CSF. Nevertheless, there is no reason to assume that our findings relating hair cortisol to cognitive performance are somehow unique to the NR condition as MPR and NR monkeys did not differ in hair cortisol values in the first six months of life. It is possible that rearing differences are not detectable in hair cortisol until later in infancy and early adolescence, an idea that is supported by preliminary data from our laboratory (Dettmer et al., 2007; Dettmer, 2009).
The nature of the relationship between cortisol and cognition is not yet well understood. Studies that show impaired cognitive function after acute cortisol administration suggest a causal mechanism that could, for example, be mediated by rapidly-acting membrane glucocorticoid receptors in the brain (Tasker, Di, and Malcher-Lopez, 2006). Such studies, however, typically produce high circulating cortisol concentrations in the stress range. In contrast, the present results were obtained from animals that were neither stressed during the learning task nor given exogenous glucocorticoids. Rather, we found that an index of endogenous circulating cortisol integrated over a period of months was predictive of object permanence learning in our infant monkeys. There is much evidence that chronic stress or other manipulations that persistently elevate glucocorticoid levels above the normal resting range have deleterious effects on key brain areas such as the hippocampus and prefrontal cortex (McEwen, 2008), both of which have been implicated in object permanence tasks (Goldman-Rakic, 1990). It is intriguing to speculate that chronically high cortisol levels even within the “normal” range exert a subtle influence on the hippocampus and prefrontal cortex, particularly during development, in a way that compromises the organism’s ability to perform optimally on tasks that engage these areas. Alternatively, hair cortisol in the present context may simply be serving as a marker for other traits that are causally related to object permanence performance, rather than cortisol itself. Further research is needed to test these different hypotheses.
In conclusion, the present study is the first to relate hair cortisol to cognitive performance in any species and at any stage of development. The results demonstrate that hair cortisol is a significant predictor of object permanence learning in infant rhesus monkeys, with high cortisol levels predicting relatively poor performance. We are currently testing infants on a battery of more complex cognitive tasks that examine prefrontal cortex and hippocampal functioning throughout the first 8–12 months of life to see whether hair cortisol is a useful predictor of performance on these tasks as well. An exciting range of studies is now possible for researchers interested in studying chronic integrated HPA activity with respect to cognition, from infancy to adolescence and into adulthood.
ACKNOWLEDGEMENTS
The studies described in this report were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the NICHD Animal Care and Use Committee. We gratefully acknowledge the assistance of Caroline Kenney, Elizabeth Kerschner, Michelle Miller, Angela Ruggiero, Lisa Darcey, Sarah Unbehagen, Elizabeth Mallott, and Daniel Hipp in data collection. This research was funded the Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health & Human Development, and by NIH Grant RR11122 to MAN. A preliminary report of this research was presented at the 31st annual meeting of the American Society of Primatologists in West Palm, Florida, 2008.
REFERENCES
- Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE. Early Adverse Rearing Experiences Alter Sleep-Wake Patterns and Plasma Cortisol Levels in Juvenile Rhesus Monkeys. Psychoneuroendocrinology. 2009;34(7):1029–1040. doi: 10.1016/j.psyneuen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguera B, Muruais C, Penalva A, Dieguez C, Casanueva FF. Dual and Selective Actions of Glucocorticoids Upon Basal and Stimulated Growth Hormone Release in Man. Neuroendocrinology. 1990;51:51–58. doi: 10.1159/000125315. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing Environment and Hypothalamic-Pituitary-Adrenal Regulation in Young Rhesus Monkeys (Macaca mulatta) Devel. Psychobiol. 2005;46(4):318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Champoux M, Coe CL, Schanber SM, Kuhn CM, Suomi SJ. Hormonal Effects of Early Rearing Conditions in the Infant Rhesus Monkeys. Am. J. Primatol. 