Abstract
Dietary restriction (DR) extends healthy lifespan in diverse organisms, and reduces fecundity 1,2. DR is widely assumed to induce adaptive reallocation of nutrients from reproduction to somatic maintenance, aiding survival of food shortages in nature 3-6. Long life under DR and high fecundity under full feeding would thus be mutually exclusive, through competition for the same, limiting nutrients. We tested this idea, by identifying the nutrients producing the responses of lifespan and fecundity to DR in Drosophila. Adding essential amino acids to a DR diet increased fecundity and decreased lifespan, similar to full feeding, with other nutrients having little or no effect. However, methionine alone increased fecundity as much as full feeding, but without reducing lifespan. Reallocation of nutrients therefore does not explain the DR responses. Lifespan was reduced by amino acids, particularly essential amino acids. Hence an imbalance in dietary amino acids away from the ratio optimal for reproduction shortens lifespan during full feeding and limits fecundity during DR. Reduced activity of the insulin/Igf signaling pathway extends lifespan in diverse organisms 7, and it protected against the shortening of lifespan with full feeding. In other organisms, including mammals, it may be possible to obtain the benefits for lifespan of DR without reduced fecundity, through a suitable balance of nutrients in the diet.
Dietary restriction (DR), a reduction in food intake without starvation, extends lifespan in many organisms: yeast 8, invertebrates 9 and mammals 1, including primates 10. DR in rodents and primates also produces a broad-spectrum improvement in health during ageing 1,10. Reduced calorie intake has been suggested to underlie extended lifespan in rodents. However, specific amino acids may be as or more important 11-13. DR lowers fecundity 2, for instance in the nematode worm Caenorhabditis elegans 14, the fruit fly Drosophila melanogaster 15 and rodents 16. The prevailing view is that DR induces an evolved response to food shortages 3,17. If somatic maintenance and reproduction compete for limiting nutrients then, with abundant food, reproduction is prioritized, and somatic maintenance is allocated only the nutrients necessary to ensure survival during the reproductive period, which, due to extrinsic hazards in the wild, falls far short of intrinsic, potential lifespan 4. With food shortage, reproduction becomes dangerous for the parent and offspring survive poorly, and nutrients are hence reallocated to somatic maintenance, thus increasing survival to reproduce successfully when the food supply returns 3,5. High survival, associated with DR, and high reproductive rate, associated with full feeding, would thus be mutually exclusive.
We have tested this prediction in Drosophila. DR is implemented by dilution of the diet, without compensation of food intake rates 18-20, resulting in increased lifespan and reduced fecundity, measured as egg laying 20. In nature, Drosophila eat yeasts 21 and, although many manipulations of dietary balance can alter lifespan 22,23, enhanced longevity by DR is modulated almost exclusively by dietary yeast, independent of calorie intake 18,22-24.
We investigated which nutrients in yeast produce high fecundity in fully-fed flies, and whether these same nutrients also decrease lifespan, as predicted by the reallocation hypothesis. The ratio and type of food components were optimised to maximise both lifespan with DR and fecundity with full feeding 24, and we examined the effect of adding back nutrients to the DR diet. Since availability of free nutrients will be higher than that in yeast, we first measured fecundity with addition of all nutrients in the ratio present in yeast (see Methods), at several concentrations. We then used the concentration that increased fecundity to the level with full feeding (Table S1). Adding back vitamins, lipids or carbohydrates did not affect fecundity or lifespan (Fig. 1), indicating that they do not limit fecundity during DR, and that increased intake of calories per se does not reduce lifespan. In contrast, addition of amino acids increased fecundity and decreased lifespan, as for full feeding (Fig. 1).
Figure 1. Amino acids mediate lifespan and fecundity changes in fly DR.
