Abstract
Background
Activation of innate immunity plays a key role in determining the outcome of an infection. Here, we investigated whether Toll-like receptors (TLRs) are involved in retinal innate response and explored the prophylactic use of TLR2 ligand in preventing bacterial endophthalmitis.
Methods
C57BL/6 (B6) mice were given intravitreal injections of Pam3Cys, a synthetic ligand of TLR2, or vehicle (PBS) 24 h prior to S. aureus (SA) inoculation. The severity of endophthalmitis was graded by slit lamp, electroretinography (ERG), histological examinations and determination of bacterial load in the retina. The expression of cytokines/chemokines and cathelicidin-related antimicrobial peptide (CRAMP) was assessed by ELISA and Western blot respectively.
Results
Intravitreal injections of Pam3Cys up-regulated TLR2 expression in the B6 retina and Pam3Cys pretreatment significantly improved the outcome of SA endophthalmitis, preserved retinal structural integrity and maintained visual function assessed with ERG in B6 mice. Furthermore, Pam3Cys pretreatment activated retinal microglia cells, induced the expression of CRAMP, and remarkably reduced the bacterial load.
Conclusions
This is the first report that highlights the existence and role of TLR2 in retinal innate immune response to SA infection and suggests that modulation of TLR activation provides a novel prophylactic approach to prevent bacterial endophthalmitis.
INTRODUCTION
Endophthalmitis is a potentially vision-threatening condition that requires prompt diagnosis and treatment to prevent vision loss [1]. Bacterial endophthalmitis, with an estimated incidence between0.07% and 0.3%, is one of the most severe complications of cataract surgery, a common surgical procedure performed in the aged population worldwide [2, 3]. In addition to cataract, increasing use of multiple periodic intravitreal injections of anti-vascular endothelial growth factor (VEGF) drugs for the treatment of age-related macular degeneration (AMD) and diabetic retinopathy also predispose patients to infectious endophthalmitis [4]. The reported incidence of endophthalmitis per eye in multicenter clinical trials with anti-VEGF therapy ranged from 0.7% to 1.6% [5].
Among bacterial pathogen Staphylococcus aureus (SA) is a leading cause of severe inflammation and destruction of the retina, resulting in poor visual prognosis even with treatment [6]. The main problem in early diagnosis of endophthalmitis is that ophthalmologists can only recognize the incident after the signs of endophthalmitis become evident. To prevent such situations, surgeons prescribe the use of topical antibiotics both prior and post surgery [7]. In a recent study a fourth-generation fluoroquinolone, moxifl oxacin was shown effective in preventing experimental SA endophthalmitis [8]. Although this class of powerful antibiotics covers a broad spectrum of organisms, they seem to be less effective against Methicillin resistant S. aureus (MRSA), making the prophylactic treatment ineffective [9]. Thus, in the light of an increasing incidence of endophthalmitis and antibiotic-resistant bacterial infection, the development of new adjunctive prophylactic/therapeutic modalities for preventing ocular surgery associated staphylococcal endophthalmitis is warranted.
Although the contribution of SA virulence factors to the pathogenesis of endophthalmitis is fairly well understood [6, 10], relatively little is known about the retinal innate response in this disease. Retina is known to be highly sensitive to inflammation-mediated tissue damage and possess strong innate defenses allowing rapid elimination of invading pathogens with simultaneous reduction of the destructive effects caused by pathogens and the host inflammatory reaction [11, 12]. Host innate immune system uses a series of pattern recognition receptors (PRRs) including the families of Toll-like receptors (TLRs) to detect the presence of pathogens thus allowing rapid initiation of host defense responses [13]. An increasing number of studies have implicated the role of TLRs in provided exciting insights into host-pathogen Interactions [14, 15]. Previous studies from our laboratory [16, 17] and others [18–21] have established an important role of TLRs in mediating innate immune responses in bacterial keratitis [22]. Although, the expression and function of TLRs in other tissues have been studied extensively; very few studies were carried out to define their involvement in the retina. Several TLRs including TLR2, 4, and 9 were found to be expressed in the retinal pigment epithelial (RPE) cells and choroid [23, 24], suggesting a potential role of these receptors in the host defense against invading pathogens in the posterior segment of the eye [24]. To date, the expression of TLRs and their role in detecting and mediating innate response to pathogens in the retina remains elusive.
MATERIAL AND METHODS
Bacterial strain and reagents
S. aureus (strain RN 6390) was maintained intryptic soy broth (TSB; Sigma-Aldrich, St. Louis, MO). Bacterial lipopeptide Pam3Cys-Ser-(Lys)4 hydrochloride (Pam3Cys), a synthetic lipopeptide, which acts as an exclusive TLR2 agonist was purchased from Calbiochem.
Infection procedure and clinical examination
Female C57BL/6 mice (8 wk) were intravitreally injected in left eye, with different amounts of Pam3Cys reconstituted in sterile PBS, or PBS alone as a sham control (1 μl volume). The intravitreal injections were performed using a 33-gauge needle (Hamilton Company, Reno, Nevada). After 24 h, mice were challenged with or without 5000 colony forming units (cfu) of SA per eye in 1 μl PBS via intravitreal inoculation. The right eye of each mouse was left untreated/uninfected, serving as an internal control. Clinical examinations were performed by an ophthalmologist in a blinded fashion using slit lamp microscopy. The ocular disease was graded, and clinical scores from 0 to 4.0 were assigned using the previously described scale [25, 26]. Mice were treated in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and all procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University.
ELISA assay for cytokines/chemokines
At the desired time point after TLR2 ligand or bacterial inoculation, eyes were enucleated. Sensory retina and RPE-choroid complex was carefully isolated, placed in 150 μL of lysis buffer and sonicated on ice for15 seconds. The lysate was centrifuged at 14,000 rpm for 15minutes at 4°C, and MIP-2, and IL-1β levels in the supernatant were determined with a mouse specific ELISA kit (R&D Systems) and normalized to total protein, according to the manufacturer’ sprotocol.
