Abstract
The prefrontal cortex (PFC) is associated with higher cognitive functions including attention and working memory and has been implicated in the regulation of impulsivity as well as the pathology of complex mental illnesses. N-methyl D-aspartate (NMDA) antagonist treatment with dizocilpine induces cell death which is greatest in the frontal cortex on postnatal day seven (P7), however the long-term structural and behavioral effects of this treatment are unknown. This study investigates both the acute neurotoxicity of P7 dizocilpine and the persistent effects of this treatment on pyramidal cells and parvalbumin interneurons in the adult PFC, a brain region involved in the regulation of impulsivity.
Dizocilpine treatment on P7 increased cleaved caspase-3 immunoreactivity (IR) in the PFC on P8. In adult mice (P82), P7 dizocilpine treatment resulted in 50% fewer parvalbumin-positive interneurons (p<0.01) and 42% fewer layer V pyramidal neurons (p<0.01) in the PFC. Double immunohistochemistry revealed cleaved caspase-3 IR in both GAD67 IR interneurons and GAD67 (-) neurons. Following dizocilpine treatment at P7, adults showed reduced time in the center of the open field suggesting increased anxiety-like behavior. These findings indicate that early brain insults affecting glutamatergic neurotransmission lead to persistent brain pathology that could contribute to impulsivity and cognitive dysfunction.
Keywords: Dizocilpine, impulsivity, schizophrenia, prefrontal cortex, parvalbumin interneurons, pyramidal neurons, anxiety
1. Introduction
The prefrontal cortex (PFC) is a critical brain region involved in executive functioning, decision making and behavioral planning. The PFC controls attention and integrates information from the limbic and other regions to appropriately manage the subject's impulsive and compulsive responses. Dysfunctional control of impulsivity and deficits in executive function, such as in schizophrenia and addiction, could be secondary to damage to the PFC. The PFC in humans and rodents is primarily defined by reciprocal projections with the medial dorsal nucleus of the thalamus (Kuroda et al., 1998; Rotaru et al., 2005; Uylings et al., 2003). PFC also receives input from substantia nigra, amygdala, olfactory cortex, ventral pallidum, and other regions (Fuster, 1997). Both human and rodent medial PFC includes anterior cingulate and infralimbic regions. Similar to humans, medial regions of the rodent prefrontal cortex are involved in regulating cognition and impulsivity through modulating the attentional aspects of decision making (Birrell and Brown, 2000; Muir et al., 1996). The PFC is a multilayer cortical structure that contains varied glutamatergic and γ-amino butyric acid (GABA)ergic neurons in varied morphology and layers. PFC cortical layers, glutamatergic excitatory pyramidal cells and inhibitory GABAergic interneurons develop over an extended prenatal and postnatal period that likely correspond with development of executive functions of the PFC.
Developmental damage to the PFC may result in altered cellular structure and connectivity that causes dysfunction in adults. In rodents the early postnatal period is known to be particularly sensitive to insults. For example, a comparison of dizocilpine (a non-competitive NMDA antagonist) and ethanol across post-natal days 3-21 (P3-P21) found marked toxicity that declined on P21 with post-natal day 7 being the peak time point for neurotoxicity in PFC (Ikonomidou et al., 2000). A later study using 2 doses of dizocilpine on P7 and investigating P60 brain found significant hippocampal neuronal loss and damage to thalamic regions, but PFC was not studied (Harris et al., 2003). A more recent study treating rats with PCP, an NMDA antagonist, on P7 and investigating adult brain (P56) found a selective loss of a subtype of GABAergic interneurons in superficial cortical layers II-IV of primary somatosensory, motor, and retrospenial cortex. (Wang et al., 2008). There were no changes found in striatum or hippocampus, but the PFC anterior cingulate and infralimbic regions were not investigated. PFC dysfunction is suspected in impulsivity and addiction (For review see Crews and Boettiger 2009, current issue) and has been implicated in schizophrenia (Powell and Miyakawa, 2006). Stefani and Moghaddam (Stefani and Moghaddam, 2005), in an effort to model schizophrenia, treated rats with PCP, for 4 days (P7-P10) and found persistent deficits in adult cognitive set shifting ability, presumably a PFC function, but they did not investigate anatomy. Interestingly, adult rats treated with the NMDA antagonist CPP directly into the PFC display increased impulsive behavior as measured by anticipatory responses in the 5-Choice Serial Reaction Time task (5-CSRT) (Baviera et al., 2008; Mirjana et al., 2004). NMDA antagonists early in life cause dysfunction but the PFC has not been investigated histologically in adulthood following this early-life insult.
We hypothesized that early post-natal NMDA antagonists will induce cell death in PFC resulting in a persistent change in PFC structure in adulthood (P82). The cortex contains VI (6) layers with important neuronal densities of GABAergic interneurons and glutamatergic pyramidal cells. PFC layer V pyramidal neurons project to several brain regions, including reciprocal connections with the medial dorsal thalamic nucleus (Kuroda et al., 1998; Molnar and Cheung, 2006). PFC has at least three types of GABAergic interneurons characterized by expression of different calcium binding proteins, e.g. parvalbumin (PV), calretinin (CR), or calbindin (CB) (Baimbridge et al., 1992; Gabbott et al., 1997) which can be distinguished immunohistochemically in adults. Many early post-natal GABAergic interneurons do not show the mature calcium binding protein phenotype, since PV and CB expression increase substantially from P7 to P21 (Lema Tome et al., 2006). Using glutamic acid decarboxylase-67 (GAD67), a marker of GABAergic neurons (Tamamaki et al., 2003), and cleaved caspase 3 immunohistochemistry, a marker of cell death (Krajewska et al., 1997), we were able to assess which neuronal phenotypes were insulted. We found both GABAergic and non-GABAergic neurons were insulted. Previous studies have found that PCP treatment of P7 rats selectively reduces adult somatosensory and motor cortical PV interneurons (Wang et al., 2008), similar to the loss of PV interneurons in human schizophrenic PFC (Beasley and Reynolds, 1997). Since, neither CB nor CR GABAergic interneurons were found to be decreased in these brain regions in young adulthood (P56) following P7 PCP treatment (Wang et al., 2008), cortical PV interneurons, rather than other GABAergic interneuron subtypes, are likely more vulnerable to this type of insult. We measured the density of PV interneurons and layer V glutamatergic pyramidal cells in the medial region of the PFC in adult animals. Pyramidal neurons were quantified in adults using a mouse that expresses a YFP transgene in cortical layer V pyramidal neurons (Feng et al., 2000). Interneurons in adults were quantified using PV immunohistochemistry. We report here for the first time that P7 treatment of mice with dizocilpine results in a persistent loss of adult (P82) PFC pyramidal neurons and PV GABAergic interneurons. These neuronal deficits were associated with decreased center time in open field behavior in adults, suggesting increased anxiety, with no changes in pre-pulse inhibition. Future studies will investigate if the persistent loss of PFC neurons disrupts executive functions and alters impulsivity.