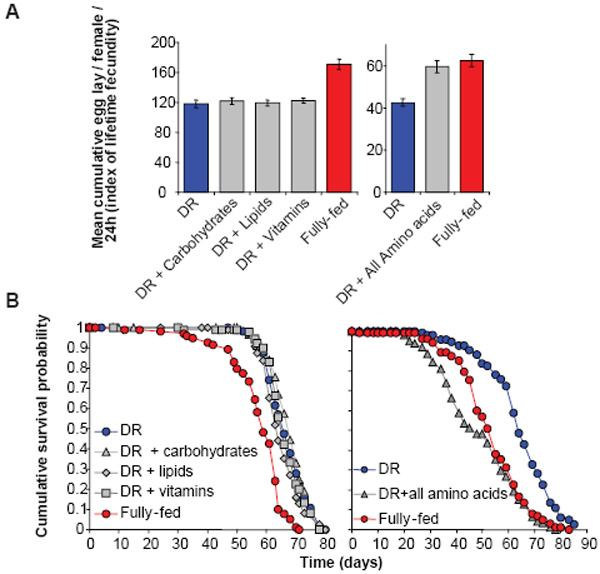
(a) Adding nutrients to DR revealed that amino acids limited fecundity and their addition rescued the level for fully-fed flies (DR+Amino Acids v Fully-fed, P = 0.5288; DR v other conditions, P>0.2). (b) Adding amino acids to DR food shortened lifespan (P<0.00001) to that of fully-fed flies (P=0.102). No other nutrient additions tested changed lifespan from the DR level (P>0.1 in all comparisons). Fecundity: mean ± s.e.m.; n=10; compared using the Wilcoxon rank sum test. Survivorship: starting n=100 per treatment; compared using the log rank test.
To test for non-nutritional toxicity of amino acids, we measured the osmolarity and pH of each diet. Compared with full feeding, amino acid additions to DR food caused small changes in osmolarity that do not correlate with lifespan (446mOsM for DR increased to 495mOsM with All AA and 1081mOsM for full feeding), and no detectable change in pH, indicating that changes in these factors do not account for the lifespan differences (Fig. S2). Furthermore, provision of excess water did not abrogate life-shortening by amino acids, but completely rescued that of 0.8% salt addition to DR food (Fig. S2), demonstrating the efficacy of water provision.
Reallocation of amino acids from reproduction to somatic maintenance could explain the responses of lifespan and fecundity to amino acid add-back. Alternatively, different amino acids could independently produce the two responses. We first investigated the 10 essential and 10 non-essential amino acids, similar in Drosophila to those in mammals 25. Adding back non-essential amino acids (N-EAAs) slightly decreased lifespan, with no effect on fecundity (Fig. 2a & b). In contrast, adding back essential amino acids (EAAs) increased fecundity as much as did all 20 amino acids or full feeding (Fig. 2a), and also substantially decreased survival, again as much as full feeding (Fig. 2b). Adding back N-EAAs increased dietary nitrogen concentration by 9% more than adding back EAAs (Table S1), suggesting that specific amino acids rather than increased dietary nitrogen were responsible. Further increasing the concentration of EAAs led to further increased fecundity and decreased survival (Fig. S3). The effects of full feeding can thus be attributed to EAAs in the diet, consistent with reallocation of EAAs from reproduction to somatic maintenance upon DR.
Figure 2. Essential amino acids cause the DR effect.
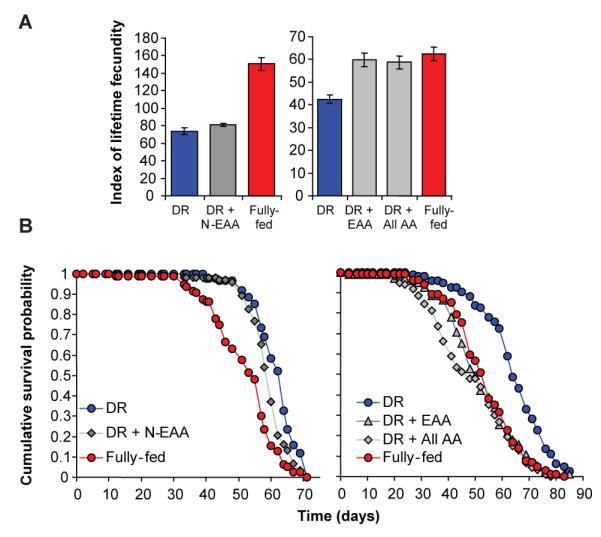
(a) Adding essential amino acids (EAA), but not non-essential amino acids (N-EAA), increased fecundity to the level with all amino acids (All AA) and fully-fed. (DR+EAA v Fully-fed, P=0.393; DR+EAA v DR+All AA, P=1). (b) Adding EAA or All AA to DR caused lifespan to decrease to the same extent as fully-fed (P>0.102). In contrast, N-EAA addition to DR shortened lifespan much less (P=0.011). Egg laying: mean ± s.e.m.; n=10; compared using the Wilcoxon rank sum test. Survivorship: starting n=100 per treatment; compared using the log rank test.
