Abstract
Previous studies of Min/+ (multiple intestinal neoplasia) mice on a sensitive genetic background, C57BL/6 (B6), showed that adenomas have lost heterozygosity for the germ-line ApcMin mutation in the Apc (adenomatous polyposis coli) gene. We now report that on a strongly resistant genetic background, AKR/J (AKR), Min-induced adenoma multiplicity is reduced by about two orders of magnitude compared with that observed on the B6 background. Somatic treatment with a strong mutagen increases tumor number in AKR Min/+ mice in an age-dependent manner, similar to results previously reported for B6 Min/+ mice. Immunohistochemical analyses indicate that Apc expression is suppressed in all intestinal tumors from both untreated and treated AKR Min/+ mice. However, the mechanism of Apc inactivation in AKR Min/+ mice often differs from that observed for B6 Min/+ mice. Although loss of heterozygosity is observed in some tumors, a significant percentage of tumors showed neither loss of heterozygosity nor Apc truncation mutations. These results extend our understanding of the effects of genetic background on Min-induced tumorigenesis in several ways. First, the AKR strain carries modifiers of Min in addition to Mom1. This combination of AKR modifiers can almost completely suppress spontaneous intestinal tumorigenesis associated with the Min mutation. Second, even on such a highly resistant genetic background, tumor formation continues to involve an absence of Apc function. The means by which Apc function is inactivated is affected by genetic background. Possible scenarios are discussed.
Germ-line mutation of the adenomatous polyposis coli (APC/Apc) tumor suppressor gene predisposes both humans and mice to intestinal tumorigenesis (reviewed in ref. 1). Familial adenomatous polyposis (FAP) is a dominantly inherited human cancer syndrome that results from germ-line APC mutation. Individuals with FAP can develop up to several thousand intestinal tumors, which are located primarily in the colon (2). The number of tumors observed in FAP individuals seems to be influenced by the site of the germ-line mutation in APC, environmental factors, and the effects of unlinked modifier loci (3–6).
ApcMin (Min, multiple intestinal neoplasia) is a nonsense mutation at codon 850 of the mouse APC homolog that predisposes heterozygotes to intestinal tumorigenesis (7, 8). On the C57BL/6J (B6) genetic background, Min/+ mice average more than 70 tumors throughout the intestinal tract and rarely live beyond 150 days of age (1). Somatic N-ethyl-N-nitrosourea (ENU) treatment of B6 Min/+ mice indicates that intestinal tumors are preferentially initiated during the first few weeks of life (9). Tumor formation in B6 Min/+ mice is invariably associated with somatic loss of the wild-type Apc allele, apparently by chromosome loss (10). On the (AKR × B6)F1 genetic background, Min/+ adenoma formation also involves Apc+ loss (9). However, not all tumors show loss of the Apc+ marker when the Min mutation is carried on certain other F1 hybrid genetic backgrounds (11). Also, regional Apc deletion or nonsense mutation frequently is observed in tumors from Min/+ mice that have been treated somatically with γ-irradiation or ENU (11, 12).
Crosses between B6 Min/+ mice and the inbred strain AKR/J (AKR) led to the identification of the Mom1 locus (13–15). Mom1 is a semidominant modifier of intestinal tumor number in Min/+ mice: one AKR allele of Mom1 (Mom1A) causes a reduction in tumor number by a factor of two; two copies of Mom1A reduce tumor multiplicity by a factor of four (16). Analysis of Min/+ mice carrying a Pla2 g2a transgene indicates that this secreted phospholipase comprises at least a portion of Mom1 (17).
Beyond Mom1, the AKR strain carries alleles of other loci that modify intestinal tumor number in Min/+ mice (14). In this paper we have examined the full extent to which the intestinal phenotype of Min/+ mice can be modified by transferring the ApcMin mutation from the sensitive B6 strain onto AKR. We have found that Min-induced intestinal tumorigenesis is almost completely suppressed on the homozygous AKR background. We have observed that a significant percentage of tumors from AKR Min/+ mice do not show Apc+ allele loss. Comparing these observations with previous analyses of the (B6 × AKR)F1 genetic background (10), we conclude that the reduced frequency of Apc+ allele loss of AKR is recessive to the high frequency conferred by B6. In this paper, we have asked further whether tumor formation on this highly resistant background requires functional inactivation of both Apc alleles. Immunohistochemical analysis has revealed that Apc gene expression is greatly reduced in all of the tumors that retain the Apc+ allelic marker. Several very different scenarios can account for this observation.
MATERIALS AND METHODS
Mice.