Analysis of retinal function
Scotopic electroretinogram (ERG) was used to track down retinal function during the course of endophthalmitis. ERGs were recorded following bilateral mydriasis and 4h of dark adaptation. Silver/nylon fiber recording corneal electrodes (DTL Plus Electrodes™, Diadnosis LLC, Lowell, MA) were used. Reference needle electrodes were placed in both ears; and a ground needle electrode was placed in the tail. Responses were acquired using data acquisition software from the UTAS visual diagnostic system and amplified using the UBA-4204 amplifier (LKC Technologies, Gaithersburg, MD). Ganzfeld light stimulus was used to present eight 10-ms flashes, with light intensities, increasing from 0.0001 to100 cd-s/m2. The ERG a-wave was measured as an amplitude between the ERG baseline and the first negative peak, and the ERG b-wave was measured as an amplitude between the first negative peak and the first positive peak.
Bacterial load and histopathological analysis
At 3 days post infection (dpi), eyes were enucleated and rinsed in sterile PBS. Individual eyes were disrupted and homogenized with metal beads in a tissue lyser (Quigen) in 1 ml PBS for 2 minutes at maximum speed. The homogenates were serially diluted, and aliquots(100 μl) were plated onto StaphChromo agar plates (BD Biosciences), and incubated overnight at 37°C. For histology eyes were enucleated, fixed in 10% formalin for 24 h and subjected to sectioning and staining with hematoxylin and eosin (H&E).
Antibacterial activity and CRAMP expression
The antibacterial activity of conditioned media derived from microglia (BV-2 cell line) challenged with Pam3Cys was tested against SA as described earlier [27]. Two milliliter of conditioned media was inoculated with 100 cfu of SA and the cultures were incubated at 37°C for 8 h and bacterial growth was monitored by measuring OD at 600. The accumulation of TNF-α and CRAMP in the same media was determined by ELISA and Dot-Blot [27].
Statistical analysis
An unpaired, two-tailed Student t test was used to determine statistical significance for data from the cytokine ELISA, and bacterial count. A nonparametric Mann-Whitney U test was performed for clinical score and multiple group comparison was performed using analysis of variance (ANOVA).
RESULTS
TLR2 is expressed in the retina and retinal cells
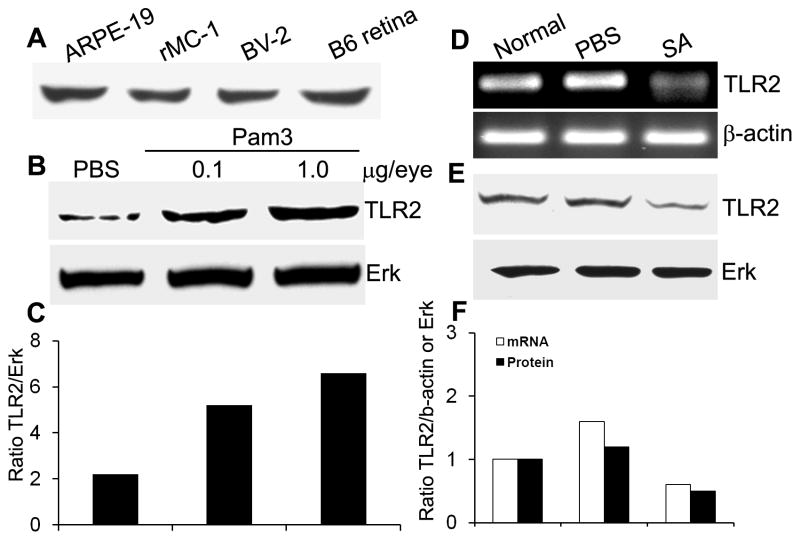
As a first step to understand the role of TLRs in the retina, we assessed the expression of TLR2, a major cell surface PRR for Gram-positive bacteria. Figure 1A shows that TLR2 is expressed in the whole mouse retina and in several types of cultured, non-neural retinal cells including retinal pigment epithelium (ARPE-19), Muller cells (rMC-1), and microglia (BV2).
Figure 1. TLR2 expression in Pam3Cys and SA challenged mouse retina and retinal cells.
(A) The indicated cultured cells and normal B6 mouse retina were lysed for Western blot analysis using anti-TLR2 antibody. To assess the modulation of TLR2 expression by Pam3Cys treatment in vivo, 24h after intravitreal injection of Pam3Cys (0.1 μg and 1 μg), PBS (B), and SA (E) TLR2 protein expression in whole B6 mouse retina was detected using Western blot. Expression of TLR2 mRNA at 24h post SA infection was assessed by RT-PCR in whole retina (D). The band intensity of western blot and PCR product was quantitated by densitometric analysis and normalized with their respective internal controls i.e. ERK and β-actin (C & F).
To determine if theTLR2-expressing retina recognizes and responds to TLR2 ligand, we challenged the mouse retina with a synthetic TLR2 ligand Pam3Cys, SA or PBS (vehicle control) by intravitreal injection. Western blot analysis revealed that exposure of the mouse retina to Pam3Cys (0.1 and 1 μg/eye) resulted in an increase in the levels of TLR2 (Figure 1B&C). In contrast, intravitreal inoculation of SA decreased the expression of TLR2 both at mRNA (Figure 1D), and protein levels (Figure 1E&F) in B6 mouse retina. Taken together these results suggest that the retina is capable of recognizing and responding to SA via TLR2.