We next determined which EAAs affected fecundity and lifespan. In rodents, lifespan can be extended by restricting either methionine or tryptophan 11-13. Adding-back EAAs except methionine and tryptophan did not increase fecundity (Fig. 3a), indicating that one of these is limiting. Adding back EAAs except methionine also did not increase fecundity from the DR condition (Fig. 3a), indicating that methionine is essential, while omission of tryptophan produced the full increase (Fig. 3a). Furthermore, adding back methionine (but not tryptophan or any other EAA) alone to a DR diet increased fecundity as much as addition of all 10 EAAs and full feeding (Fig. 3b & S3). Methionine alone is thus necessary and sufficient for the increase in fecundity. Importantly, egg quality, as indicated by hatching of larvae, was normal upon methionine addition (Fig. S5). Elevated fecundity with amino acid addition could have resulted from increased food intake. However, direct feeding observations and dye accumulation assays 19 showed that feeding behaviour and rate of food intake were unaltered (Fig. S6). Adding back a range of methionine concentrations (0.07mM to 13mM) increased female fecundity to a plateau (Fig. 3c and Fig. S7a) and only addition of other, now limiting, EAAs could increase fecundity further (Fig. S8). Thus methionine probably does not act as a signal to determine fecundity, because its effects depended upon the ratio of methionine to other EAAs, suggesting instead that it acts through nutritional limitation of reproduction.
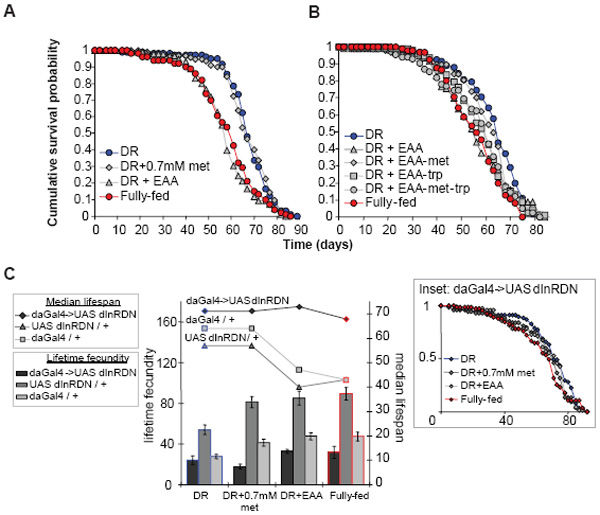
Figure 3. Methionine is necessary and sufficient to increase DR egg laying.
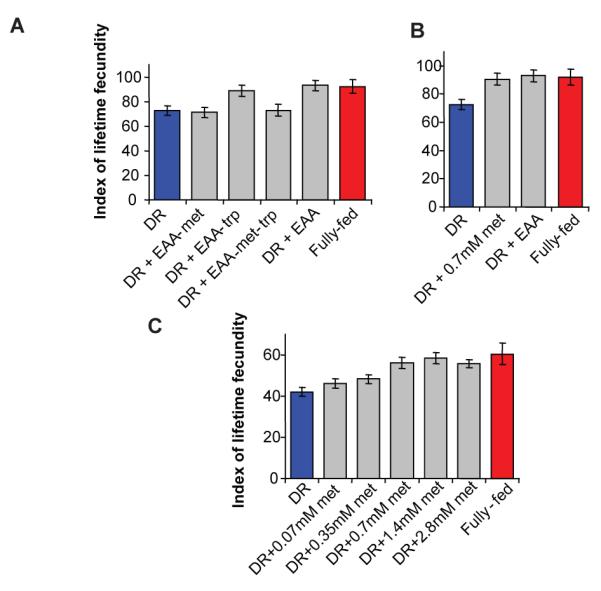
(a) Adding EAA except methionine (−met) did not increase fecundity from DR (P=0.796), however EAA without tryptophan (−trp) did to the level of DR+EAA or Fully-fed (DR v DR+EAA-trp, P=0.00893; DR+EAA-trp v DR+EAA, P=0.4359; DR+EAA-trp v Fully-fed, P=0.7394; DR+EAA-met-trp v DR+EAA-met, P=0.796). (b) Methionine alone increased fecundity to that of DR+EAA and fully-fed (DR+met v DR+EAA, P=0.5288; DR+met v fully-fed, P=0.9118). (c) Fecundity increased with methionine addition (significant ≥0.35mM, P=0.02323), reaching fully-fed at 0.7mM (P=0.393). Egg laying: mean ± s.e.m.; n=10; compared using the Wilcoxon rank sum test.