2. Methods
2.1. Subjects
Transgenic mice expressing Thy1/yellow fluorescent protein (YFP) line H mice on a C57BL/6 background were bred in the University of North Carolina at Chapel Hill (UNC-CH) animal facility (Feng et al., 2000). Layer V pyramidal neurons express the YFP transgene in cerebral cortex of these mice. Transgene expression was confirmed by PCR. Genomic DNA was extracted from tail biopsies of mice no later than postnatal day five and analyzed by PCR using PCR primers (5′ to 3′) specific for Thy1F1 (TCTGAGTGG CAAAGGACC TTAGG) and EYFPR1 (CCGTCGCCGATGGGGGTGTT). Heterozygous males were mated with homozygous negative females. For long-term studies, only pups that were homozygous for the transgene were used. Approximately 40% of pups bred from heterozygous parents were homozygous for YFP. Other than the YFP expression, animals appeared normal. Animals were maintained in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facilities and experiments were approved by the UNC-CH Institutional Animal Care and Use Committee in accordance with the Congressional Animal Welfare Act.
2.2. Experimental design and P7 dizocilpine treatment
Mice were treated on P7 with either saline (N=10) or dizocilpine (N=10) (1mg/kg, i.p.) every eight hours (t = 0, 8, 16 hours) as described previously (Ikonomidou et al., 1999). There were no significant differences in weight between treatment groups during the treatment period or prior to observation in adulthood (see Supplemental Figure 1). Half of the animals (group 1) were sacrificed 8 hours after the last injection to assess the acute effects of dizocilpine on cleaved caspase-3 immunoreactivity (IR). Mice from group 1 were sacrificed by decapitation on P8, and the brain was removed. The whole brain was submerged in 4% paraformaldehyde (PFA) for 24 hours at 4°C. Coronal sections were prepared using a vibratome at a thickness of 40 μm. The second group of animals (group 2) matured under normal housing conditions and underwent behavioral assessment on P80, followed by sacrifice at P82 for immunohistochemical analysis. On P82, mice in group 2 were first mildly anesthetized using vaporized isoflurane, followed by intra-peritoneal pentobarbital injection. Animals were then perfused transcardially with 0.1M phosphate buffered saline (PBS) followed by 4% PFA. Brains were incubated in 4% PFA for 24 hours at 4°C. Coronal sections were prepared on a vibratome at a thickness of 100 μm in order to optimize visualization of YFP.
2.3. Cleaved caspase-3 immunohistochemistry on P8
Caspase-3 cleavage was assessed via cleaved (19 kD) caspase-3 immunoreactivity (IR) using established methods (Jarskog et al., 2007; Lema Tome et al., 2006). Briefly, every fourth section from animals in group 1 was mounted on Superfrost Plus® slides, washed in PBS, and incubated in 0.3% hydrogen peroxide for 30 minutes. This resulted in 3-4 sections per animal that contained the PFC. Following subsequent washes in PBS, sections were blocked in 5% goat serum in 0.3% Triton X-100 for 1 hour followed by overnight incubation with primary antibody for cleaved caspase-3 (1:200, Cell Signaling) at 4°C in a humidification chamber. After washing, the sections were incubated for 1 hour with anti-rabbit secondary antibody (1:200, Vector Labs) at room temperature in 5% goat serum. Immunostaining was performed using the avidin-biotin (ABC) method (Vectastain Elite Kit, Vector Labs) with diaminobenzidine (DAB)/Nickel enhancement for 10 minutes. Nissl counterstaining was performed to visualize the cortical layers. Sections were then dehydrated in a series of ethanol dilutions, immersed in xylene and cover-slipped.
2.4. Cleaved caspase-3 and GAD67 co-labeling on P8, double immunohistochemistry
Free-floating sections from mice in group 1 (P8) were prepared for immunoflourescent double labeling as described (Nixon and Crews, 2002). Briefly, sections were washed in PBS followed by 30 minutes incubation in 0.1% hydrogen peroxide to reduce endogenous fluorescence. Sections were then incubated with cleaved caspase-3 antibody with an Alexa-Fluor 488 conjugate (1:10, Cell Signaling) and an anti-GAD67 antibody (1:100, Santa Cruz) overnight at 4°C in a blocking solution containing 0.1% Triton X-100 and 3% rabbit serum. The following day sections were washed in PBS and incubated in Alex-Fluor 594 rabbit anti-goat secondary (1:1000) for 1 hour at room temperature.
2.5. Parvalbumin and YFP immunohistochemistry in adulthood (P82)
The same procedure was performed as above with the following modifications. Free-floating sections from adult mice (group 2) were washed three times in PBS, followed by incubation in 0.1% hydrogen peroxide (He and Crews, 2006). This thickness was chosen in order to optimize visualization of YFP fluorescent neurons. However, since quantification of fluorescence was unreliable, sections were visualized using the ABC-DAB method as described above. Floating sections were incubated overnight in either anti-parvalbumin (1:2000, Sigma) or anti-EGFP/EYFP [6AT316] (1:1000, ABCAM/fisher) at 4°C followed by washing and appropriate secondary antibody incubation the following day. Following ABC and DAB exposure, sections were mounted, allowed to air dry over night at room temperature, and cover-slipped.
2.6. Anatomical boundaries of the PFC
The medial regions of the PFC (anterior cingulate (Cg), pre-limbic, and infra-limbic cortices) were investigated. Since it was difficult to distinguish between the pre-limbic and infra-limbic regions, they were combined and analyzed as the limbic region (LI). The boundaries of the anterior cingulate and the limbic cortices (Figure 1A) were as guided by the mouse atlas (Franklin and Paxinos, 2001) as well as guidelines in a previous immunohistochemical study (Grobin et al., 2003). Coronal sections were identified by comparison with the mouse atlas between bregma 1.98, the appearance of the forceps minor corpus callosum (fmi), and bregma 1.10, the genu of the corpus callosum being the antero-posterior boundaries. The dorsal boundary of the anterior cingulate was defined as the diagonal parallel to the dorso-lateral curvature of the fmi, beginning at the medial peak of the fmi to the medial pial surface. The ventral boundary of the anterior cingulate cortex was defined as the diagonal parallel to the dorsal boundary, beginning one fourth of the maximum length of the fmi ventral from the peak of the fmi to the medial pial surface. The limbic region of the medial PFC was defined as previously from the medial pial surface laterally to the fmi, ventral to the anterior cingulate cortex.
Figure 1. P7 Dizocilpine Increases Caspase-3 Cleavage.