All mice were bred at the McArdle Laboratory for Cancer Research. Parental AKR mice were purchased from The Jackson Laboratory. All of the AKR Min/+ mice used in these experiments had been backcrossed to AKR for 5–9 generations. Thus, these AKR Min/+ mice were homozygous for AKR alleles at approximately 97% of all loci. The tumor multiplicity of these mice is comparable to that of mice that are now at the 11th backcross generation (data not shown). Animals were genotyped for the presence of the ApcMin mutation by a previously described PCR-based assay (14). For mutagenesis studies, all mice from a litter (consisting of AKR Min/+ and +/+ mice) were given an i.p. injection of ENU from a single sample as described previously (9). To control further for ENU variability, litters from each age group were included in each round of ENU treatment. Litters of 10- to 15-day-old B6 Min/+ mice also were included in each round of ENU treatment. Tumor multiplicity in these control mice was comparable to results previously published (9).
Tumor Scoring and Collection.
Intestinal tumors were scored at ×10–30 magnification as previously described (9). For experiments requiring unfixed tissue, tumor and normal intestinal tissues were carefully dissected, with a fresh scalpel blade for each sample. All other samples were fixed overnight in 10% buffered formalin or, for immunohistochemistry, for 1 hr in periodate-lysine-paraformaldehyde (18).
Quantitative PCR Analysis of Apc.
Apc allelic status was assessed by a quantitative PCR assay described previously (10, 12). This assay generates 123-bp and 144-bp products from the Apc+ and ApcMin alleles, respectively. The Apc+/ApcMin ratio was determined by quantitation of 32P-labeled products (10). To be consistent with previously reported results, the allelic ratios were not corrected for the difference in the sizes of the ApcMin and Apc+ amplimers (10, 12). All samples were independently amplified by PCR at least twice. Only samples that gave repeatable values (within 10%) with signals at least 10-fold above background were included in the results.
Examination of Apc for Truncation Mutations.
Codons 680-1230 of the Apc ORF were examined for truncation mutations with an in vitro synthesized protein assay as described previously (12). This region also includes the germ-line Min nonsense mutation, which served as a positive control for the assay.
Immunohistochemical Analysis of Apc Expression.
Tissue samples used for immunohistochemistry were fixed for 1 hr in periodate-lysine-paraformaldehyde. Antibody staining was performed essentially as described by Merritt et al. (19). Affinity-purified rabbit polyclonal antibody APC2, raised against amino acids 1035–2130 of human APC (a gift from Paul Polakis, Onyx Pharmaceuticals, Richmond, CA), was used at a dilution of 1:100 (20). Rabbit polyclonal antibody 3122, raised against amino acids 8–347 of human APC, was used at a dilution of 1:500 (21). The regions of APC used to generate the APC2 and 3122 antibodies are more than 87% identical to mouse Apc at the amino acid level (8). Slides were lightly counterstained with hematoxylin before dehydration and coverslipping. Staining of each sample always was accompanied by a negative control sample in which the primary antibody was omitted. No staining was observed in any of these control samples (data not shown).
RESULTS
Intestinal Tumor Multiplicity in AKR Min/+ Mice.
When counting intestinal tumors in B6 Min/+ mice, we typically score four regions representing approximately 40% of the intestine. These regions consist of the entire colon and 4-cm segments from the proximal, middle, and distal small intestine. We consistently have found that B6 Min/+ mice scored in this manner develop 30 ± 10 tumors (1). To examine further the effects of genetic background on Min-dependent intestinal tumorigenesis, we introduced the Min mutation onto the AKR background (see Materials and Methods).
A summary of the intestinal tumor incidence and multiplicity for AKR Min/+ mice is shown in Table 1. Owing to the low tumor multiplicity of AKR Min/+ mice, the entire length of the small and large intestine was scored for tumors in these animals. Only 43 of 170 AKR Min/+ mice developed even a single intestinal tumor. In total, 53 intestinal tumors were identified from this set of 170 AKR Min/+ mice, yielding a multiplicity of 0.3 tumors per mouse. All of these tumors were located in the small intestine. Correcting for the difference in length of intestine scored, the tumor multiplicity of AKR Min/+ mice represents a reduction by approximately two orders of magnitude from that observed in B6 Min/+ mice (Table 1). Only one intestinal tumor was found in a total of 37 AKR Apc+/+ mice.
Table 1.