Surprisingly, adding back methionine did not decrease lifespan (Fig. 4a), even when it was added back at much higher concentrations than that limiting for fecundity (Fig. S7b & c). Hence, reduction in lifespan upon full feeding does not result from reallocation of nutrients from somatic maintenance to reproduction, because the nutrient that increased fecundity, methionine, did not reduce lifespan. Furthermore, the fact that high fecundity and high lifespan can co-occur is inconsistent with the idea that any aspect of reproduction directly inflicts damage on the soma to shorten lifespan 26. We obtained identical results using a fly diet made with another yeast commonly used for fly DR studies 24, indicating that these results are not diet-specific (Fig. S9). Nor can decreased lifespan with full feeding be attributed to unidentified toxins in the food 20,24. Instead, the responses of lifespan and fecundity to full feeding are independently mediated by different amino acids.
Figure 4. Amino acids, insulin signaling and DR.
(a) Adding methionine to DR did not shorten lifespan (P=0.683) but EAA did (DR v DR+EAA, P=0.014; DR+EAA v Fully-fed, P=0.323). (b) Adding EAA without methionine (-met) rescued DR longevity (DR v DR+EAA-met, P=0.183) but tryptophan removal (-trp) did not (DR+EAA-trp v Fully-fed, P=0.115). (c) Reduced insulin signaling (daGal4->InRDN) extended DR lifespan (v controls, P<0.00001) and reduced flies’ response to AA or yeast addition (lifespan P>0.064 and fecundity P>0.3, DR v other foods; inset: survival curves for daGal4->InRDN). Controls responded similarly to wild-types. Lines connect median lifespans. Experimental setup and statistics as previously described.
Adding-back each EAA individually did not decrease lifespan while, again, methionine alone increased fecundity (Fig. S4). Interestingly, adding-back all EAAs except methionine restored lifespan to the DR level, whereas omission of tryptophan had no effect (Fig. 4b). Notably, restriction of methionine alone also increases lifespan in rodents 12,13. Methionine thus acts in combination with one or more other EAAs to shorten lifespan upon full feeding. Full feeding thus increases fecundity and decreases lifespan through different nutrients in Drosophila, the former through methionine alone and the latter through a combination of methionine and other EAAs (Fig. S1). There is thus an imbalance in the ratio of amino acids in yeast relative to what the fly requires for the high fecundity from full feeding, and some consequence of this imbalance decreases lifespan.
Genetic interventions that reduce insulin / insulin-like growth factor signalling (IIS) also extend lifespan of worms, flies and mice 7. There has been debate on the role of IIS in lifespan-extension by DR 9. Drosophila has a single IIS receptor, dInR, which mediates both the growth and metabolic functions of IIS 27. We tested the role of IIS in the responses to DR and EAAs, by over-expressing a dominant-negative form of dInR (InRDN), which extends fly lifespan 28. InRDN-expressing flies were longer-lived than controls even with DR and, like that of controls, their lifespan was unchanged by the addition of methionine (Fig. 4c & S10). However, in sharp contrast to controls, lifespan was hardly (trial 1) or not at all (trial 2) reduced by EAA add-back or full feeding (Fig. 4c & S10). InRDN expression also reduced the responses of egg-laying to methionine and full feeding. Thus, reduced IIS can both extend lifespan beyond the maximal response to DR, showing that mechanisms additional to those of DR are involved, but it can also protect against the lifespan-shortening effects of amino acid imbalance upon full feeding and EAA addition, showing that IIS is required for the lifespan-shortening.