Intravitreal injection of Pam3Cys protects mice from SA endophthalmitis
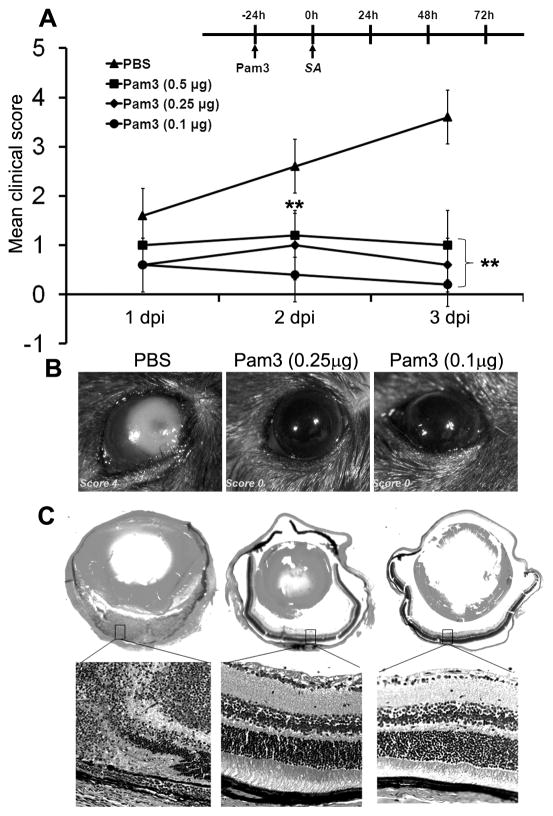
Having shown that the retina expresses TLR2 and is responsive to its agonist, we next investigated whether TLR2 ligand can enhance the innate immunity of the retina. We adapted a B6 mice model of SA-induced endophthalmitis [25, 28] and confirmed that mice receiving 5,000 cfu SA by intravitreal injection showed a complete destruction of the retina (clinical score 4.0). To assess the protective effect of TLR2 activation on the development of staphylococcal endophthalmitis, mice were given intravitreal injections of Pam3Cys, 24 h prior to challenge with 5,000 cfu of SA. As shown in Figure 2A, no significant difference was observed in clinical scores in the control vs Pam3Cys treated groups (p >0.05) at 1 dpi. At 2 dpi, the average clinical scores, 1.2 (0.5 μg), 1.0 (0.25μg) and 0.4 (0.1 μg) for the Pam3Cys-pretreated mice, were significantly lower than the PBS-pretreated controls with an average clinical score of 2.6. At 3 dpi, while 60% (3/5) of the control eyes scored 4.0, 80% (4/5) of eyes pretreated with 0.1μg Pam3Cys scored 0, suggesting a Pam3Cys pretreatment-mediated protection. Overall, clinical scores of all Pam3Cys treated groups were significantly (ANOVA, p<0.001) lower than the control both at 2 and 3 dpi.
Figure 2. Intravitreal injection of Pam3cys prevents the development of endophthalmitis and preserves retinal architecture.
(A) C57BL/6 mice (n=5 each group) were administered PBS or indicated concentrations (μg/eye) of Pam3Cys by intravitreal injection (see Methods for detail). After 24 h, eyes were inoculated with 5000 cfu of SA (RN6390). A clinical score (range 0 to 4) of Pam3Cys-pretreated and PBS injected mice on 1, 2 and 3 days post infection (dpi) was assigned for each infected eye by an ophthalmologist and presented as mean clinical scores. ANOVA was performed to compare all groups at 2 (p<0.001) and 3 dpi (p<0.001) and a nonparametric Mann-Whitney U test was performed to compare each Pam3Cys treatment to the PBS group (** p<0.001). Eyes pretreated with indicated concentration of Pam3Cys or PBS control were enucleated at 3 dpi. Light microscopy examination (B) and histopathological analysis (C) was performed on representative eyes. H&E-stained retinal tissue of PBS-pretreated and SA -infected mice showed massive infiltration as well as destruction of retinal architecture (left panel); histology of Pam3Cys-pretreated and SA-infected mice showed intact retinal architecture (right panels). Magnification, whole eyes (2.5 ×) and the retina (20 ×).
As shown by light microscopy (Figure 2B), PBS-pretreated mice exhibited dense opacity and clear signs of severe inflammation and infection at 3 dpi. In contrast, the eyes pre-exposed to Pam3Cys were clear with no sign of infection or inflammation (score 0). Histopathological analysis (Figure 2C) revealed that in PBS pretreated eye, infection caused complete destruction of the retina with heavy inflammatory infiltrates (Figure 2C, left panel). In contrast, pre-exposure of the retina to Pam3Cys preserved retinal structural integrity and reduced tissue damage associated with SA infection (Figure 2C, right panels). Thus, TLR2 ligand pretreatment protects the retina from tissue damage caused by SA in B6 mice.