Comparison of intestinal tumor multiplicity in AKR and B6 Min/+ mice
| Mice* | Number of mice with tumors | Tumor multiplicity, average ± SD | Average age, days |
|---|---|---|---|
| B6 Min/+ | 68/68 (100%) | 29.2 ± 9.4 | 85 |
| B6 Mom1A/A Min/+ | 21/21 (100%) | 7.8 ± 3.4 | 120 |
| AKR Min/+ | 43/170 (25%) | 0.3 ± 0.5† | 138‡ |
For AKR Min/+ mice, the entire length of the small and large intestine was scored for tumors. For B6 Min/+ and B6 Mom1A/A Min/+ mice, four representative regions comprising approximately 40% of the entire intestinal tract were scored. The entire intestinal trace was also scored for a total of 37 AKR Apc+/+ mice (average age of 182 days). One tumor was found in the small intestine of one of these mice. No tumors were found in the entire intestinal tract of 25 B6 Apc+/+ mice (average age of 99 days).
In total, 53 intestinal tumors were found in 170 AKR Min/+ mice. All tumors were in the small intestine.
This lifespan is limited by the predisposition of AKR to lymphomagenesis.
The modifier effects of the Mom1 locus previously were studied by using a strain in which the AKR allele of Mom1 had been introgressed onto the B6 background (15). B6 Mom1A/A Min/+ mice averaged 7.8 ± 3.4 intestinal tumors, and the proportion of Min/+ mice with tumors remained 100% (Table 1) (15). Thus, Mom1 can account for only a small portion of the reduction in tumor multiplicity between B6 Min/+ and AKR Min/+ mice.
Stimulation of Intestinal Adenoma Formation in AKR Min/+ Mice by Treatment with ENU.
We previously have shown that ENU treatment of B6 Min/+ mice at 5–14 days of age increased tumor multiplicity approximately 3.8-fold, whereas treatment between 20 and 35 days resulted in only a 1.6-fold increase (9). These results suggest that intestinal tumors in B6 Min/+ mice are preferentially initiated during the first few weeks of life. To investigate the timing of tumor induction in AKR Min/+ mice, we injected Min/+ and +/+ mice with a single dose of ENU at either 9–16 or 27–42 days of age. Mice were sacrificed approximately 150 days after treatment, and tumors were scored along the entire length of the small and large intestine.
ENU treatment significantly increased both the incidence and multiplicity of tumors for each treatment group relative to untreated AKR Min/+ mice (P < 0.001 for comparisons of tumor number, Wilcoxon rank sum test, Table 2). However, a much stronger effect was seen for mice treated at 9–16 days of age. One hundred percent of these mice developed intestinal tumors, with an average multiplicity of 12.8. By contrast, 79% of AKR Min/+ mice treated at 27–42 days of age developed tumors, with an average multiplicity of only 1.9 (Table 2). These average tumor numbers represent, respectively, 25.6- and 3.8-fold increases over untreated mice. Note that no intestinal tumors were found in any of the 39 ENU-treated AKR Apc+/+ mice. These results indicate that, as observed for B6 Min/+ mice, intestinal tumors in AKR Min/+ mice are preferentially initiated before weaning. Colonic tumorigenesis was strongly suppressed even in ENU-treated AKR Min/+ mice. Only one colonic tumor was found, in an AKR Min/+ mouse treated with ENU at 16 days of age.
Table 2.
Intestinal tumor multiplicity in ENU-treated AKR Min/+ mice
| Age at ENU treatment | Number of Min/+ mice with tumors | Tumor multiplicity, average ± SD |
|---|---|---|
| 9–16 days | 18/18 (100%) | 12.8 ± 5.9 |
| 27–42 days | 19/24 (79%) | 1.9 ± 1.6 |
| Untreated | 9/23 (39%) | 0.5 ± 0.8 |
Entire litters were treated with ENU as described in Materials and Methods. The entire length of the small and large intestine was scored. The average age (post-ENU) for the ENU-treated mice was 156 days. The average age for the untreated mice was 152 days. No tumors were found in any of 39 ENU-treated AKR Apc+/+ mice, 12 of which were treated at 9–16 days of age and the remaining 27 mice at 27–42 days of age. The average post-ENU age of the Apc+/+ mice was 138 days.
Analysis of Apc Allelic Status in Intestinal Tumors from AKR Min/+ Mice.
All spontaneous intestinal tumors examined from B6 Min/+ and (AKR × B6)F1 Min/+ mice show loss of the Apc+ allele, apparently resulting from chromosome loss (10). This pathway for inactivating tumor suppressor function is designated loss of heterozygosity (LOH). Because there were major differences in intestinal tumor multiplicity between B6 Min/+ and AKR Min/+ mice, we investigated whether there are also differences in the somatic events associated with tumor formation.