Amino acids that are not used in reproduction in the flies could shorten lifespan through metabolic costs of their removal, through consequent damage, for instance to the excretory malpighian tubules, or through other life-shortening physiological responses. Nutrient imbalance in the diet could also account for the responses of lifespan and fecundity to DR in other organisms, including mammals, if specific nutrients in their diet are also limiting for full physiological function. Indeed, protein quality is implicated in human health, because the ratio of amino acids in the diet can affect traits important for aging, such as glucose homeostasis and bone health 29. The mechanisms that influence lifespan are conserved over the large evolutionary distances between invertebrates and mammals 7, and our results hence imply that in mammals too the benefits of DR for health and lifespan may be obtained without impaired fecundity and without DR itself, by a suitable balance of nutrients in the diet.
Methods summary
Nutritional analysis of the yeast was provided by MP Biomedicals, Solon OH, USA. Stock solutions for the different nutrient add-back treatments were prepared as outlined in Table S1 and were added to the DR medium containing 100 g.l−1 yeast, 50 g.l−1 sucrose, 15 g.l−1 agar and preservatives 24, after the food had cooled to 65°C.
Fly stocks and maintenance
All experiments were performed using the wild-type, outbred strain Dahomey, which was originally collected in 1970 from Dahomey (now the Republic of Benin) and has since been maintained in stock cages with overlapping generations at 25°C on a 12:12 hour light:dark cycle. Insulin-signalling mutant flies expressed a dominant negative form of the insulin receptor with a single amino acid substitution in the kinase domain (UAS-dInRA1409K). Adult flies expressing this transgene are approximately 32% lighter than controls, similar to the effect of reducing insulin ligand production30. To drive ubiquitous expression of the transgene, a daughterless-Gal4 driver was used. Control lines contained either the driver or UAS transgene alone. All flies were backcrossed into the wild-type wDah background as described 28.
Lifespan and fecundity protocols
Flies were reared at standard density and were allowed to mate for 48 hours24. Under CO2 anaesthesia, females were collected and randomly allocated to glass vials containing the different add-back treatments, at a density of 10 flies per vial and 10 vials per treatment (n=100). Flies were transferred to fresh medium 3 times per week and deaths recorded. Egg counts were performed over an 18-24 hour period at several intervals throughout the experiment (see supplementary methods) until daily egg laying reached a low plateau late in life.
Methods
Preparation of add-back solutions
To establish the ratios of nutrients present in yeast, we combined data from the literature 31 and chemical analyses32. Free nutrients are likely to be at higher effective concentrations compared with yeast so we measured the effects on fecundity of an all nutrient addition, in the ratios in which they are found in yeast (Table S1), at several concentrations. We used the concentration that produced the same increase in fecundity as full-feeding (Fig. 1a). To check for toxicity, these levels were doubled during N-EAA add back (Fig 2a & b) and for single EAA additions (Fig. S4). The individual ingredients were weighed out and dissolved in appropriate solvent to make stock solutions, as outlined in Table S1. Phosphatidylcholine was chosen as the lipid source because it is a major phospholipid of eukaryotic cells and contains choline, which is essential for adult Drosophila 33. DR food contains sufficient levels of nutrients from yeast for healthy lifespan of Drosophila 32. All add-back reagents were from Sigma, Dorset, UK. Amino acid purity was at least 98%; order numbers are: arg: A5131; ala: A7627; asn: A0884; asp: A6683; cys: C1276; glu: G1251; gln: G3126; gly: G7126; his: H8000; ile: I2752; leu: L8912; lys: L5626; met: M9625; phe: P2126; pro: P0380; ser: S4500; thr: T8625; trp: T0254; tyr: T3754; val: V0500.