Pam3Cys pretreatment maintains retinal function
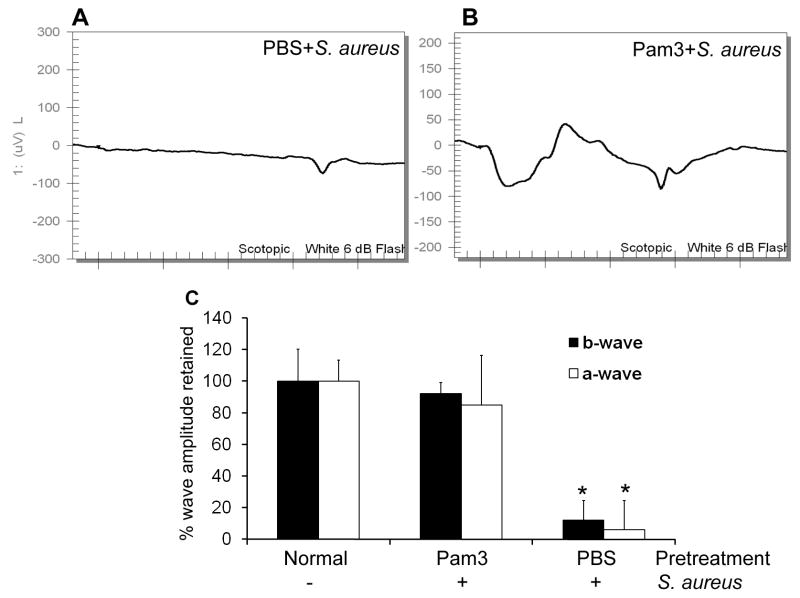
The foregoing results demonstrate that Pam3Cys pretreatment diminished the pathology of staphylococcal endophthalmitis at the tissue level. To determine if Pam3Cys pretreatment also preserves retinal function in the infected eyes, we measured scotopic ERG responses in PBS- and Pam3Cys-pretreated and SA infected mice. Representative ERG traces at a flash intensity of 6-dB (10 cd-s/m2) are shown in Figure 3. ERG response showed normal a-wave (the response generated from photoreceptors) and b-wave (the response generated from the inner retina, mostly the bipolar and Muller cells) in the PBS- and Pam3Cys- pretreated eyes without infection (data not shown). Control mice that received sterile PBS intravitreal injections prior to SA inoculation demonstrated the loss of retinal function with a significant decrease in both a -wave (90%) and b-wave (94%) amplitudes (panels A & C) at 3 dpi. Remarkably, mice pretreated with Pam3Cys, maintained their normal ERG with no significant decline in a- or b-wave amplitude (panels B & C). Taken together these results suggest that the intravitreal injection of Pam3Cys at low doses preserves ERG during bacterial infection.
Figure 3. Pam3Cys pretreatment maintained normal retinal function in B6 mice eyes following SA infection.
ERG response to a 6-dB flash were recorded at 3 dpi in mice were pretreated via intravitreal injection of PBS (A) or 0.1 μg of Pam3Cys (B) followed by SA infection. The percentage amplitude of a- and b-wave retained in mice pretreated with PBS or Pam3Cys were compared to normal and presented as mean ± SD ERG amplitudes (C). *P<0.001 (student’s t-test), significant decline in amplitude of a-and b-wave compared to both Pam3Cys pretreated and normal eyes.
Pam3Cys pretreatment reduced the bacterial load in B6 mice eyes
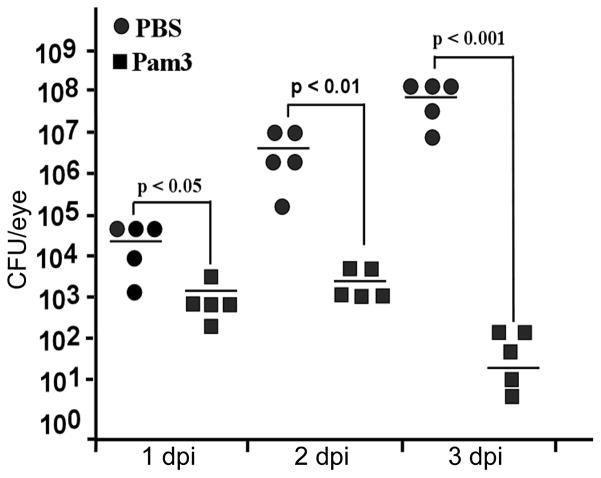
The observation that Pam3Cys-pretreated eyes were protected from endophthalmitis suggests an enhanced bacterial clearance in these eyes. We next assessed the effect of Pam3Cys-pretreatment on bacterial load in the eyes. As shown in Figure 4, at 1 dpi there were on an average 5 × 104 cfu/eye in the control mice and 2 × 103 in Pam3Cys-pretreated mice, which represents a 15-fold difference from the control and a 2.5-fold reduction from the total number of bacteria injected into the vitreous. At 2 dpi, an average of 5 × 106 cfu per eye was detected in the control mice and 3 × 103 in Pam3Cys-pretreated mice. More strikingly, at 3 dpi, the average bacterial load in the control group was 2 × 108 and in Pam3Cys-pretreated eyes was reduced to 1 × 102 cfu Thus, Pam3Cys pretreatment greatly enhanced bacterial clearance.
Figure 4. Pam3cys pretreatment reduces bacterial load in infected B6 mice eyes.
Mice were treated with PBS (n = 5) or 0.1 μg of Pam3cys/eye (n = 5) as described in the text, followed by intravitreal inoculation of 5000 cfu of SA per eye. At the indicated days of post-infection, the whole eyes were enucleated under sterile conditions, homogenized in 1ml PBS and the bacterial load was determined by serial dilution plate count. ANOVA was performed for all group at 3 dpi (p< 0.01), indicated p values were generated using paired Student’s t test.
