Abstract
We characterized the age-related change in large and small artery compliance in 137 healthy subjects between 9 and 77 years of age. Large artery compliance, measured by diastolic pulse contour analysis, had a sharp positive linear trend (0.89 ml × mmHg−1× year−1) highly correlated with age in subjects younger than 30 years (r = 0.76, p < 0.0001), had a slight negative trend (−0.10 ml × mmHg−1× year−1) not significantly associated with age (r = −0.11, p = 0.532) in middle-aged subjects, and had a sharper negative trend (−0.19 ml × mmHg−1× year−1) significantly associated with age (r = −0.30, p = 0.023) in subjects beyond 50 years. Similar results were found for small artery compliance. Large and small artery compliance increase in children, adolescents, and young adults, reach plateaus near age 30, and then decline beyond 30 years of age in those free of cardiovascular disease and risk factors.
Keywords: Arterial Compliance, Age
INTRODUCTION
Early detection of vascular dysfunction is an important clinical objective to identify those at risk for subsequent cardiovascular events, and to target these individuals for medical and behavioral interventions to reduce cardiovascular risk. Arterial stiffness, or reduced arterial compliance, is a non-invasive marker predictive of occlusive cerebrovascular disease, coronary disease, and peripheral vascular disease.1,2 Arterial compliance is reduced with modifiable risk factors such as smoking,3 hypertension,3 elevated levels of triglycerides,4 and diabetes,5 and is increased with exercise.6-9 Consequently, arterial compliance may be an early marker for cardiovascular disease,1,10 and possibly even a new cardiovascular risk factor.11,12
Age-related decreases in large artery compliance occur even in the absence of coexisting diseases13,14 due to structural changes within the arterial wall. These changes include increased fragmentation and decreased density of elastin,15 increased concentration of collagen,16 hypertrophy of vascular smooth muscle,16 and decreased nitric oxide production.17 Less is known about the age-related changes in small artery compliance. We recently reported that in a group of middle-aged and older subjects with cardiovascular risk factors, small artery compliance was lower in individuals older than 70 years of age.13
Although aging negatively impacts arterial compliance, it is not clear when compliance begins to decline in the large and small arteries in relatively healthy subjects without cardiovascular disease and risk factors. Furthermore, the rates of change in both large and small arterial compliance are not well described. Therefore, the purpose of this study was to characterize the age-related change in arterial compliance in both the large and small arteries in apparently healthy subjects free of cardiovascular disease and risk factors across a wide age span.
METHODS
Subjects
Recruitment
A total of 137 subjects ranging in age from 9 to 77 years participated in this study. The subjects were recruited by local newspaper advertisements and informational flyers distributed in Oklahoma City and surrounding areas. At the beginning of the study visit, subjects agreed to participate by signing the informed consent form approved by the Institutional Review Boards at the University of Oklahoma, and the University of Oklahoma Health Sciences Center.
Medical Screening
Subjects were evaluated during a medical history and physical examination. Demographic information, height, weight, body mass index, waist and hip circumferences, cardiovascular risk factors, co-morbid conditions, blood samples, and a list of current medications were obtained. Subjects were excluded from this study if they were taking medications or had a history of the following conditions: cardiovascular disease, congestive heart failure, cerebrovascular disease, peripheral arterial disease, renal disease, hypertension, dyslipidemia, diabetes, and current smoking. Additionally, subjects who were not taking medications and who did not have a history of these conditions were excluded for the following measurements obtained during the medical screening: systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg triglycerides ≥ 150 mg/dl, cholesterol > 200 mg/dl, high-density lipoprotein cholesterol < 40 mg/dl in men and < 50 mg/dl in women, glucose ≥ 110 mg/dl, creatinine > 1.2 mg/dl, blood urea nitrogen > 21 mg/dl, and obesity defined as a body mass index ≥ 30 kg/m2.