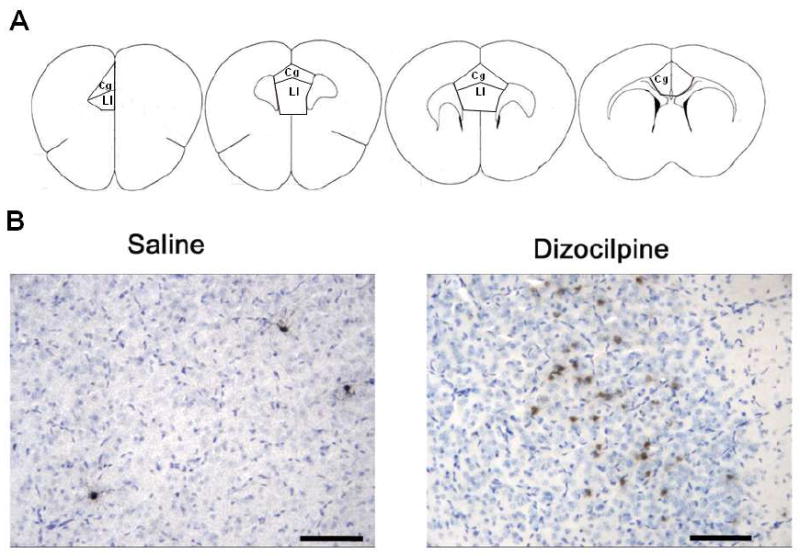
(A.) Diagrams of coronal brain sections between bregma +1.98 to bregma +1.10 that identify the areas of mouse PFC quantified for cleaved caspase-3 immunohistochemistry (Franklin and Paxinos, 2001). Briefly, the dorsal boundary of the anterior cingulate was defined as the diagonal parallel to the dorso-lateral curvature of the fmi (arrows), beginning at the medial peak of the fmi to the medial pial surface. The ventral boundary of the anterior cingulate cortex was defined as a diagonal parallel to the dorsal boundary, beginning one fourth of the total medial length of the fmi, ventral from the peak of the fmi, across to the medial pial surface. (B.) Cleaved Caspase-3+IR. Mice were treated on postnatal day 7 with either saline or dizocilpine (1 mg/kg, 3×24h, i.p.) and assessed for cleaved caspase-3 immunoreactivity (IR) in the prefrontal cortex (PFC) 24 hours after the initial injection. Representative images of cleaved caspase-3 staining with Nissl counterstain (blue) in PFC following either saline or dizocilpine. Note dizocilpine treatment caused an increase in multiple+IR brown cellular profiles indicating cleaved caspase-3+IR. Arrows denote cleaved caspase-3 + IR. Scale bar denotes 50μm.
2.7. Quantification of immunopositive cells
Immunopositive cells were visualized with a CCD camera connected to an Olympus microscope. The PFC was traced and the area traced measured using the Bioquant Image Analysis system as previously described (He et al., 2005). Labeled cells were counted within the region of interest, divided by the area of the section, and expressed as cells/mm2. For each animal, PFC cell counts in 3-4 sections coursing the PFC (bregma +1.98 to +1.1). Counts were determined for each hemisphere individually, and an average value for both hemispheres of each section was calculated. Next, the average value across all sections for each animal was determined. Lastly, the average density for each treatment group (i.e. control or dizocilpine) was calculated compared statistically. We have previously shown that this profile counting method and stereological estimations show identical results in percentage change (Crews et al., 2004; Nixon and Crews, 2002, 2004).
2.8. Open field exploration in adulthood (P80)
Reduced exploratory behavior is an index of anxiety-like behavior (Crawley, 1999). We evaluated exploratory behavior of adult mice (P80) in a novel environment following P7 saline or dizocilpine treatment. Mice were placed in an open field chamber crossed by a grid of photobeams (VersaMax system, AccuScan Instruments) as described (Mohn et al., 1999). Both total distance traveled and time spent in the center were evaluated. Values were collected every five minutes.
2.9. Inhibition of the acoustic startle response (PPI) in adulthood (P80)
The acoustic startle response is a measure of the whole-body flinch reflex following a sudden noise. PPI occurs when a low pre-stimulus leads to a reduced startle in response to a subsequent louder noise. Adult mice (P80) treated on P7 with either saline or dizocilpine were tested in a San Diego Instruments SR-Lab system, as described previously (Moy et al., 2006; Paylor and Crawley, 1997). Briefly, a softer pre-pulse stimulus (74, 78, 82, 86, or 90 dB) was given 100 ms prior to the startle stimulus (120 dB). The peak startle response during the 65-msec period following the startle stimulus was recorded. The PPI for each mouse was calculated using the following equation: (100 - [(response amplitude for pre-pulse stimulus and startle stimulus together / response amplitude for startle stimulus alone) × 100]).
All behavioral tests were performed at the Mouse Neurodevelopmental Research Behavioral Measurement Core facility at UNC. Both pre-pulse inhibition (PPI) and locomotor testing were performed on the same day.
2.10. Statistical Analysis
For the immunohistochemistry studies, the average number of immunopositive cells per area for treatment and control groups (calculation described above) was compared using Student's t-test. Behavioral tests were analyzed using repeated measures ANOVA, with the variable treatment (vehicle or drug) and the repeated measure (five-minute interval in the activity test and pre-pulse sound level in the acoustic startle test). Significance level was set at p < 0.05.
3.0 Results
3.1. Cleaved caspase-3 immunoreactivity and double immunohistochemistry with GAD67 on P8
Treatment with dizocilpine on P7 increased caspase-3+IR in PFC on P8 (Figure 1B). Caspase-3+IR in anterior cingulate cortex was increased over 4 fold (4 ± 1 to 17 ± 4 caspase-3+IR cells/mm2 in control and dizocilpine respectively, mean ± standard error p<0.03, n = 5 per group) and in the limbic cortex 7 fold (5 ± 1 to 34 ± 11 caspase-3+IR cells/mm2 in control and dizocilpine respectively, p<0.03, n = 5 per group) (Figure 2). Thus, dizocilpine treatment causes a significant increase in PFC caspase-3+IR, which is evidence of an acute increase in apoptotic cell death.
Figure 2. Quantification of Cleaved Caspase-3 Immunohistochemistry.
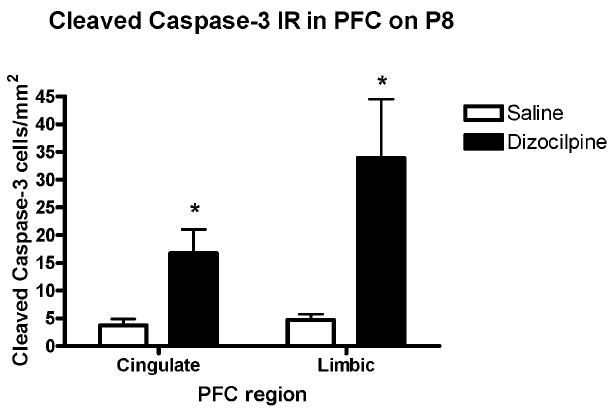
The number of cleaved caspase-3 + immunoreactive (IR) neurons was counted per unit of cortical area as described in the methods. Data shown has been divided into PFC cingulated and PFC limbic as shown in Fig. 1A and described in the methods. Overall PFC cleaved caspase-3+IR cells were increased about 4-7 fold. Within subregions of PFC the anterior cingulate cortex (Cg) increased 4-5 fold and the limbic cortex (LI) increased 6-7 fold (Mean ± standard error, *p<0.03, t-test). Number of mice per treatment group: Saline, n = 5; Dizocilpine, n = 5.