Pam3Cys induces cytokine and antimicrobial peptide expression in the retina
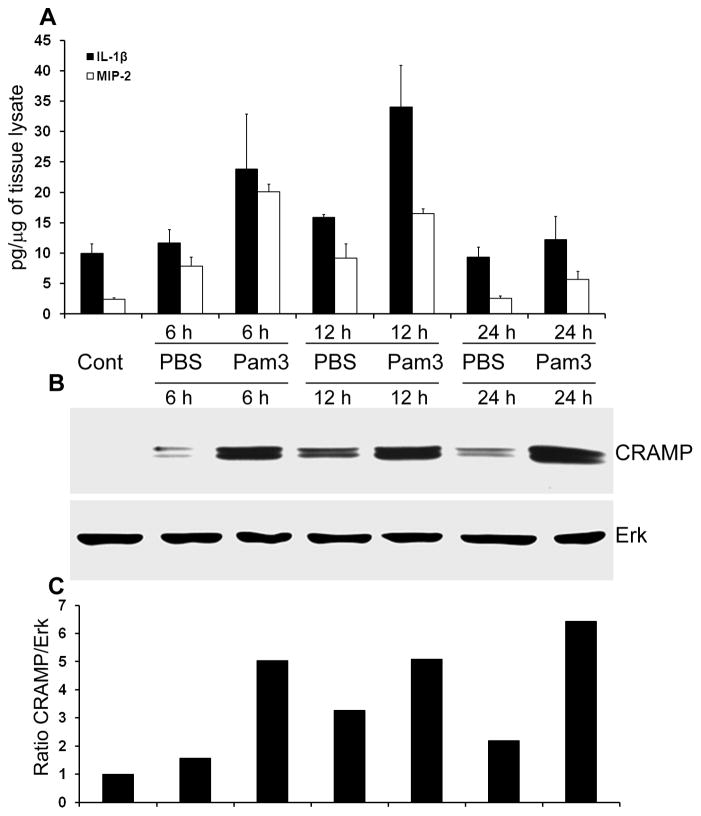
Having shown that Pam3Cys-pretreatment prevented the development of SA endophthalmitis and enhanced bacterial clearance in B6 mice eyes, we next investigated the effect of Pam3Cys on the innate response in vivo by assessing the expression of pro-inflammatory cytokines and the antimicrobial peptide, CRAMP. In the PBS injected eyes, there was slight increase in the levels of both MIP-2 and IL-1β, probably due to needle injury (Figure 5 A). In Pam3Cys-treated eyes, there were moderate increases for these two cytokines compared to the PBS-treated eyes, at 6 and 12 h post treatment. However, by 24 h, the levels of IL-1β in the Pam3Cys-treated eyes were similar to that of the control and PBS injected eyes, while the levels of MIP2 were slightly higher (Figure 5 A). Interestingly, while PBS injection resulted in the induction of CRAMP expression at low to moderate levels, intravitreal injection of Pam3Cys induced abundant CRAMP expression as early as 6 h post-treatment. Remarkably, at 24 h CRAMP levels remained elevated or even higher in Pam3Cys treated eyes, while cytokine levels returned to basal levels, as seen in untreated, normal eyes (Figure 5 B & C).
Figure 5. Pam3Cys induces the expression of proinflammatory cytokines and CRAMP in the mouse retina.
B6 mice were given intravitreal injection of sterile PBS or Pam3cys (0.1μg/eye). At indicated time points, the eyes (n=3 each group) were enucleated and the retinal protein lysate was used for MIP-2 and IL-1β detection by ELISA (A) and western blot analysis of CRAMP expression (B). The band intensity of western blot was quantitated by densitometric analysis and normalized with ERK, used as a protein loading control (C).
Pam3Cys induces the activation of retinal microglia and production of CRAMP
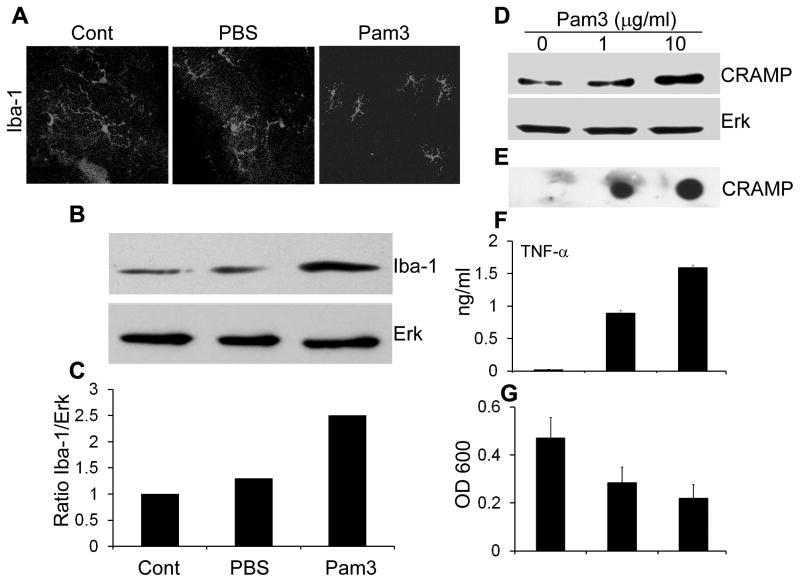
We reasoned that microglia cells, as neural macrophages, might be activated by Pam3Cys pretreatment and used microglia ramification as readout to assess their activation in vivo. Figure 6 shows representative retinal wholemount immunostained with Iba1, a marker for microglial cells. In the control and PBS injected eyes, retinal microglial cells exhibited more ramified morphology, and in the Pam3Cys-treated retina, microglial cells appeared less ramified and started assuming an amoeboid morphology (activated). The numbers of microglial cells in the Pam3Cys-treated retina also appeared to be increased, compared to the control and PBS-injected eyes (Figure 6 A). Moreover, we assessed the levels of Iba-1 expression in the retina using Western blot. The eye treated with Pam3Cys had much higher Iba-1 protein compared to PBS and untreated controls (Figure 6 B & C), suggesting that Pam3Cys induces the activation/proliferation of retinal microglia.
Figure 6. Pam3Cys induces activation of retinal microglia in vivo and production of CRAMP in vitro.
(A) Mice were given intravitreal injection of sterile PBS or Pam3Cys (0.1 μg). After 24 h, the eyes were enucleated, and retinal wholemounts were prepared and stained for microglia specific marker Iba-1. Protein lysate was used for the Western blot detection of Iba-1(B) and densitometric analysis was performed after normalizing with total ERK levels (C). Cultured mouse microglia (BV-2) cells were challenged with indicated concentration of Pam3Cys. After 24 h, cell lysate was used to detect expression of CRAMP by Western blot (D), culture media was used to assess the secretion CRAMP by Dot-blot (E) and TNF-α by ELISA (F). Antibacterial activity of Pam3Cys -challenged media was assessed against SA and presented as mean ± SD of OD 600 at 8h (G).