1989;19(2):111–117. doi: 10.1002/ajp.1350190204. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric Stress: Hormonal Mediators and Human Development. Horm. Res. 2003;59:161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions Between the Hypothalamic-Pituitary-Adrenal Axis and the Female Reproductive System: Clinical Implications. Ann. Intern. Med. 1998;129:229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Clarke AS. Social Rearing Effects on HPA Axis Activity Over Early Development and in Response to Stress in Rhesus Monkeys. Dev. Psychobiol. 1993;26(8):433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of Endogenous Cortisol Concentrations in the Hair of Rhesus Macaques. Gen. Comp. Endocrinol. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. A Rhesus Monkey Model of Self-Injury: Effects of Relocation Stress on Behavior and Neuroendocrine Function. Biol. Psychiatry. 2008;63(10):990–996. doi: 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Crouthamel BJ, Ruppenthal GC, Sackett GP. Differences in Object Permanence Performance by Baboons (P. Anubis/cynocephalus) and Pigtailed Macaques (M. nemestrina) Am. J. Primatology. 2003;60 Suppl 1:119–120. [Google Scholar]
- Dettmer AM, Ruggiero AM, Novak MA, Suomi SJ, Meyer JS. Program No. 732.17. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. The Use of Hair Cortisol to Measure Chronic Hypothalamic-Pituitary-Adrenal Activity in Differently-Reared Infant Rhesus Monkeys (Macaca mulatta) 2007. Online. [Google Scholar]
- Dettmer AM. Doctoral Dissertation. University of Massachusetts; 2009. Early Rearing Experience, Hypothalamic-Pituitary-Adrenal (HPA) Activity, and Serotonin Transporter Genotype: Influences on the Development of Anxiety in Infant Rhesus Monkeys (Macaca mulatta) [Google Scholar]
- Glazer D. Child Abuse and Neglect and the Brain – A Review. J. Child. Psychol. Psychiatry. 2000;41(1):97–116. [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP. Clinical and Biochemical Manifestations of Depression. Relation to the Neurobiology of Stress. 1. N. Engl. J. Med. 1988;319:348–353. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cortical Localization of Working Memory. In: McGaugh JL, Weinberger NM, Lynch G, editors. Brain Organization and Memory: Cells, System, and Circuits. New York: Oxford University Press; 1990. [Google Scholar]
- Haley DW, Weinberg J, Grunau RE. Cortisol, Contingency Learning, and Memory in Pre- and Full-Term Infants. Psychoneuroendocrinology. 2005;31:108–117. doi: 10.1016/j.psyneuen.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A Longitudinal Assessment of CSF Monoamine Metabolite and Plasma Cortisol Concentrations in Young Rhesus Monkeys. Biol. Psychiatry. 1992;32(2):127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Gwirtsman HE, George DT, Ebert MH, Jimerson DC, Tomai TP, Chrousos GP, Gold PW. Elevated Cerebrospinal Fluid Levels of Immunoreactive Corticotropin-Releasing Hormone in Anorexia Nervosa: Relation to State of Nutrition, Adrenal Function, and Intensity of Depression. J. Clin. Endocrinol. Metab. 1987;64:203–208. doi: 10.1210/jcem-64-2-203. [DOI] [PubMed] [Google Scholar]
- Kelly BJ, Novak MA, Meyer JS. To Bite or Not to Bite: Interrelationships Between Self-Injurious Behavior (SIB), Plasma Cortisol and Task Performance in Rhesus Monkeys (Macaca mulatta) Am. J. Primatol. 2007;69(S1):130. [Abstract] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a Retrospective Calendar of Cortisol Production – Increased Cortisol Incorporation into Hair in the Third Trimester of Pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress-and Treatment-Induced Elevations of Cortisol Levels Associated with Impaired Declarative Memory in Healthy Adults. Life Sci. 1996;58(17):1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lecours AR, Lussier I, Schwartz G, Vair NPV, Meaney MJ. Basal Cortisol Levels and Cognitive Deficits in Human Aging. J. Neurosci. 1994;14(5):2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol Levels During Human Aging Predict Hippocampal Atrophy and Memory Deficits. Nature Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working Memory is More Sensitive than Declarative Memory to the Acute Effects of Corticosteroids: A Dose-Response Study in Humans. Behav. Neurosci. 1999;113(3):420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress Inoculation-Induced Indications of Resilience in Monkeys. J. Traumatic Stress. 