Measurements
Pulse Contour Analysis (PCA)
Arterial compliance measurements were obtained in the morning following an overnight fast of at least eight hours, and prior to engaging in any strenuous physical activity. The large artery elasticity index (LAEI) and small artery elasticity index (SAEI) were obtained by an HDI/Pulsewave™ CR-2000 Cardiovascular Profiling System (Hypertension Diagnostic, Inc., Eagan, Minnesota, USA) following 5 to 10 minutes of rest in the supine position as previously described.13,18-20 To convert values to whole numbers, the units for LAEI (ml × mmHg−1) were multiplied by 10 and the units for SAEI (ml × mmHg−1) were multiplied by 100. An appropriately sized blood pressure cuff was placed around the subject's left upper-arm, and a rigid plastic wrist stabilizer was placed on the subject's right wrist to minimize wrist movement and stabilize the radial artery during the measurement. An Arterial Pulsewave™ Sensor was placed on the skin directly over the radial artery at the point of the strongest pulse, while the arm rested in a supine position. The non-invasive acoustic sensor was adjusted to the highest relative signal strength, and arterial waveforms were recorded for 30 seconds and the diastolic portion was digitized at 200 samples per second to determine large artery and small artery elasticity indices.21 Measurements were averaged over three continuous 30-second trials. The test-retest intraclass reliability coefficient is R = 0.87 for large artery compliance and R = 0.83 for small artery compliance.18
Statistical Analyses
As initial step, a polynomial regression was found that described the central tendency of the data cloud of LAEI over age. Using linear regression models, the linear trend for LAEI as a function of age was found for each of the following three age intervals: 9 to 30 years, 30 to 49 years, and 50 to 77 years. The corresponding slopes, intercepts, and correlation coefficients together with their associated p values were obtained. Analysis of the SAEI data was done in analogous manner. All analyses were performed using the NCSS statistical package. Statistical significance was set at p < 0.05.
RESULTS
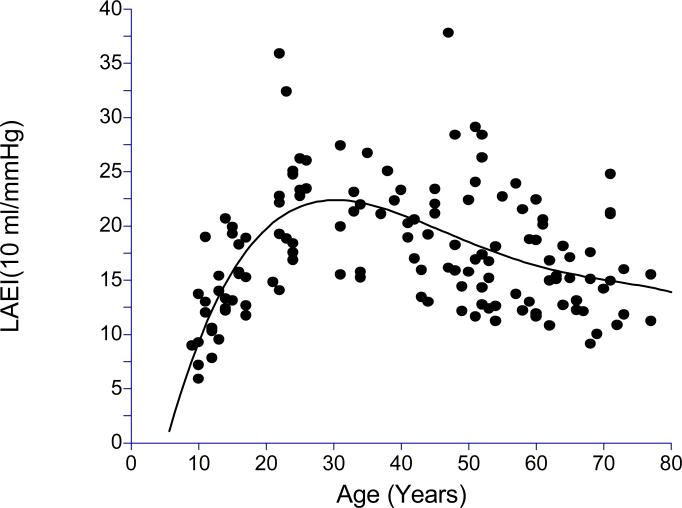
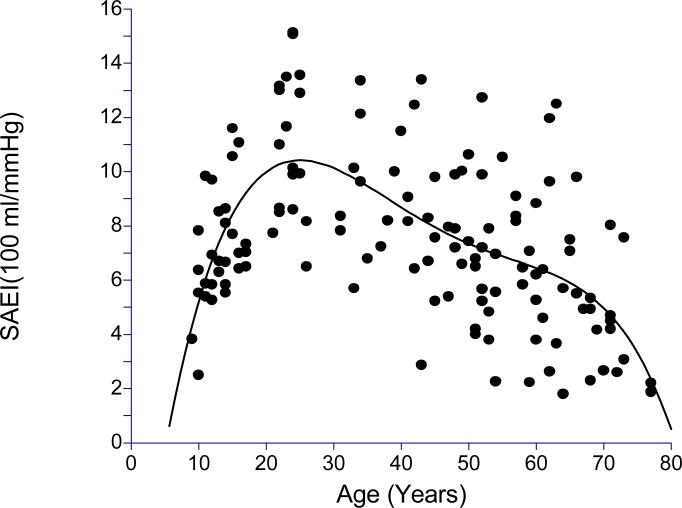
The clinical characteristics of the subjects are displayed in Table 1. The sample consisted of similar numbers of male and female subjects who were predominantly Caucasian. The subjects were apparently healthy with normal values for blood pressure and BMI. Scatter plots together with corresponding best fitting fourth degree polynomials of LAEI and SAEI across age are shown in Figures 1 and 2, respectively. In examination of these plots, both LAEI and SAEI increased with age in the younger subjects, reaching a plateau near 30 years of age, followed by a decline in subjects older than 30 years of age.