We next sought to determine whether microglia is a source of Pam3Cys-induced expression of CRAMP and proinflammatory cytokines. BV-2 cells were challenged with Pam3Cys for 24 h and we found that Pam3Cys markedly increased the production and secretion of CRAMP as detected by Western blot (Figure 6 D) and dot-blot (Figure 6 E) respectively. ELISA analysis of cultured media revealed that Pam3Cys significantly increased the secretion of TNF-α (Figure 6 F). To assess the biological function of induced CRAMP expression, we performed antibacterial assay against SA. The conditioned media derived from Pam3Cys-challenged microglia inhibited the growth of SA by ~40% (1μg Pam3) and 55% (10μg Pam3) respectively, as compared to untreated control cells (Figure 6 G). Taken together our data suggest that microglia contributes to Pam3Cys-induced expression of CRAMP and antibacterial defense in the retina.
DISCUSSION
In the present study, we showed that the retina and retinal cells express TLR2, and that signaling via this TLR results in the further induction of its expression. Using a well-characterized mouse model of SA endophthalmitis [25, 28], we demonstrated a novel protective mechanism(s) induced by TLR2 activation against bacterial infection in the retina. We showed that intravitreal injection of Pam3Cys prior to bacterial inoculation significantly attenuated the pathology of SA endophthalmitis, greatly reduced the bacterial load in the retina, and preserved the visual function in B6 mice.
Activation of the innate immune response is the first line of defense after tissue injury and infection [29, 30]. When an infectious agent enters the retina through surgery or injection site, it is critically important for this inflammation-sensitive tissue to have a rapid response system to eliminate invading pathogens, thus minimizing tissue damage to the non-regenerating retina [24]. Recent studies have demonstrated that the innate immune system composed of TLRs is a primary rapid response system to infection [15, 18]. Studies investigating the role of TLRs in retinal innate immunity were initiated recently [24], and TLRs have been linked to viral retinitis, uveitis [31, 32], and geographic atrophy in AMD [33, 34]. To understand the innate response of the retina to infection, we showed that TLR2 is expressed in the retina, suggesting that this neural tissue possesses the ability to recognize Gram-positive bacteria. Since the cells in the retina are at a sterile environment, one might expect that TLRs are expressed at the basal level in the resting state, and that recognition of the pathogen or activation of the TLR will result in up-regulation of the same or different TLRs in the retina. Indeed, we showed that exposure of the retina to TLR2 agonist resulted in the up-regulation of TLR2, whereas SA down regulated its expression. While the reason for the opposite effects of Pam3Cys and SA on TLR2 expression remains elusive, the observed effect of SA is similar to evaded recognition by innate receptors in patients with bacterial sepsis [35]. Thus, we suggest that exposure of the retina to TLR ligands may serve as a danger signal to alert the retinal innate immune system including the up-regulation of TLRs, and this alerted retina is better prepared to combat invading pathogens.
The foregoing conclusion suggests that activation of TLR2 may induce protective mechanisms in a sterile environment such as the retina. We chose Pam3Cys because it is a known activator of TLR2 [27] and a synthetic compound with less cytotoxicity than other bacterial components [36]. Previous studies have shown a protective role of Pam3Cys pretreatment in mouse model of bacterial sepsis [37] and colitis [38]. Interestingly, a recent study showed that intravitreal injection of Pam3Cys has neuroprotective role in a rat model of optic nerve injury [39]. In this study, we observed that at high dosages (1 μg/eye or higher), Pam3Cys caused noticeable inflammation. However, at lower dosages (0.1–0.5 μg/eye), Pam3Cys induced transient and low-level inflammation that disappeared rapidly. More importantly, we showed that Pam3Cys pretreatment attenuated the development of SA endophthalmitis and preserved intact retinal architecture and normal ERG response. Hence, post-surgical application of Pam3Cys or other TLR2 ligands might be used as an adjuvant therapy to antibiotics for prophylactic treatment of ocular surgeries. We recently found that intravitreal injection of Pam3Cys, alone or with antibiotics (vancomycin and ceftazidime) 24 h post SA infection significantly improved the outcome of endophthalmitis in B6 mice (unpublished data). However, further studies are warranted to delineate the underlying mechanisms of use of Pam3Cys for prophylactic and therapeutic actions in the retina.
How might Pam3Cys pretreatment reduce or prevent endophthalmitis? One major factor is the rapid elimination of the invading pathogens in the pretreated retina. We showed that Pam3Cys pretreatment reduced the bacterial load in the retina, indicating that bacterial growth was inhibited in an environment, where rapidly proliferating bacterial population was expected. Hence, the exposure of the retina to TLR ligands results in the induction of a strong, endogenous protective mechanism. The increased bacterial clearance, especially at the early stage of the infection, is a key factor for the eradication of invaded bacteria, marked reduction of inflammation and tissue damage. This, in turn, allows functional recovery of the retina, we detected in Pam3Cys-pretreated eyes after SA infection.
One major group of innate defense molecules induced upon TLR activation is AMPs including defensins and cathelicidin [40–42]. While the TLR-mediated expression of cathelicidin in non-neuronal tissues is well documented [40, 41], its expression and regulation in neural systems are less clear. Here, we showed that Pam3Cys induced an abundant and persistent (24 h, tested) expression of CRAMP. Presence of high levels of CRAMP and/or other antimicrobial peptides within the retina or secreted into the vitreous, in response to TLR activation, may result in greatly enhanced bacterial killing upon contact with pathogens. We speculated that retinal residential cells could be the source of CRAMP, thus we focused our attention on retinal microglia, which are blood-derived resident immune cells of the retina and play an important role in host defense against invading microorganisms, initiation of inflammatory processes, and tissue repair [43]. We hypothesized that Pam3Cys pretreatment should activate microglial cells expressing TLR2 at cell surface [44, 45] and that activated microglia may contribute towards innate killing of the invading pathogens. Consistent with this, we showed that Pam3Cys induces activation/proliferation of retinal microglia. Interestingly, human LL-37 was found in the cerebrospinal fluid of patients with bacterial meningitis but not in healthy control individuals [46]. In an animal model of bacterial meningitis, rat CRAMP was localized in the glia, choroid plexus, and ependymal cells [46]. Our in-vitro studies, using mouse microglia cell line, revealed that Pam3Cys induced the expression of CRAMP in BV-2 cells and the culture media of Pam3Cys-stimulated cells inhibited SA growth. Thus we conclude that Pam3Cys challenged microglial cells contribute to increased expression of CRAMP in the retina. A recent study showed TLR2 activation increases the phagocytotic activity of microglia against bacteria [47], suggesting another potential mechanism of microglia to kill invading pathogens.