2007;20(4):423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Lopez JM, Yang C, Shcatzberg AF. Stress-Level Cortisol Treatment Impairs Inhibitory Control of Behavior in Monkeys. J. Neurosci. 2000;20(20):7816–7821. doi: 10.1523/JNEUROSCI.20-20-07816.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacConnie SE, Barkan A, Lampman RM, Schork MA, Beitins IZ. Decreased Hypothalamic Gonadotropin-Releasing Hormone Secretion in Male Marathon Runners. N. Engl. J. Med. 1986;315:411–417. doi: 10.1056/NEJM198608143150702. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Gomez MT, Rose SR, Chrousos GP. Suppressed Spontaneous and Stimulated Growth Hormone Secretion in Patients with Cushing’s Disease Before and After Surgical Care. J. Clin. Endocrinol. Metab. 1994;78:131–137. doi: 10.1210/jcem.78.1.7507118. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central Effects of Stress Hormones in Health and Disease: Understanding the Protective and Damaging Effects of Stress and Stress Mediators. Eur. J. Pharmacol. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CM, Widom CS. Childhood Victimization and Long Term Intellectual and Academic Outcomes. Child Abuse & Neglect. 1994;18:617–633. doi: 10.1016/0145-2134(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzber AF, Lyons DM. Mild Early Life Stress Enhances Prefrontal-Dependent Response Inhibition in Monkeys. Biol. Psychiatry. 2005;57(8):848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC. Survey of Protocols for Nursery Rearing Infant Macaques. In: Ruppenthal GC, editor. Nursery Care of Nonhuman Primates. New York: Plenum Press; 1979. [Google Scholar]
- Ruppenthal GC, Sackett GP. Research Protocol and Technician’s Manual: A Guide to the Care, Feeding, and Evaluation of Infant Monkeys. 1992 Retrieved November 8, 2005, from the University of Washington, Seattle, Regional Primate Research Laboratory Web site: http://www.rprc.washington.edu/iprl/
- Sackett GP, Ruppenthal GC, Hewitson L, Simerly C, Schatten G. Neonatal Behavior and Infant Cognitive Development in Rhesus Macaques Produced by Assisted Reproductive Technologies. Devel. Psychobiol. 2006;48(3):243–265. doi: 10.1002/dev.20132. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in Diurnal Cortisol Rhythm and Acoustic Startle Response in Nonhuman Primates with Adverse Rearing. Biol. Psychiatry. 2005;57(4):373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Schneider ML. Delayed Object Permanence Development in Prenatally Stressed Rhesus Monkey Infants (Macaca mulatta) Occupational Ther. J. of Res. 1992;12(2):96–110. [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing Condition and Plasma Cortisol in Rhesus Monkey Infants. Am. J. Primatol. 1998;46(4):311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Smith ELP, Batuman OA, Trost RC, Coplan JD, Rosenblum LA. Transforming Growth Factor-Beta-1 and Cortisol in Differentially Reared Primates. Brain, Behavior, and Immunity. 2002;16(2):140–149. doi: 10.1006/brbi.2001.0629. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopez R. Minireview: Rapid Glucocorticoid Signaling via Membrane-Associated Receptors. Endocrinology. 2006;147(12):5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LA, Trevathan WR. Cortisol Reactivity, Maternal Sensitivity, and Learning in 3-Month Old Infants. Infant Beh. Dev. 2008;31:92–106. doi: 10.1016/j.infbeh.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. Addison-Wesley Series in Behavioral Science: Quantitative Methods. Reading, Mass: Addison-Wesley; 1977. Exploratory Data Analysis. [Google Scholar]
- Walker CG, Ruppenthal GC, Kimpo CL, Sackett GP. Sex and Rearing Condition Differences in the Development of Object Permanence in Olive Baboons and Pigtailed Macaques. Am. J. Primatology. 1992;27(1):62. [Google Scholar]
- Wand GS, Dobs AS. Alterations in the Hypothalamic-Pituitary-Adrenal Axis in Actively Drinking Alcoholics. J. Clin. Endocrinol. Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing Effects on Cerebrospinal Fluid Oxytocin Concentration and Social Buffering in Rhesus Monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Wise KL, Wise LA, Zimmerman RR. Piagetian Object Permanence in Infant Rhesus Monkey. Devel. Psychology. 1974;10:429–437. [Google Scholar]
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. Hair Cortisol as a Potential Biologic Marker of Chronic Stress in Hospitalized Neonates. Neonatology. 2007;92:42–49. doi: 10.1159/000100085. [DOI] [PubMed] [Google Scholar]