Table 1.
Clinical characteristics of 137 subjects. Values are means, SD, minimum and maximum, or percentages.
| Variables | Mean | SD | Minimum - Maximum |
|---|---|---|---|
| Age (years) | 41 | 20 | 9 - 77 |
| Height (cm) | 167.8 | 10.5 | 133.0 – 188.0 |
| Weight (kg) | 66.0 | 14.9 | 28.8 - 100.4 |
| Body Mass Index | 23.1 | 3.5 | 14.8 – 29.0 |
| Waist-Hip Ratio | 0.84 | .09 | 0.70 - 1.21 |
| Systolic Blood Pressure (mmHg) | 116 | 10 | 98 -129 |
| Diastolic Blood Pressure (mmHg) | 66 | 8 | 49 – 84 |
| Large Artery Elasticity | 18.0 | 7.8 | 5.9 - 74.4 |
| Index (ml × mmHg−1) × 10 | |||
| Small Artery Elasticity | 7.9 | 4.3 | 1.8 - 39.1 |
| Index (ml × mmHg−1) × 100 | |||
| Sex (% male) | 48 | ||
| Race (% Caucasian) | 82 |
Figure 1.
Scatter plot of large artery elasticity index (LAEI) across age with fourth degree polynomial regression (R2 = 0.3381, p<0.0001).
Figure 2.
Scatter plot of small artery elasticity index (SAEI) across age with fourth degree polynomial regression (R2 = 0.2536, p<0.0001).
Subsequent analyses were performed to better assess the association of LAEI and SAEI with age in younger, middle-aged, and older subjects by examining the linear trends within three age groups. Results for LAEI are displayed in Table 2. LAEI had a sharp positive linear trend with age (0.89 ml × mmHg−1× year−1) that was highly correlated with age in younger subjects (r = 0.76, p < 0.0001) In middle-aged subjects, LAEI had a slight negative trend (−0.10 ml × mmHg−1× year−1) and was not significantly associated with age (r = −0.11, p = 0.532), whereas older subjects had a sharper negative trend in LAEI (−0.19 ml × mmHg−1× year−1) significantly associated with age (r = −0.30, p = 0.023). The SAEI results shown in Table 3 indicate trends similar to that for LAEI. SAEI had a sharp positive linear trend with age (0.36 ml × mmHg−1× year−1) that was significantly associated with age (r = 0.66, p < 0.0001) in younger subjects. In middle-aged subjects, SAEI had a slight negative trend (−0.10 ml × mmHg−1× year−1) and was not significantly correlated with age (r = −0.24, p = 0.189), whereas older subjects had a slightly steeper negative trend in SAEI (−0.13 ml × mmHg−1× year−1) significantly associated with age (R = −0.36, p = 0.006).
Table 2.
Regression estimates for linear trend of large artery elasticity index regressed on age in three age groups.
| Age Groups | Intercept (95% CI) (ml × mmHg−1) | Slope (95% CI) (ml × mmHg−1× year−1) | R | R2 | P Value |
|---|---|---|---|---|---|
| < 30 years | 1.39 (−2.75 to 5.53) | 0.89 (0.66 to 1.12)** | 0.76 | 0.57 | < 0.0001 |
| 30-50 years | 24.40 (10.68 to 38.11)** | −0.10 (−0.44 to 0.23) | −0.11 | 0.01 | 0.532 |
| ≥ 50 years | 28.39 (18.24 to 38.53)** | −0.19 (−0.36 to −0.03)* | −0.30 | 0.09 | 0.023 |
p < 0.05
p < 0.001.