In summary, we demonstrate that intravitreal injection of Pam3Cys was efficient in inducing retinal innate immunity in a mouse model of staphylococcal endophthalmitis. We suggest that local delivery of TLR ligands can be explored as a prophylactic/therapeutic strategy to prevent the development of endophthalmitis in patients undergoing ocular surgeries.
Acknowledgments
This work was supported by in part by grants from National Institute of Health, Fight for Sight, Midwest Eye-Banks and an unrestricted Grant from the Research to Prevent Blindness to the Department of Ophthalmology, Wayne State University School of Medicine. Authors thank Dr. David Thomas (VA medical center, Wayne State University) for providing BV-2 cell line, Dr. Robert Galo (University of California San Francisco, CA) for anti-CRAMP antibody, Dr. Vijay Sarthy (Northwestern University) for rMC-1 cell line, and Dr. Jamin Brown (Kresge Eye Institute) for his help in clinical examination of mice. We also thank the lab members for the inspiration and insightful discussion during the study.
Footnotes
Conflict of interest: Authors have no conflict of interest.
REFRENCES
- 1.Cebulla CM, Flynn HW., Jr Endophthalmitis after open globe injuries. Am J Ophthalmol. 2009;147:567–8. doi: 10.1016/j.ajo.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Chiquet C, Cornut P-L, Benito Y, et al. Eubacterial PCR for Bacterial Detection and Identification in 100 Acute Postcataract Surgery Endophthalmitis. Invest Ophthalmol Vis Sci. 2008;49:1971–1978. doi: 10.1167/iovs.07-1377. [DOI] [PubMed] [Google Scholar]
- 3.Krause L, Bechrakis NE, Heimann H, Kildal D, Foerster MH. Incidence and outcome of endophthalmitis over a 13-year period. Can J Ophthalmol. 2009;44:88–94. doi: 10.3129/i08-160. [DOI] [PubMed] [Google Scholar]
- 4.Diago T, McCannel CA, Bakri SJ, Pulido JS, Edwards AO, Pach JM. Infectious endophthalmitis after intravitreal injection of antiangiogenic agents. Retina. 2009;29:601–5. doi: 10.1097/IAE.0b013e31819d2591. [DOI] [PubMed] [Google Scholar]
- 5.Scott IU, Flynn HW., Jr Reducing the risk of endophthalmitis following intravitreal injections. Retina. 2007;27:10–2. doi: 10.1097/IAE.0b013e3180307271. [DOI] [PubMed] [Google Scholar]
- 6.Callegan MC, Gilmore MS, Gregory M, et al. Bacterial endophthalmitis: Therapeutic challenges and host-pathogen interactions. Progress in Retinal and Eye Research. 2007;26:189–203. doi: 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd JC, Braga-Mele R. Incidence of postoperative endophthalmitis in a high-volume cataract surgicentre in Canada. Can J Ophthalmol. 2009;44:288–92. doi: 10.3129/i09-052. [DOI] [PubMed] [Google Scholar]
- 8.Kowalski RP, Romanowski EG, Mah FS, Sasaki H, Fukuda M, Gordon YJ. A comparison of moxifloxacin and levofloxacin topical prophylaxis in a fluoroquinolone-resistant Staphylococcus aureus rabbit model. Jpn J Ophthalmol. 2008;52:211–6. doi: 10.1007/s10384-008-0530-1. [DOI] [PubMed] [Google Scholar]
- 9.Deramo VA, Lai JC, Winokur J, Luchs J, Udell IJ. Visual Outcome and Bacterial Sensitivity After Methicillin-Resistant Staphylococcus aureus-Associated Acute Endophthalmitis. Am J Ophthalmol. 2008 doi: 10.1016/j.ajo.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Callegan MC, Engelbert M, Parke DW, 2nd, Jett BD, Gilmore MS. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev. 2002;15:111–24. doi: 10.1128/CMR.15.1.111-124.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory M, Callegan MC, Gilmore MS. Role of bacterial and host factors in infectious endophthalmitis. Chem Immunol Allergy. 2007;92:266–75. doi: 10.1159/000099277. [DOI] [PubMed] [Google Scholar]
- 12.Tezel G the Fourth ARVO/Pfizer Ophthalmics Research Institute Conference Working Group. The Role of Glia, Mitochondria, and the Immune System in Glaucoma. Invest Ophthalmol Vis Sci. 2009;50:1001–1012. doi: 10.1167/iovs.08-2717. [DOI] [PubMed] [Google Scholar]
- 13.Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev. 2005;18:521–40. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–37. doi: 10.2174/156652406776894572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu FS, Hazlett LD. Toll-like Receptors and the Eye. Invest Ophthalmol Vis Sci. 2006;47:1255–63. doi: 10.1167/iovs.05-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearlman E, Johnson A, Adhikary G, et al. Toll-like receptors at the ocular surface. Ocul Surf. 2008;6:108–16. doi: 10.1016/s1542-0124(12)70279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueta M. Innate immunity of the ocular surface and ocular surface inflammatory disorders. Cornea. 2008;27(Suppl 1):S31–40. doi: 10.1097/ICO.0b013e31817f2a7f. [DOI] [PubMed] [Google Scholar]