Media preparation
DR diet (1.0SY) contained per litre: 100g autolysed yeast powder (MP Biomedicals, Solon, OH, USA), 50g sucrose (Tate & Lyle Sugars, London, UK), 15g agar (Sigma, Dorset, UK), 30 ml Nipagin (100g.l−1 in 95% ethanol; Clariant UK Ltd, Pontypridd, UK) and 3ml propionic acid (Sigma, Dorset, UK). This was used as base for all treatments. The fully-fed condition (2.0SY) was the same as DR, except that the autolysed yeast content was 200g.l−1. This diet is optimized calorically and nutritionally for DR experiments with Drosophila 32,34. Separate 1 l cooks were performed for each treatment. In all cases the food was prepared as described in 32 and when the temperature fell below 65°C, the add-back solution was added with any remaining water to adjust the volume, as well as preservatives. The food was dispensed into glass vials in 4ml aliquots. Fresh food was prepared approximately every three weeks throughout the course of the experiments.
osmolarity determination
A slurry of sugar and yeast at the concentration in the DR and fully fed conditions was made in 50ml water. These were heated to 100°C and cooled to mimic cooking. A small sampled was taken for osmolarity determination using an Advanced model 3300 micro-osmometer (Advanced Instruments West Sussex, UK).
pH determination
Food vials from the same cook as those for experiments. 1ml water was added to the surface of the food and allowed to equilibrate overnight. The water was then removed and the pH measured.
Lifespan experiments
Larvae were reared at a standard density in 200ml bottles containing 70ml of 1.0SY laboratory medium 35. Flies emerging over a 24h period were transferred into fresh bottles where they were kept to mate for 48h. Females were then separated from males under light CO2 anaesthesia and systematically distributed between food treatments at a density of 10 flies per vial with at least 100 flies per experimental condition. Flies were transferred to fresh vials at least three times per week and deaths scored on those days.
Fecundity assays (index of lifetime fecundity)
18-24h after transferring flies to fresh vials the eggs in each vial were counted by hand under a dissecting microscope. For each vial, the data were expressed as eggs per female per 24h. At the end of the experiment, the values for each vial were summed to give an index of lifetime fecundity. Typically, the counts were performed on day 6, 8, 15, 22, 29 and 35 after the start of treatment.
Feeding assays
Dye-calibrated feeding observation feeding assays were performed as described in 36. This involves the following two procedures.
Blue-dye feeding assay combined with observations
To determine if there was a correspondence between observed feeding activity and actual food consumption, the behavioural assay was calibrated. Once-mated, female flies were reared and maintained as for lifespans and housed at 5 flies per vial. On day 7 of adult life, flies were transferred to food containing 2.5% blue dye (w/w; FD&C Blue No.1). During the initial 30 minutes of access to the blue food, feeding observations were recorded for each of the vials. After 30 minutes, flies were frozen and the amount of blue dye consumed measured spectrophotometrically. Data were quantified by reference to a standard curve generated from a known amount of blue-dyed food. The relationship between observed feeding events and blue-dye consumption was then analysed. There was a significant linear relationship between the proportion of flies feeding and the amount of dye consumed.
Feeding observations
To measure fly feeding under conditions resembling those for lifespans, feeding behaviour observations were made on undisturbed flies. On the days before measurement, flies were transferred to fresh vials, the labelling coded by another lab member and the vials arranged on viewing racks. One hour after lights on (11am) feeding observations were made for 90 minutes as described in 36. Data is presented as the proportion of flies feeding (Fig. S6). This is the sum of scored feeding events divided by the total number of feeding opportunities, where feeding opportunities = number of flies in the vial × number of vials in the group × number of observations per vial.
Statistical analyses
All statistical tests were performed using JMP (version 5.1; SAS Institute, Cary, NC) and R (v2.2.1 37). Survivorships were compared using the log-rank test and fecundity using the Wilcoxon rank sum test. To assess the relationship between proboscis extension behaviour and accumulation of blue dye, a linear mixed effects model was used 36. This modelled dye accumulation as a function of proportion of time observed feeding. To compare the effect of dietary composition on feeding frequency, we used generalised linear models (with binomial error structure and logit link function, the deviances were scaled to correct for over-dispersion, and F-tests used for analysis of signficiance). Simplification of the factor levels was performed by evaluating whether combining factor levels into a single level led to a significant increase in deviance of the model, using F-tests 38.
Supplementary Material
Acknowledgements
We acknowledge funding from a Wellcome Trust Strategic Award to LP (MDWP and LP) and Research into Ageing (RCG and LP). We would also like to thank Matthew Hoddinott for excellent technical support as well as Scott Pletcher and Eric Blanc for assistance with statistical analyses.