Using double immunohistochemistry and confocal microscopy on P8 following dizocilpine treatment, we found caspase-3+IR was detected in both GAD67 immunopositive and GAD67 immunonegative cells (Figure 3). A neuron that is positive for both cleaved caspase-3 and GAD67 displays nuclear swelling and cytoplasmic shrinkage, suggesting ongoing cell death is pictured (Figure 3C, arrow) (Obernier et al., 2002). Thus, caspase-3 cleavage associated with P7 dizocilpine treatment occurs in both GABAergic and non-GABAergic neurons in frontal cortex.
Figure 3. Cleaved Caspase-3 and GAD67 Immunoflourescent+ IR.
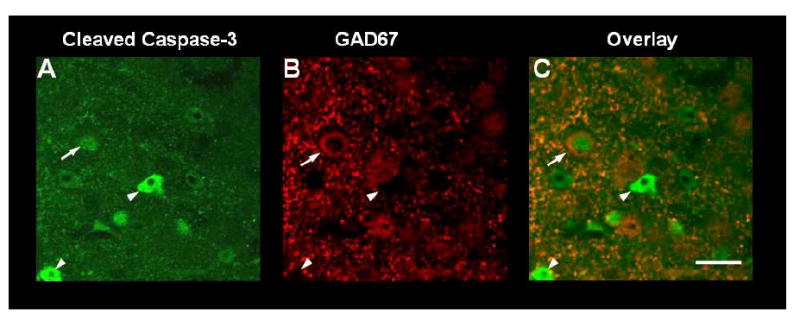
Shown are images of PFC from double immunohistochemistry for cleaved caspase-3 (A, green), for the GABAergic enzyme GAD-67 (B, red) and for the merged images (C). Mice were treated on postnatal day 7 with dizocilpine and assessed for colocalization of cleaved (19 kD) caspase-3 + IF and GAD67 + IR in PFC. on P8. Since parvalbumin is not yet expressed on P8, GAD67 was chosen to identify GABA interneurons. Arrow denotes a swollen nuclear form in a cleaved caspase-3+IR cell (A) that is also GAD67+IR (B) and merges to show the same figure has both +IR. Swollen nuclei often identify dying cells (Obernier et al., 2002). Arrowheads denote caspase-3 positive cells that do not show GAD67+IR and are therefore not GABAergic neurons. Scale bar denotes 20 μm.
3.2. P7 dizocilpine reduces PFC PV interneurons in adults (P82)
The number of GABAergic PV+ IR cells in adults (P82) was assessed following P7 dizocilpine demonstrating visibly fewer PV+IR in PFC (Figure 4). Saline treated animals had nearly twice as many PV + IR neurons (106 ± 19 PV positive cells/mm2) compared to dizocilpine (58 ± 10 PV+IR cells/mm2) across the medial regions of the PFC, p = 0.05, t-test (Figure 5). More detailed analysis of cortical subregions, found a 54% loss of PV cells in anterior cingulate cortex (saline, 113 ± 14 PV + IR cells/mm2n = 4; dizocilpine, 64 ± 9; n = 5, p<0.02), and a 51% decrease limbic cortex (saline, 98 ± 29; dizocilpine, 50 ± 10; p=0.13) which in limbic cortex was not statistically significant. These studies indicate that GABAergic neurons are insulted on P7 resulting in about a 50% loss of PFC PV GABAergic interneurons in adulthood.
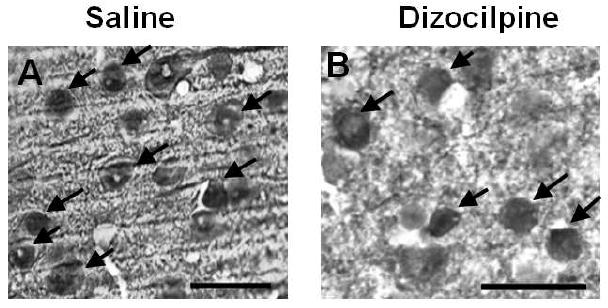
Figure 4. P7 Dizocilpine Reduces Parvalbumin (PV) Immunoreactivity in Adults.
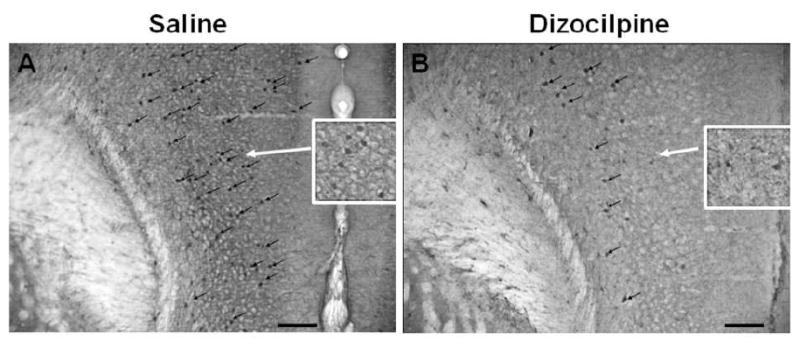
Shown are sections of PFC from mice treated on postnatal day 7 (P7) with either saline or dizocilpine and assessed for PV + immunoreactivity in adulthood (P82). Dizocilpine treatment results in reduced PV + immunoreactive (IR) neurons in the prefrontal cortex (PFC) of adult animals (P82). Representative images (10X) of parvalbumin + IR. Black arrows denote parvalbumin positive immunoreactive neurons. Inserts show higher magnification of PV + IR neurons. Scale bar denotes 100μm.
Figure 5. Quantification of Parvalbumin (PV) Immunohistochemistry.
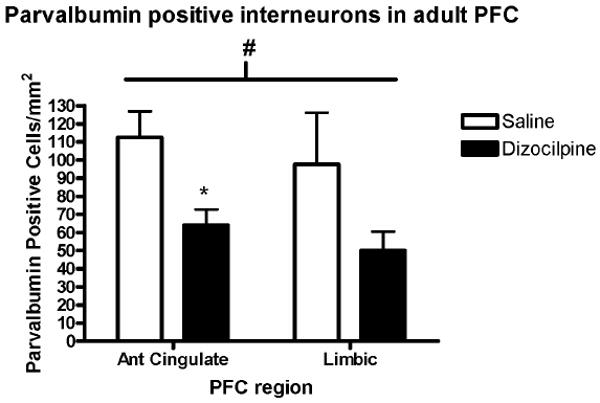
The number of PV + immunoreactive (IR) neurons in the prefrontal cortex (PFC) per unit of cortical area was determined in adult mice on postnatal day 82 (P82) as described in the methods. In the entire PFC dizocilpine treatment on P7 caused a 45% reduction in the number of adult PFC (P82) PV + immunoreactive (IR) (# p=0.05). Dividing PFC into subregions indicated that the anterior cingulate cortex lost about 50% of PV+IR cells (*p<0.02), and the limbic cortex lost about 50% which did not reach statistical significance. (Saline, 98 ± 29; Dizocilpine, 50 ± 10, p=0.13). Mean ± standard error. Number of mice per treatment group: Saline, n = 4; Dizocilpine, n = 5.