- 20.Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90:103–8. doi: 10.1136/bjo.2005.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, Barrett RP, McClellan SA, Hazlett LD. Silencing Toll-like Receptor-9 in Pseudomonas aeruginosa Keratitis. Invest Ophthalmol Vis Sci. 2005;46:4209–16. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Zhang J, Yu F. TLR3 mediates Poly (I:C)-induced antiviral response In human corneal epithelial cells. Immunology. 2006;117:11–21. doi: 10.1111/j.1365-2567.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kindzelskii AL, Elner VM, Elner SG, Yang D, Hughes BA, Petty HR. Toll-like receptor 4 (TLR4) of retinal pigment epithelial cells participates in transmembrane signaling in response to photoreceptor outer segments. J Gen Physiol. 2004;124:139–49. doi: 10.1085/jgp.200409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiston EA, Sugi N, Kamradt MC, et al. alphaB-crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infect Immun. 2008;76:1781–90. doi: 10.1128/IAI.01285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramadan RT, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006;31:955–65. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Zhang J, Yu F-SX. Toll-like receptor 2-mediated expression of [beta]-defensin-2 in human corneal epithelial cells. Microbes and Infection. 2006;8:380–389. doi: 10.1016/j.micinf.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelbert M, Gilmore MS. Fas ligand but not complement is critical for control of experimental Staphylococcus aureus Endophthalmitis. Invest Ophthalmol Vis Sci. 2005;46:2479–86. doi: 10.1167/iovs.04-1139. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi ME, Manfredi AA. IMMUNOLOGY: Dangers In and Out. Science. 2009;323:1683–1684. doi: 10.1126/science.1172794. [DOI] [PubMed] [Google Scholar]
- 30.DeLeo FR, Diep BA, Otto M. Host Defense and Pathogenesis in Staphylococcus aureus Infections. Infectious Disease Clinics of North America. 2009;23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooks JJ, Nagineni CN, Hooper LC, Hayashi K, Detrick B. IFN-{beta} Provides Immuno-Protection in the Retina by Inhibiting ICAM-1 and CXCL9 in Retinal Pigment Epithelial Cells. J Immunol. 2008;180:3789–3796. doi: 10.4049/jimmunol.180.6.3789. [DOI] [PubMed] [Google Scholar]
- 32.Chang JH, Hampartzoumian T, Everett B, Lloyd A, McCluskey PJ, Wakefield D. Changes in Toll-like Receptor (TLR)-2 and TLR4 Expression and Function but Not Polymorphisms Are Associated with Acute Anterior Uveitis. Invest Ophthalmol Vis Sci. 2007;48:1711–1717. doi: 10.1167/iovs.06-0807. [DOI] [PubMed] [Google Scholar]
- 33.Kleinman ME, Yamada K, Takeda A, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards AO, Chen D, Fridley BL, et al. Toll-like Receptor Polymorphisms and Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2008;49:1652–1659. doi: 10.1167/iovs.07-1378. [DOI] [PubMed] [Google Scholar]
- 35.Schaaf B, Luitjens K, Goldmann T, et al. Mortality in human sepsis is associated with downregulation of Toll-like receptor 2 and CD14 expression on blood monocytes. Diagnostic Pathology. 2009;4:12. doi: 10.1186/1746-1596-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moyle PM, Toth I. Self-adjuvanting lipopeptide vaccines. Curr Med Chem. 2008;15:506–16. doi: 10.2174/092986708783503249. [DOI] [PubMed] [Google Scholar]
- 37.Feterowski C, Novotny A, Kaiser-Moore S, et al. Attenuated pathogenesis of polymicrobial peritonitis in mice after TLR2 agonist pre-treatment involves ST2 up-regulation. Int Immunol. 2005;17:1035–46. doi: 10.1093/intimm/dxh282. [DOI] [PubMed] [Google Scholar]
- 38.Cario E, Gerken G, Podolsky DK. Toll-Like Receptor 2 Controls Mucosal Inflammation by Regulating Epithelial Barrier Function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 39.Hauk TG, Leibinger M, Muller A, Andreadaki N, Knippschild U, Fischer D. Intravitreal application of the Toll-like receptor 2 agonist Pam3Cys stimulates axon regeneration in the mature optic nerve. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-4203. [DOI] [PubMed] [Google Scholar]
- 40.Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends in Molecular Medicine. 2007;13:117–124. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 42.Kumar A, Hazlett LD, Yu FS. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect Immun. 2008;76:89–96. doi: 10.1128/IAI.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seung U, Kim JdV. Microglia in health and disease. Journal of Neuroscience Research. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- 44.Chen K, Zhang L, Huang J, Gong W, Dunlop NM, Wang JM. Cooperation between NOD2 and Toll-like receptor 2 ligands in the up-regulation of mouse mFPR2, a G-protein-coupled A{beta}42 peptide receptor, in microglial cells. J Leukoc Biol. 2008;83:1467–1475. doi: 10.1189/jlb.0907607. [DOI] [PubMed] [Google Scholar]
- 45.Yoon HJ, Jeon S-B, Kim I-H, Park EJ. Regulation of TLR2 Expression by Prostaglandins in Brain Glia. J Immunol. 2008;180:8400–8409. doi: 10.4049/jimmunol.180.12.8400. [DOI] [PubMed] [Google Scholar]
- 46.Brandenburg LO, Varoga D, Nicolaeva N, et al. Role of glial cells in the functional expression of LL-37/rat cathelin-related antimicrobial peptide in meningitis. J Neuropathol Exp Neurol. 2008;67:1041–54. doi: 10.1097/NEN.0b013e31818b4801. [DOI] [PubMed] [Google Scholar]
- 47.Ribes S, Ebert S, Czesnik D, et al. Toll-Like Receptor Prestimulation Increases Phagocytosis of Escherichia coli DH5{alpha} and Escherichia coli K1 Strains by Murine Microglial Cells. Infect Immun. 2009;77:557–564. doi: 10.1128/IAI.00903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]