Footnotes
Author information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
The authors declare no competing financial interests.
Supplementary information is linked to the online version of the paper at www.nature.com/nature. A figure summarizing the main result of this paper is available in Supplementary Information.
Reference List
- 1.Weindruch R, Walford RL. the retardation of aging and disease by dietary restriction. Thomas; Springfield, Ill.: 1988. [Google Scholar]
- 2.Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120(4):461. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioessays. 1989;10(4):125. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- 4.Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. American Naturalist. 1966;100:687. [Google Scholar]
- 5.Kirkwood TB. Evolution of ageing. Nature. 1977;270(5635):301. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 6.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727-54:727. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 7.Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat. Rev. Mol. Cell Biol. 2007;8(9):681. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 8.Kaeberlein M, Burtner CR, Kennedy BK. Recent developments in yeast aging. PLoS Genet. 2007;3(5):e84. doi: 10.1371/journal.pgen.0030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piper MD, Bartke A. Diet and aging. Cell Metab. 2008;8(2):99. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Marte ML, Enesco HE. Influence of low tryptophan diet on survival and organ growth in mice. Mech. Ageing Dev. 1986;36(2):161. doi: 10.1016/0047-6374(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman JA, et al. Nutritional control of aging. Exp. Gerontol. 2003;38(1-2):47. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 13.Miller RA, et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4(3):119. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 1977;6(6):413. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 15.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc. R. Soc. Lond B Biol. Sci. 1996;263(1371):755. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 16.Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7(5):622. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison DE, Archer JR. Natural selection for extended longevity from food restriction. Growth Dev. Aging. 1989;53:3. [PubMed] [Google Scholar]
- 18.Mair W, Piper MD, Partridge L. Calories do not explain extension of lifespan by dietary restriction in Drosophila. 2005;7:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong R, et al. Quantification of food intake in Drosophila. PLoS One. 2009;4(6):e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piper MD, Partridge L. Dietary restriction in Drosophila: Delayed aging or experimental artefact? PLoS. Genet. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spieth HT. Courtship behaviour in Drosophila. Annu. Rev. Entomol. 1974;19:385. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- 22.Skorupa DA, et al. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7(4):478. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KP, et al. Ageing and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2498. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bass TM, et al. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2007;6:1071. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sang JH, King RC. Nutritional requirements of axenically cultured Drosophila melanogaster adults. J. Exp. Biol. 1961;38:793. [Google Scholar]
- 26.O’Brien DM, et al. Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Curr. Biol. 2008;18(4):R155–R156. doi: 10.1016/j.cub.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez R, et al. The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J. 1995;14(14):3373. doi: 10.1002/j.1460-2075.1995.tb07343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeya T, et al. The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proc. R. Soc. Lond B Biol. Sci. 2009 doi: 10.1098/rspb.2009.0778. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millward DJ, et al. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008;87(5):1576S. doi: 10.1093/ajcn/87.5.1576S. [DOI] [PubMed] [Google Scholar]
- 30.Ikeya T, et al. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 2002;12(15):1293. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 31.Lange HC, Heijnen JJ. Statistical reconciliation of the elemental and molecular biomass composition of Saccharomyces cerevisiae. Biotechnol. Bioeng. 2001;75(3):334. doi: 10.1002/bit.10054. [DOI] [PubMed] [Google Scholar]
- 32.Bass TM, et al. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2007;6:1071. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sang JH. The Nutritional Requirements of Drosophila. In: Ashburner M, Wright TRF, editors. The genetics and biology of Drosophila. Academic Press; London: 1978. pp. 159–192. [Google Scholar]
- 34.Grandison RC, et al. Effect of a Standardised Dietary Restriction Protocol on Multiple Laboratory Strains of Drosophila melanogaster. 2009;4(1):e4067. doi: 10.1371/journal.pone.0004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clancy DJ, Kennington WJ. A simple method to achieve consistent larval density in bottle cultures. Dros. Inf. Serv. 2001;84:168. [Google Scholar]
- 36.Wong R, et al. Quantification of food intake in Drosophila. PLoS One. 2009;4(6):e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2005. [Google Scholar]
- 38.Crawley MJ. statistics: an introduction using r. John Wiley & Sons, Ltd.; Chichester, UK: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.