An examination of potential microglial activation using the microglial marker Iba-1+IR showed no apparent differences in PFC microglia following P7 dizocilpine (see Supplemental Figure 2). Thus, P7 dizocilpine results in the loss of PV+ GABAergic interneurons without affecting adult microglia numbers in the adult PFC.
3.3. P7 dizocilpine results in reduced layer V pyramidal neurons in adults
To investigate PFC pyramidal neurons we used immunohistochemistry staining for YFP in transgenic mice expressing YFP in layer V pyramidal neurons. We used immunohistochemistry for YFP, since it resulted in clearer images and more sustained visualization than native fluorescence for YFP. Though there are available markers for glutamatergic neurons, these markers do not specifically label layer V pyramidal neurons. Therefore, we used this transgenic mouse to clearly label this population of neurons. Differences in the number of YFP positive pyramidal neurons between P7 saline treated and dizocilpine treated animals were readily observable (Figure 6). Quantification revealed that dizocilpine treatment on P7 resulted in a 43% reduction of YFP positive layer V pyramidal neurons across the entire PFC (saline, 446 ± 82 YFP + IR neurons/mm2; dizocilpine, 254 ± 20, p < 0.05, n=3 per group) (Figure 7). Thus, both PV GABAergic interneurons and layer V glutamatergic pyramidal neurons are reduced in adult PFC following P7 dizocilpine.
Figure 6. P7 Dizocilpine Reduces Layer V Pyramidal Neurons in Adults.
Shown are representative images of YFP+IR. As described in the methods, the C57BL/6 mice studied express YFP in layer V pyramidal neurons. Arrows highlight YFP+IR in layer V pyramidal neurons. Dizocilpine treatment on P7 reduced YFP + IR layer V pyramidal neurons in adult mice (P82). Representative images of YFP + IR in adult PFC (40X), following (A) P7 saline or (B) P7 dizocilpine treatment. Scale bar denotes 20μm.
Figure 7. Quantification of Yellow Fluorescent Protein (YFP) Positive Layer V Pyramidal Neuron Immunohistochemistry.
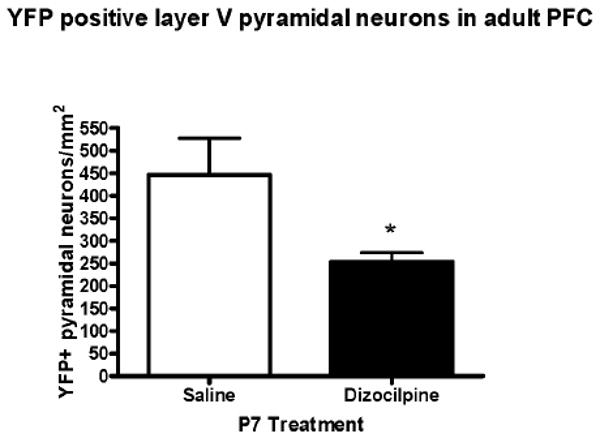
The number of YFP + immunoreactive (IR) layer V pyramidal neurons per cortical area in the PFC was determined as described in the methods. Dizocilpine treatment on P7 caused a significant 43% reduction of layer V pyramidal neurons at P82 across the PFC (*p<0.05). Mean ± standard error. Number of mice per treatment group: Saline, n = 3; Dizocilpine, n = 3.
3.4. P7 dizocilpine alters open field behavior in adults (P80) without disrupting PPI
Center time in the open field, overall locomotor activity, and PPI of acoustic startle responses were evaluated in mice at P80. PPI is an index of sensorimotor gaiting and open field activity is a global measure of motor activity, exploration and overall locomotion. There were no significant effects of the early exposure to dizocilpine on overall locomotor activity (Figure 8A, main effect of treatment, F1,7=0.20, p = 0.67), nor on the time course for habituation i.e. the reduced activity with time in the open field (treatment × time interaction, F23,161=1.19, p = 0.26). Additional information was obtained from open field activity by assessing time in the center. Comparisons of total center time showed controls spent about twice as much time in the center during the first 50 minutes of the test, prior to habituation (Figure 8B). Controls spent an average of 36± 7 seconds/5 min observation period, whereas dizocilpine treated mice spend an average of 19± 3 seconds/5min (2-way ANOVA with repeated measures: F1,7= 5.81, * p< 0.05) in the center of the open field. The tendency to avoid the center open field, or thigmotaxis, has been used as a standard measure of anxiety-like behavior (Crawley, 1999), that is enhanced by anxiogenic drugs and reduced by anxiolytic drugs (Simon et al., 1994; Treit and Fundytus, 1988). Treatment with dizocilpine on P7 did not lead to changes in PPI in adult (P80) animals (Figure 9, repeated measures ANOVA, main effect of treatment, F1,7=1.64, p = 0.24; treatment × decibel level interaction, F4,28=1.16, p = 0.35). Thus, P7 dizocilpine significantly reduced time spent in the center region of the chamber, without altering overall locomotor activity or pre-pulse inhibition.
Figure 8. Open Field Activity of P80 Adult Mice With or Without P7 Dizocilpine.
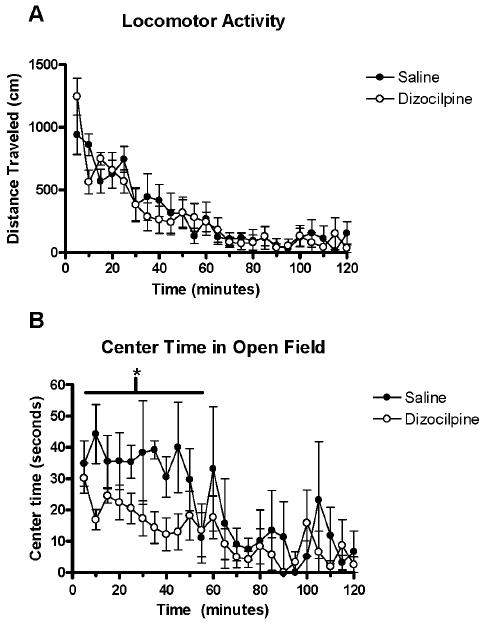
Mice were placed in an open field chamber and activity automatically recorded when light beams were broken. (A) Overall Open Field Locomotor activity (5 minute periods). Note high exploratory activity at early time points that declines over time as the animal habituates to the novelty of the new environment. Saline and dizocilpine mice displayed no differences in their overall locomotor activity (F1,7= 0.2, p = 0.67). Mice habituated to the test chamber during the first 50 minutes. There was no difference in the time course for habituation between groups (Treatment × time interaction F23,161= 1.19, p = 0.26). (B) Center time open field activity. Note mice treated on P7 with dizocilpine show less center time during most 5 minute periods during the 50 minute exploratory phase. Dizocilpine treated mice spent about half as much time exploring the center of the open field as mice treated with saline on P7 over the first 50 minutes, prior to habituation (F1,7= 5.81, p<0.05). Saline, n = 4; Dizocilpine, n = 5.