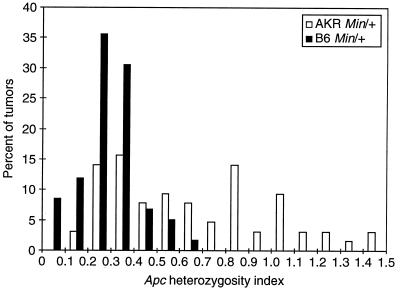
The allelic status of the Apc locus was examined by a quantitative PCR assay of DNA from 16 intestinal tumors from untreated AKR Min/+ mice. Tumors from the AKR Min/+ mice tend to be quite small; however, the amount of stromal cell infiltration seemed comparable to that observed for tumors from B6 Min/+ mice. To obtain samples enriched for normal or tumor cells, we prepared DNA from cells that were scraped from fixed and sectioned tissue samples. The index of heterozygosity at the Apc locus, HetApc, was defined as the Apc+/ApcMin band ratio of the tumor DNA sample, normalized to the band ratio determined in parallel for the DNA sample from adjacent normal tissue (see Fig. 1). If the HetApc was ≥0.64, we concluded that the Apc+ allelic marker persisted. By this criterion, 37% (6/16) of the fixed tumors from untreated AKR Min/+ mice maintained heterozygosity for the Apc marker (Fig. 1).
Figure 1.
Apc heterozygosity index for adenomas in B6 Min/+ and AKR Min/+ animals. The Apc heterozygosity index, HetApc, was defined by the Apc+/ApcMin band intensity ratio for tumor DNA, normalized to that ratio for DNA from adjacent normal tissue. For 59 tumors arising spontaneously in B6 Min/+ animals (filled bars), the values of HetApc are distributed approximately as Gaussian with mean 0.32 and SD 0.17. For 64 tumors (22 spontaneous and 42 arising after ENU treatment) in AKR Min/+ animals (empty bars), the distribution of HetApc differs strongly from that of B6 Min/+ tumors (P = 3.5 × 10−9 by the Wilcoxon rank sum test). The AKR Min/+ indices are well approximated by a mixture of two Gaussian distributions, with 53% of the tumors centered around 0.32 (the LOH class) and 47% of the tumors around 0.95 as determined by the method of maximum likelihood. With a definition of maintenance of heterozygosity as HetApc ≥ 0.64, the probability of misclassifying a tumor with LOH is only 3%. No significant differences in the distribution of HetApc for tumors from AKR Min/+ mice were observed as a function of ENU treatment or method of tumor dissection and DNA isolation.
Tumors from AKR Min/+ mice that maintained the Apc+ allelic marker might have acquired mutations within the Apc+ allele. We previously have found that at least 27% (25/91) of intestinal tumors from ENU-treated B6 Min/+ mice that fail to show LOH instead carry a detectable somatic Apc truncation mutation (12). To begin to address this possibility for the AKR Min/+ case, we analyzed tumors from both untreated and ENU-treated mice for Apc truncation mutations, using the in vitro synthesized protein (IVSP) assay. In this initial analysis we focused on the region of Apc between codons 680 and 1230, because all of the 25 truncation mutations found in tumors from ENU-treated B6 Min/+ mice were found in that region of the gene. A set of frozen tissue samples was collected for these experiments, because the IVSP assay is incompatible with nucleic acids prepared from fixed tissue.
Frozen tumors from untreated and ENU-treated AKR Min/+ mice were analyzed. Twenty tumors (2/6 and 18/36, respectively) maintained Apc heterozygosity (Fig. 1). Of these, two untreated and 10 ENU-treated tumors were successfully examined by the in vitro synthesized protein assay. In contrast to ENU-treated B6 Min/+ mice, where 25/91 of the tumors that maintained the Apc+ marker showed truncations (9), none of the 12 tumors in AKR Min/+ mice that maintained the Apc+ marker showed truncations. The truncation product produced by the Min allele was observed in all samples, serving as a positive control for the assay. In the absence of evidence for LOH at the Apc locus or for truncation mutations in the Apc+ allele, is Apc+ expressed in tumors from the AKR Min/+ stock?
Analysis of Apc Expression in Intestinal Tumors from AKR Min/+ Mice.
To explore the Apc expression in intestinal tumors from AKR Min/+ mice more thoroughly, we examined the Apc polypeptide by immunohistochemistry, using the amino-terminal 3122 and central APC2 polyclonal antibodies (see Materials and Methods).
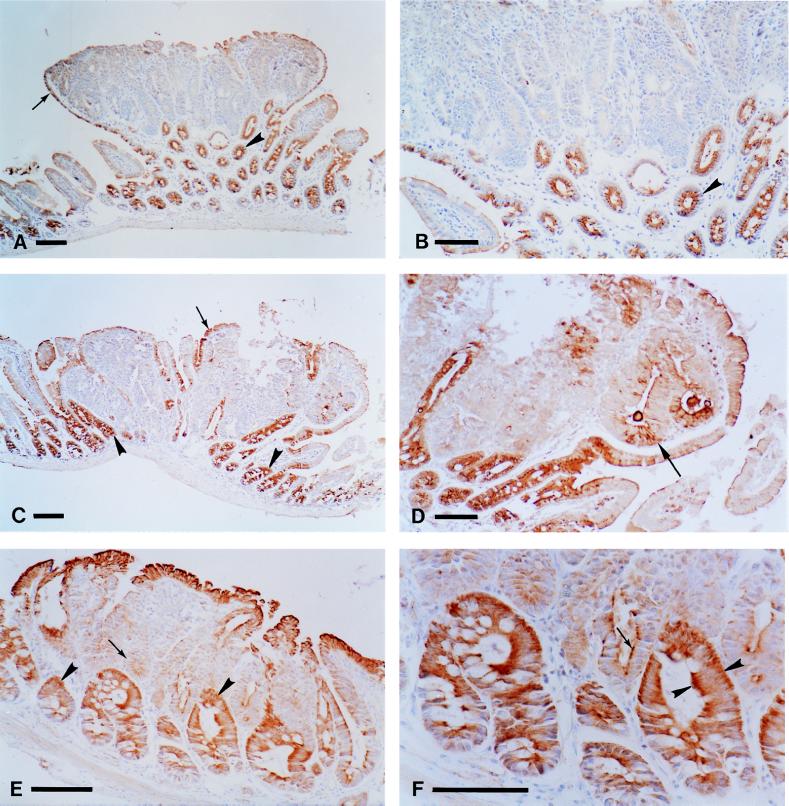
Cytoplasmic Apc staining was observed in cells from normal intestinal epithelia of B6 Min/+ and AKR Min/+ mice (Fig. 2). Apc staining was more intense for cells in the upper half of crypts than at the base. The columnar epithelial cells of the intestinal villi showed light, but consistent, Apc staining (Fig. 2). Both 3122 and APC2 gave similar results, and this pattern of expression is consistent with previous reports of APC/Apc expression (18, 22–24). However, there were some differences between 3122 and APC2 with respect to intracellular localization of Apc. Each antibody detected Apc expression in the basal, lateral, and apical cytoplasm (Fig. 2). However, 3122 stained the apical cytoplasm much more intensely than the basal cytoplasm, whereas APC2 strongly stained both the basal and the apical cytoplasm (compare Fig. 2 B and F).
Figure 2.
Analysis of Apc expression in tumors from the small intestine of Min/+ mice. Periodate-lysine-paraformaldehyde-fixed tissues were stained with anti-Apc antibodies as described in Materials and Methods. The tumors in A, B, E, and F were classified by quantitative PCR as showing LOH, whereas the tumor in C and D maintained heterozygosity at Apc. A tumor from an untreated B6 Min/+ mouse stained with the 3122 antibody is shown at low (A) and high (B) magnification. Apc staining is observed in normal crypts (A, arrowhead) and in the normal cells that encapsulate the tumor (A, arrow). Note the strong Apc staining in the apical cytoplasm in the cells from the normal crypts (B, arrowhead). A tumor from an ENU-treated AKR Min/+ mouse stained with 3122 is shown in C and D. Apc staining is seen in normal crypts (C, arrowheads) and in the cells that encapsulate the tumor (C, arrow). A different, serial section of a region of the tumor in C is shown at higher magnification in D. Low levels of staining are observed in some of the tumor cells (D, arrow). A tumor from an untreated AKR Min/+ mouse stained with the APC2 antibody is shown at low (E) and high (F) magnification. Apc staining is much stronger in the cells of the normal crypts (E, arrowheads) relative to the tumor cells (E, arrow). Staining also is seen in the normal cells that encapsulate the tumor. Note the apical and strong basal cytoplasmic staining in the normal crypts (F, arrowheads, compare with B). Some residual staining can be seen in the apical cytoplasm of some of the tumor cells (F, arrow). (Bars, 100 μm for A, C, and E; 75 μm for B, D, and F.)