Figure 9. P7 Dizocilpine Does Not Change Pre-Pulse Inhibition (PPI) of Acoustic Startle Responses in Adult Mice.

Mice were treated with either saline or dizocilpine on P7 and PPI was measured on P80. PPI, a measure of sensorimotor gating, was not changed by dizocilpine (repeated measures 2-way ANOVA (main effect of treatment F1,7 = 1.64, p<0.24; treatment × decibel level interaction, F4,28=1.163, p = 0.35). Number of mice per treatment group: Saline, n = 4; Dizocilpine, n = 5.
4.0. Discussion
We found that post-natal day 7 NMDA antagonism increases cleaved caspase-3 IR in the PFC in both GABAergic and non-GABAergic cells. Our observation of a 4-7 fold increase in the density of cleaved caspase-3 IR cells in the PFC (Figure 2) is consistent with previous studies that show NMDA antagonist-induced cell death in other brain regions (Harris et al., 2003; Ikonomidou et al., 1999; Lema Tome et al., 2006; Wang and Johnson, 2007). In their groundbreaking work, Ikonomidou et al demonstrated by TUNEL staining that P7 dizocilpine (0.5-1.0mg/kg, i.p., 1 injection every 8 hours, 3 total injections) robustly increases the number of degenerating neurons in thalamic, hypothalamic, hippocampal, parietal, retrosplenial, cingulate, and frontal layers II and IV, with 2-22 fold increase in the number degenerating neurons in frontal and cingulate regions (Ikonomidou et al., 1999). This study, however, did not differentiate between different frontal cortical regions. Lema Tome et al identified this observation in the somatosensory cortex, with one subcutaneous injection of dizocilpine (1mg/kg) on P7 increasing the number cleaved caspase-3 IR cells by 20 fold, 16 hours after the injection (Lema Tome et al., 2006). Cleaved caspase-3 IR is a putative marker for dying cells (Krajewska et al., 1997). Olney et al found that the pattern of cleaved caspase-3 IR closely corresponds to the pattern of argyrophilic neurodegeneration following P7 dizocilpine treatment in rodents (Olney et al., 2002). Wang et al showed that following 1 injection of the NMDA antagonist PCP (1, 3, or 10 mg/kg), cleaved caspase-3 IR was found to co-localize with TUNEL positive neurons up to nine hours after the injection (Wang and Johnson, 2007). Broad inhibition of caspases reduces dizocilpine-induced cell death in neuronal cultures by 60-80% (Yoon et al., 2003). Thus, cleaved caspase-3 likely identifies cells undergoing cell death in this paradigm.
Though it is clear that many neurons are dying following P7 NMDA antagonism, it was previously unknown which neurons are vulnerable. We have extended our studies into this area and show that some of the PFC neurons expressing cleaved caspase-3 also express GAD67 (Figure 3), a marker for GABAergic interneurons. GAD67 is expressed prior to P7 (Kiser et al., 1998; Tamamaki et al., 2003) and neurons expressing GAD67 on P7 mature into various GABAergic interneurons e.g. PV, CR, and somatostatin (Tamamaki et al., 2003). Interestingly, Wang et al found almost no co-localization of the cell death markers cleaved caspase-3 or TUNEL with GABAergic interneuron markers CB or CR in dorsal subiculum, retrosplenial cortex, motor cortex, cingulate, hippocampus, or somatosensory cortex 16 hours after injection on P7 with PCP (10 mg/kg) (Wang et al., 2008). In this study they did not study parvalbumin co-localization with cleaved caspase-3 due to its low expression on P7. Likewise, Lema Tome et al found very little co-localization of cleaved caspase-3 with PV, CB, or CR in different brain regions following P7 dizocilpine (Lema Tome et al., 2006). In fact, developmental calcium binding protein expression coincided with a decline in dizocilpine induced caspase-3 cleavage and the number of cortical neurons expressing CB and PV increased substantially from P7 to P21 (10 fold and 60 fold respectively). Therefore, GAD67 is probably a better marker for identifying dying GABAergic interneurons during early post-natal life. We demonstrate for the first time that P7 dizocilpine treatment causes a robust increase in cleaved caspase-3 IR in the PFC, including some that are GABAergic interneurons.
In adult animals we found a striking, persistent reduction in PV GABAergic interneurons and layer V pyramidal neurons in PFC following P7 treatment with dizocilpine. The density of adult PV interneurons and layer V pyramidal neurons was reduced by 45% and 43% respectively (Figure 5). Wang et al showed persistent reductions in of PV interneurons in the somatosensory (63%), motor (36%), and retrosplenial (21%) cortices, with no measureable reductions in the striatum, hippocampus, or cingulate in young adult rats (P56) following one P7 injection of PCP (10 mg/kg) (Wang et al., 2008). Our studies, however, show a robust (43%) reduction of PV interneurons in the anterior cingulate (Figure 5). A possible reason for this difference is that Wang et al did saggittal sections so that their investigation spanned the entire antero-posterior length of the cingulate (i.e. bregma +3.7 to -1.4 in the rat). In our studies, however, we did coronal sections and only measured PV neurons in the anterior cingulate region associated with the PFC (i.e. bregma +1.98 to +1.10 in the mouse). Previous studies have not investigated the effect of P7 NMDA antagonism on layer V pyramidal neuron numbers. The use of the YFP mouse allowed us to investigate these important neurons and we found a 45% reduction in adult PFC. In the rodent PFC parvalbumin identifies wide arbor basket and chandelier interneurons (Conde et al., 1994; Gabbott and Bacon, 1996) which converge onto pyramidal neurons in both layers III and V modulate their activity patterns. Layer V pyramidal neurons represent the major glutamatergic projections from the PFC to contralateral cortex, striatum, subcortical structures and posteromedial thalamus (Hattox and Nelson, 2007; Molnar and Cheung, 2006). Layer V pyramidal neurons also send reciprocal projections to the medial dorsal thalamus (though layer III pyramidal neurons receive the majority of medial dorsal thalamic input) (Kuroda et al., 1998). Thus, the reduction of these neurons that we observed could result in dysregulation of PFC functions including cognition and the control of impulsivity.
The findings of persistent reductions PV interneurons and layer V pyramidal neurons in PFC may also be pertinent for human cognitive disorders. Deficits in PV interneurons in the prefrontal cortex have been found in postmortem tissue in patients with schizophrenia (Beasley and Reynolds, 1997) and major depressive disorder (Rajkowska et al., 2007). A recent study has shown a reduction in the density of layer III and V calmodulin (+) pyramidal neurons in the postmortem human schizophrenic PFC (Broadbelt and Jones, 2008). Thus, the persistent reduction in PV interneurons and layer V pyramidal neurons we observed in adults following P7 dizocilpine treatment reflects the neuropathology found in some mental diseases.
Consistent with this line of reasoning, we observed persistent behavioral changes in adults as seen by reduced exploration of the center in the open field test following P7 dizocilpine. Although the layer V pyramidal YFP mouse allowed histological determination of PFC layer V pyramidal cells the breeding limited the availability of animals. We used 4-5 mice per group which may have underpowered the behavioral studies, nevertheless, we still detected significant reductions in the time spent in the center of the open field. Reduced center time in the open field, or thigmotaxis, is a standard index of non-conditioned anxiety-like behavior, as high anxiety animals spend less time in the center (Belzung and Griebel, 2001; Crawley, 1999; Heisler et al., 1998). This anxiety-like behavior is reversed by anti-anxiety drugs that do not impair overall locomotor activity (Hoplight et al., 2005; Simon et al., 1994; Treit and Fundytus, 1988). We observed that mice treated with dizocilpine on P7 spent about half the time in the center of the open field apparatus as control animals (Figure 8). The magnitude of the change is similar to the reduced center time reported for high-anxiety transgenic mice lacking functional serotonin-1A receptors (Heisler et al., 1998). Thus, our findings suggest early post-natal NMDA antagonists induce persistent adult anxiety.
Clinical and animal observations suggest that anxiety is probably related to impulsivity. Heightened anxiety is a common co-morbid feature of several psychiatric conditions displayed reduced impulsive control such as attention deficit hyperactivity disorder (ADHD) (Bowen et al., 2008; Schatz and Rostain, 2006), schizophrenia (Braga et al., 2005), and alcohol dependence (Brady and Lydiard, 1993). Rodent studies also suggest an association between anxiety and impulsivity. Thiebot demonstrated that serotonin uptake inhibitors, modern anxiolytic drugs, reduce the choice of rats for a small reward rather than a larger delayed reward by nearly 40% (i.e. they reduce impulsivity) (Thiebot et al., 1985), while benzodiazepines had the opposite effect. More recent studies showed that 5-HT1A agonism and 5-HT2A antagonism, which are modern anxiolytic approaches, reduce anticipatory, and perseverative responses in the 5-CSRT (i.e. reduced impulsivity) to control levels following enhancement of impulsive behavior by acute NMDA antagonism directly into the PFC of adults (Carli et al., 2006; Mirjana et al., 2004). These studies suggest that anxiety and impulsivity might be directly related. Further, it has been theorized that impulsive behavior may be in part due to an imbalance between the impulsive drive from the amygdala and the inhibitory response from the PFC (Bechara, 2005). The PFC is also considered to counteract “pro-anxiety” drives coming from the amygdala by promoting swift recovery from negative emotional stimuli and inhibiting output from the amygdala (Davidson, 2002). Thus, anxiety and impulsivity may both prove to be parallel features of imbalanced PFC-amygdala interactions. Therefore, our observation of heightened anxiety-like behavior in adults pretreated with dizocilpine on P7 suggests that this treatment might also increase impulsive behaviors.
Though other studies have not looked at anxiety-like behavior in adults following early post-natal NMDA antagonism, other behaviors associated with PFC function have been investigated. For example, Stefani and Moghaddam found that young adult rats (P60) treated post-natally with dizocilpine for four days (P7-P10) made fewer correct choices in a four arm maze following a change in the cue associated with the reward from brightness to texture (Stefani and Moghaddam, 2005). This ability to respond to a shift in the perceptual dimension of a cue associated with a reward (such as brightness to texture) has been shown to require proper function of medial regions of the PFC in rats (Birrell and Brown, 2000). To demonstrate this, adult rats were first trained to find a reward hidden in a bowl by associating a defined odor with the correct bowl. Once they had learned successfully, the cue associated with the correct bowl was changed from odor to texture. Rats with medial PFC lesions learned the first association (i.e. with an odor) equally as well as controls, and they also performed equally as well when the quality of the odor was changed. However, lesioned rats required 40% more trials to successfully reach criterion once the cue associated with the reward was changed from odor to texture. In studies investigating the role of PFC in impulsivity, adult rats treated with the NMDA antagonist CPP (50ng/μl) directly into the adult PFC display made four times as many anticipatory responses and three times as many perseverative responses in the 5-Choice Serial Reaction Time task (5-CSRT) than controls signifying increased impulsive behavior (Baviera et al., 2008; Mirjana et al., 2004). Thus, NMDA antagonism both during post-natal life as well as adulthood can alter PFC behaviors associated with cognitive function and impulsivity.
We found no disruption in PPI in adult animals following P7 dizocilpine (Figure 9). Pre-pulse inhibition is a measure of sensorimotor gating, which is mediated primarily a pontine reflex, and modulated by several brain regions e.g. PFC, hippocampus, thalamus, and others (Caine et al., 1992; Davis et al., 1982; Fendt et al., 2001; Schwabe and Koch, 2004). A similar inability of PCP on P7 to produce later deficits in PPI in animals (P25) has been shown previously (Anastasio and Johnson, 2008). Harris et al showed that two injections of dizocilpine (0.5 mg/ml) on P7 caused a disruption in PPI in adult female rats only (55%), while males were unaffected. Since PPI is primarily a brain stem reflex, the absence of a disruption of PPI in our study may be due to insufficient damage to either brain stem nuclei or other regions as a result of this insult (Wang et al., 2008). This idea is supported by the fact that repeated NMDA antagonist treatment regimens do disrupt PPI in older animals (e.g. PCP (10 mg/kg) on P7, P9, and P11 causes a 55% reduction in PPI on P25 (Wang et al., 2001) and CGP on P1, P3, P6, P9, P12, P15, P18, and P21causes a 74% reduction in PPI on P60 (Wedzony et al., 2008)). Thus, the inability of one day of dizocilpine treatment on P7 to disrupt PPI in adults may be due to the lack of damage to pontine nucleus accompanied by sufficient accommodation from other brain regions to account for the damage to the PFC.
This study is the first to show that NMDA antagonism with dizocilpine on P7 causes cleavage of caspase-3 in both GABAergic and non-GABAergic neurons in the PFC. This is associated with persistent reductions of approximately 45% PV GABAergic interneurons and layer V pyramidal neurons in PFC as well as a 50% reduction in center exploration in the open field test. The PFC cellular losses and anxiety-like behavior suggest that this early post-natal insult might result in increased impulsivity in adulthood. Future studies will directly investigate the effects of the loss of these PFC neurons on impulsive behavioral phenotypes.