In intestinal tumors from B6 Min/+ mice, Apc expression was essentially undetectable with either antibody (Fig. 2 A and B). Because the 3122 antibody should be capable of recognizing the ApcMin polypeptide, the lack of 3122 staining in tumors from B6 Min/+ mice was surprising. Indeed, Western blot analysis, with a mAb specific for amino acids 1–29 of APC, detects the polypeptide fragment from the ApcMin allele in colonic tumors from B6 Min/+ mice (1). Therefore, the native conformation of the ApcMin fragment polypeptide may be unrecognizable by the 3122 antibody or the fragment may be expressed at levels not detectable by immunohistochemistry. Altogether, immunohistochemistry was used to examine 18 tumors from the small intestine of both untreated (nine tumors) and ENU-treated (nine tumors) AKR Min/+ mice (Fig. 2 C–F). For three of the 18 samples, the APC2 signal was too light to be reliably interpreted in normal and tumor cells. Twelve of the 15 scorable tumors also were analyzed by quantitative PCR: eight maintained heterozygosity for the Min marker (two untreated and six ENU-treated). In the cells of all scorable samples, Apc expression detected by immunohistochemistry was significantly reduced relative to staining levels in adjacent normal cells (Fig. 2 C–F). However, some residual Apc staining was observed in all but two of the AKR Min/+ tumors. This residual staining was not found in the most dysplastic regions of the tumors. Because this residual signal was observed even in tumors classified as involving LOH, it may be nonspecific.
Taken together, these results indicate that normal Apc function(s) must be attenuated for both spontaneous and ENU-induced intestinal tumor formation in AKR Min/+ mice. However, the mechanism(s) of Apc inactivation in tumors of AKR Min/+ mice frequently differs from that observed for B6 Min/+ mice.
DISCUSSION
The Strong Dependence of a Mouse Cancer Model on Genetic Background.
Manipulation of the genetic background alone can reduce intestinal tumor multiplicity in mice carrying the ApcMin mutation by about two orders of magnitude (Table 1). Indeed, 75% of Min/+ mice on the AKR genetic background were tumor free at 6 months of age. This finding contrasts sharply with the B6 background, where 100% of Min/+ animals develop numerous intestinal tumors by 2–3 months of age (7). Furthermore, from the entire set of 170 AKR Min/+ mice examined, only one colonic tumor was identified—in an ENU-treated animal. These results highlight the importance of evaluating genetic background when studying the effects of any germ-line mutation in the mouse. To appreciate fully the phenotype(s) associated with a gene, it seems imperative to study each germ-line mutation for that gene on several different genetic backgrounds.
Even on the highly resistant AKR genetic background, intestinal tumors in Min/+ mice appear to initiate preferentially early in life. A 26-fold increase in tumor number was observed for mice treated with ENU at 9–16 days, whereas mice treated at 27–42 days showed only a 3.8-fold increase in tumor number compared with the untreated set. The enhanced susceptibility of younger B6 and AKR Min/+ mice to tumor formation may be influenced by developmental changes that occur in the intestine during the first few weeks of life. Intestinal crypt purification and expansion are two changes that may be important contributors to this susceptibility. The effect of the AKR genetic background on tumor multiplicity in Min/+ mice does not seem to involve alteration of the age dependence of tumorigenesis.
The mechanism of somatic Apc inactivation also was influenced by the genetic background. In contrast to the 100% LOH observed for untreated B6 mice, 36% (8/22) of the tumors from untreated AKR Min/+ mice failed to show LOH by our assay. Also, in our previous analysis of ENU-treated B6 Min/+ mice, 25/91 tumors lacking LOH had shown truncations, all between codons 700 and 1215 (12). No such truncation mutations in Apc were observed in 12 tumors from AKR Min/+ mice. Consider the null hypothesis that the AKR pattern does not differ from the B6 pattern. On this hypothesis, the probability that no truncations would be found in this sample is 0.036 (Fisher exact test). Thus ENU treatment on the AKR background either alters the Apc mutational spectrum relative to B6 or stimulates tumorigenesis by influencing loci other than Apc. Mutational activation of β-catenin would be one possibility for this latter scenario, but note that, in contrast to the reported case (25), the Apc locus always is silenced in the cases we have studied.
There are numerous intriguing possibilities raised by these results. First, it is reasonable to expect that tumor multiplicity would be influenced by factors that affect the rate or frequency of somatic Apc segregation. It is worth noting that even a small change in mitotic fidelity could have a large effect on tumor number in a tissue as mitotically active as the intestinal epithelium. The possibility of differences between the B6 and AKR strains in modifier loci that influence mitotic segregation requires further investigation.
At least in respect to the Apc locus and mouse chromosome 18, we classify B6 as mitotically unstable and AKR as relatively more stable. The instability generated by B6 is dominant to the stability of AKR, because adenomas in (AKR×B6)F1 animals show a 100% incidence of LOH (10). These germ-line differences in frequency of LOH are interesting to compare with recent observations on cell lines derived from human colon cancer. Lengauer and his colleagues (26) have described two classes of cell lines from human colonic tumors, one class karyotypically unstable and the other karyotypically stable. They have suggested that the normal stem cell precursor for colonic tumors is karyotypically stable (27). Cell fusion analysis indicates that karyotypic instability is at least partially dominant to stability (26). One cause adduced for dominant karyotypic instability is mutation of the mitotic checkpoint gene, Bub1 (28). Several lines of further investigation are required to ascertain whether the difference in LOH frequency between the AKR and B6 backgrounds reflects generalized karyotypic instability, a difference in stem cell source for tumors (29), or germ-line differences at mitotic checkpoint loci such as Bub1.