Supplementary Material
Acknowledgments
The authors would like to sincerely thank the NIAAA (R01 AA06069, 5P60-AA011605, F30-AA018051), NIMH (1 P50 MH064065, K08 MH10752), and NICHD (P30 HD03110), the UNC-Bowles Center for Alcohol Studies and the UNC Neurodevelopmental Research Center for their support. Also, we thank Swarooparani Vadlamudi and Randal Nonneman for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anastasio NC, Johnson KM. Atypical anti-schizophrenic drugs prevent changes in cortical N-methyl-D-aspartate receptors and behavior following sub-chronic phencyclidine administration in developing rat pups. Pharmacol Biochem Behav. 2008;90:569–577. doi: 10.1016/j.pbb.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Baviera M, Invernizzi RW, Carli M. Haloperidol and clozapine have dissociable effects in a model of attentional performance deficits induced by blockade of NMDA receptors in the mPFC. Psychopharmacology (Berl) 2008;196:269–280. doi: 10.1007/s00213-007-0959-9. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen R, Chavira DA, Bailey K, Stein MT, Stein MB. Nature of anxiety comorbid with attention deficit hyperactivity disorder in children from a pediatric primary care setting. Psychiatry Res. 2008;157:201–209. doi: 10.1016/j.psychres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Brady KT, Lydiard RB. The association of alcoholism and anxiety. Psychiatr Q. 1993;64:135–149. doi: 10.1007/BF01065866. [DOI] [PubMed] [Google Scholar]
- Braga RJ, Mendlowicz MV, Marrocos RP, Figueira IL. Anxiety disorders in outpatients with schizophrenia: prevalence and impact on the subjective quality of life. J Psychiatr Res. 2005;39:409–414. doi: 10.1016/j.jpsychires.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Broadbelt K, Jones LB. Evidence of altered calmodulin immunoreactivity in areas 9 and 32 of schizophrenic prefrontal cortex. J Psychiatr Res. 2008;42:612–621. doi: 10.1016/j.jpsychires.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR. Hippocampal modulation of acoustic startle and prepulse inhibition in the rat. Pharmacol Biochem Behav. 1992;43:1201–1208. doi: 10.1016/0091-3057(92)90503-8. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Franklin, Paxinos . The Mouse Brain in Stereotaxic Coordinates. 2001. [Google Scholar]
- Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J Comp Neurol. 1996;364:567–608. doi: 10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Heenan EJ, Lieberman JA, Morrow AL. Perinatal neurosteroid levels influence GABAergic interneuron localization in adult rat prefrontal cortex. J Neurosci. 2003;23:1832–1839. doi: 10.1523/JNEUROSCI.23-05-01832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur J Neurosci. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- He J, Crews F. Neurogenesis Decreases during Brain Maturation from Adolescence to Adulthood. Pharmacol Biochem Behav. 2006 doi: 10.1016/j.pbb.2006.11.003. Submitted June 2006. [DOI] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoplight BJ, Vincow ES, Neumaier JF. The effects of SB 224289 on anxiety and cocaine-related behaviors in a novel object task. Physiol Behav. 2005;84:707–714. doi: 10.1016/j.physbeh.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Gilmore JH, Glantz LA, Gable KL, German TT, Tong RI, Lieberman JA. Caspase-3 activation in rat frontal cortex following treatment with typical and atypical antipsychotics. Neuropsychopharmacology. 2007;32:95–102. doi: 10.1038/sj.npp.1301074. [DOI] [PubMed] [Google Scholar]
- Kiser PJ, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of rat somatosensory barrel cortex. J Comp Neurol. 1998;402:62–74. [PubMed] [Google Scholar]
- Krajewska M, Wang HG, Krajewski S, Zapata JM, Shabaik A, Gascoyne R, Reed JC. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res. 1997;57:1605–1613. [PubMed] [Google Scholar]
- Kuroda M, Yokofujita J, Murakami K. An ultrastructural study of the neural circuit between the prefrontal cortex and the mediodorsal nucleus of the thalamus. Prog Neurobiol. 1998;54:417–458. doi: 10.1016/s0301-0082(97)00070-1. [DOI] [PubMed] [Google Scholar]
- Lema Tome CM, Bauer C, Nottingham C, Smith C, Blackstone K, Brown L, Hlavaty C, Nelson C, Daker R, Sola R, Miller R, Bryan R, Turner CP. MK801-induced caspase-3 in the postnatal brain: inverse relationship with calcium binding proteins. Neuroscience. 2006;141:1351–1363. doi: 10.1016/j.neuroscience.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Mirjana C, Baviera M, Invernizzi RW, Balducci C. The serotonin 5-HT2A receptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology. 2004;29:1637–1647. doi: 10.1038/sj.npp.1300479. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Moy SS, Perez A, Koller BH, Duncan GE. Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain Res. 2006;1089:186–194. doi: 10.1016/j.brainres.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res. 2002;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiatry. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, Barrionuevo G, Sesack SR. Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: synaptic relationships to subclasses of interneurons. J Comp Neurol. 2005;490:220–238. doi: 10.1002/cne.20661. [DOI] [PubMed] [Google Scholar]
- Schatz DB, Rostain AL. ADHD with comorbid anxiety: a review of the current literature. J Atten Disord. 2006;10:141–149. doi: 10.1177/1087054706286698. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Koch M. Role of the medial prefrontal cortex in N-methyl-D-aspartate receptor antagonist induced sensorimotor gating deficit in rats. Neurosci Lett. 2004;355:5–8. doi: 10.1016/j.neulet.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psychiatry. 2005;57:433–436. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Thiebot MH, Le Bihan C, Soubrie P, Simon P. Benzodiazepines reduce the tolerance to reward delay in rats. Psychopharmacology (Berl) 1985;86:147–152. doi: 10.1007/BF00431700. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: implications for schizophrenia. Neuroscience. 2001;107:535–550. doi: 10.1016/s0306-4522(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. The role of caspase-3 activation in phencyclidine-induced neuronal death in postnatal rats. Neuropsychopharmacology. 2007;32:1178–1194. doi: 10.1038/sj.npp.1301202. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Fijal K, Mackowiak M, Chocyk A, Zajaczkowski W. Impact of postnatal blockade of N-methyl-d-aspartate receptors on rat behavior: A search for a new developmental model of schizophrenia. Neuroscience. 2008;153:1370–1379. doi: 10.1016/j.neuroscience.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Yoon WJ, Won SJ, Ryu BR, Gwag BJ. Blockade of ionotropic glutamate receptors produces neuronal apoptosis through the Bax-cytochrome C-caspase pathway: the causative role of Ca2+ deficiency. J Neurochem. 2003;85:525–533. doi: 10.1046/j.1471-4159.2003.01724.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