Mom1 is the first mapped polymorphic modifier locus that affects intestinal tumor number in Min/+ mice. However, Mom1 cannot fully account for the reduced tumor multiplicity observed in AKR Min/+ mice (Table 1). Homozygosity for the AKR-resistance allele of Mom1 reduces tumor multiplicity by a factor of four relative to B6 Min/+ mice (16). Thus, tumor number is reduced by an additional factor of 60 by making the Min mutation congenic on the AKR background. Clearly, the AKR strain carries alleles at loci other than Mom1 that strongly influence Min-induced tumorigenesis.
Apc as a Canonical Tumor Suppressor Locus.
The canonical genetic pathway for inactivating a “tumor suppressor” function involves two hits, one affecting each allele of an autosomal locus (30). When this pathway is inefficient, other mechanisms can come into play. We identified 32 tumors from AKR Min/+ mice in which Apc did not appear to be somatically inactivated either by allele loss or by truncation mutation (Fig. 1 and above). The Apc-negative status of this set of tumors cannot be readily explained by missense mutations in the wild-type allele. Missense substitutions should not simultaneously affect the multiple epitopes detected by the 3122 and APC2 antibodies. But note that the stimulation of adenoma formation on the AKR background by ENU is observed only in ApcMin/+ heterozygotes. Thus, a two-hit hypothesis remains tenable: a germ-line lesion in Apc combined with a somatic mutation in an interacting locus. The initial two-hit hypothesis of Knudson (30) did not restrict the hits to the two alleles of one locus. The complexities of the genetics of Wilms’ tumor have been interpreted by such two-locus models (31).
Although the primary mechanism of Apc inactivation may differ between B6 and AKR Min/+ mice, all intestinal tumors examined from both untreated and ENU-treated AKR Min/+ mice showed a dramatic deficiency in Apc expression relative to normal cells (Fig. 2). We can only speculate at this stage as to how the Apc+ allele becomes silent in the apparent absence of LOH or somatic mutation (32). This result demonstrates that lack of Apc tumor suppressor function is critical for tumor formation in both strains. These findings support the hypothesis of APC/Apc as a unique “gatekeeper” gene that functions as a critical negative regulator mediating intestinal epithelial homeostasis (33). Whether all neoplastic pathways are negatively regulated by a unique “gatekeeper” is an interesting issue for the future.
Acknowledgments
We thank Paul Polakis for providing the APC2 antibody and for valuable critique of the manuscript. Anita Merritt provided assistance with the immunohistochemistry and helpful comments on the manuscript. We also thank Henry Pitot for histological consultation. Natalie Borenstein, Melinda Grove, Cheri Pasch, Dana Olson, and Jane Weeks provided expert technical assistance with these experiments. Andrea Bilger, Robert Cormier, Norman Drinkwater, Karen Gould, Kevin Haigis, Richard Halberg, Ilse Riegel, and Alexandra Shedlovsky have provided critical help in finalizing the manuscript, which Mary Jo Markham and Kristen Adler have skillfully processed. This work was supported by Core Grant CA-07175, Training Grant CA-09135, and Research Grants R01CA-50585, R01CA-63677, and R29CA64364-02 from the National Cancer Institute.
ABBREVIATIONS
- APC/Apc
adenomatous polyposis coli gene of the human/mouse
- B6
C57BL/6J
- ENU
N-ethyl-N-nitrosourea
- HetApc
Apc heterozygosity index
- LOH
loss of heterozygosity
- Min
multiple intestinal neoplasia
- Mom1
modifier of Min 1
- Mom1A
AKR allele of Mom1
References
- 1.Shoemaker A R, Gould K A, Luongo C, Moser A R, Dove W F. Biochim Biophys Acta. 1997;1332:F25–F48. doi: 10.1016/s0304-419x(96)00041-8. [DOI] [PubMed] [Google Scholar]
- 2.Haggitt R C, Reid B J. Am J Surg Pathol. 1986;10:871–887. doi: 10.1097/00000478-198612000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Leppert M, Burt R, Hughes J P, Samowitz W, Nakamura Y, Woodward S, Gardner E, Lalouel J-M, White R. N Engl J Med. 1990;322:904–908. doi: 10.1056/NEJM199003293221306. [DOI] [PubMed] [Google Scholar]
- 4.Spirio L, Olschwang S, Groden J, Robertson M, Samowitz W, Joslyn G, Gelbert L, Thliveris A, Carlson M, Otterud B, et al. Cell. 1993;75:1–20. doi: 10.1016/0092-8674(93)90538-2. [DOI] [PubMed] [Google Scholar]
- 5.Paul P, Letteboer T, Gelbert L, Groden J, White R, Coppes M J. Hum Mol Genet. 1993;2:925–931. doi: 10.1093/hmg/2.7.925. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez E, La Vecchia C, D’Avanzo B, Negri E, Franceschi S. Brit J Cancer. 1997;75:1381–1384. doi: 10.1038/bjc.1997.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moser A R, Pitot H C, Dove W F. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 8.Su L K, Kinzler K W, Vogelstein B, Preisinger A C, Moser A R, Luongo C, Gould K A, Dove W F. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 9.Shoemaker A R, Moser A R, Dove W F. Cancer Res. 1995;55:4479–4485. [PubMed] [Google Scholar]
- 10.Luongo C, Moser A R, Gledhill S, Dove W F. Cancer Res. 1994;54:5947–5952. [PubMed] [Google Scholar]
- 11.Luongo C, Dove W F. Genes Chromosomes Cancer. 1996;17:194–198. doi: 10.1002/1098-2264(199611)17:3<194::aid-gcc2870170302>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Shoemaker A R, Luongo C, Moser A R, Marton L J, Dove W F. Cancer Res. 1997;57:1999–2006. [PubMed] [Google Scholar]
- 13.Moser A R, Dove W F, Roth K A, Gordon J I. J Cell Biol. 1992;116:1517–1526. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich W F, Lander E S, Smith J S, Moser A R, Gould K A, Luongo C, Borenstein N, Dove W F. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 15.Gould K A, Dietrich W F, Borenstein N, Lander E S, Dove W F. Genetics. 1996;144:1769–1776. doi: 10.1093/genetics/144.4.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould K A, Luongo C, Moser A R, McNeley M K, Borenstein N, Shedlovsky A, Dove W F, Hong K, Dietrich W F, Lander E S. Genetics. 1996;144:1777–1785. doi: 10.1093/genetics/144.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cormier R T, Hong K H, Halberg R B, Hawkins T L, Richardson P, Mulherkar R, Dove W F, Lander E S. Nat Genet. 1997;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- 18.Wong M H, Hermiston M L, Syder A J, Gordon J I. Proc Natl Acad Sci USA. 1996;93:9588–9593. doi: 10.1073/pnas.93.18.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merritt A J, Potten C S, Watson A J M, Loh D Y, Nakayama K, Nakayama K, Hickman J A. J Cell Sci. 1997;108:2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- 20.Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain S, Masiarz F R, Munemitsu S, Polakis P. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 21.Midgley C A, White S, Howitt R, Save V, Dunlop M G, Hall P A, Lane D P, Wyllie A H, Bubb V J. J Pathol. 1997;181:426–433. doi: 10.1002/(SICI)1096-9896(199704)181:4<426::AID-PATH768>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Smith K J, Johnson K A, Bryan T M, Hill D E, Markowitz S, Willson J K V, Paraskeva C, Petersen G M, Hamilton S R, Vogelstein B, Kinzler K W. Proc Natl Acad Sci USA. 1993;90:2846–2850. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathke I S, Adams C L, Polakis P, Sellin J, Nelson W J. J Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyashiro I, Senda T, Matsumine A, Baeg G-H, Kuroda T, Shimano T, Miura S, Noda T, Kobayashi S, Monden M, et al. Oncogene. 1995;11:89–96. [PubMed] [Google Scholar]
- 25.Morin P, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 26.Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 27.Potten C S, Loeffler M. Development (Cambridge, UK) 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 28.Cahill D P, Lengauer C, Yu J, Riggins G J, Willson J K V, Kinzler K E, Vogelstein B. Nature (London) 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 29.Brown K, Strathdee D, Bryson S, Lambie W, Balmain A. Curr Biol. 1998;8:516–524. doi: 10.1016/s0960-9822(98)70203-9. [DOI] [PubMed] [Google Scholar]
- 30.Knudson A G., Jr Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haber D A, Buckler A J, Glaser T, Call K M, Pelletier J, Sohn R L, Douglass E C, Housman D E. Cell. 1990;61:1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- 32.Dove W F, Cormier R T, Gould K A, Halberg R B, Merritt A J, Newton M A, Shoemaker A R. Philos Trans R Soc London B. 1998;353:915–923. doi: 10.1098/rstb.1998.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinzler K W, Vogelstein B. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]