Table 3.
Regression estimates for linear trend of small artery elasticity index regressed on age in three age groups.
| Age Groups | Intercept (95% CI) (ml × mmHg−1) | Slope (95% CI) (ml × mmHg−1× year−1) | R | R2 | P Value |
|---|---|---|---|---|---|
| < 30 years | 2.44 (0.28 to 4.60)* | 0.36 (0.24 to 0.48)** | 0.66 | 0.44 | < 0.0001 |
| 30-50 years | 12.52 (6.39 to 18.64)** | −0.10 (−0.25 to 0.05) | −0.24 | 0.06 | 0.189 |
| ≥ 50 years | 14.01 (8.38 to 19.64)** | −0.13 (−0.22 to −0.04)* | −0.36 | 0.13 | 0.006 |
p < 0.01
p < 0.001.
DISCUSSION
A novel finding in this study was that large artery compliance was positively correlated with age in healthy, non-obese subjects younger than age 30, indicating that large artery compliance increased by 0.89 units per year with physical maturation. Although several studies have examined the role of obesity on large artery compliance in children and adolescents,22-24 the association with age has not been reported in this age group. We found that large artery compliance declined beyond age 30, and was negatively correlated with age in healthy adults 50 years of age and older . The age-related decline in large artery compliance supports an earlier report that found large artery compliance was negatively associated with age in healthy adults between 21 and 82 years of age,21 and another study that found arterial stiffness, as measured by aortic pulse wave velocity and augmentation index, increased with age in healthy adults between 21 and 96 years of age.9 Other investigations have found increased large artery stiffness in asymptomatic subjects above 55 years of age,14 and decreased large artery compliance in asymptomatic subjects above 70 years of age.13
Another unique finding in this investigation was that small artery compliance increased with age in subjects younger than age 30, similar to the results for large artery compliance. Thus, the compliance of the microvasculature increases with physical maturation in healthy, non-obese young subjects, a finding that has not been reported in previous studies. Beyond 30 years of age, small artery compliance declined and was negatively correlated with age in those age 50 and older. This supports a previous study reporting a correlation of −0.551 between small artery compliance and age in subjects between 21 and 82 years of age,21 and an earlier investigation in our laboratory showing that subjects over 70 years of age had a 27% lower small artery compliance than those younger than 70.13 The negative association between small artery compliance and age was not as strong in the current study, probably because the middle-aged and older subjects were carefully screened for cardiovascular risk factors. Collectively, these studies demonstrate that a decline in microvasculature function occurs with age even in healthy adults, particularly beyond age 70.
There are several limitations to this study. The cross-sectional research design of this study does not allow causality to be established when examining the relationship between arterial compliance and age. Another limitation is that diastolic PCA is a non-invasive technique to determine large and small artery compliance.21,25,26 However, this technique has been validated with invasive measures of arterial compliance25 and provides reliable measurement of arterial compliance.18 The present findings are also limited to healthy, non-obese subjects free of cardiovascular disease and cardiovascular risk factors. This rather strict inclusion of subjects was done to better assess the association between age and arterial compliance by minimizing the impact that comorbid conditions have on arterial compliance.18,19
In conclusion, both large and small artery compliance increase in children, adolescents, and young adults, reach plateaus near 30 years of age, and then decline gradually in those older than 30 years of age who are free of cardiovascular disease and risk factors. Future studies should focus on whether different trajectories of large and small artery compliance occur in subjects with cardiovascular and metabolic risk factors to better understand when interventions may be most optimal to improve arterial compliance.
Acknowledgments
This research was supported by the University of Oklahoma Health Sciences Center General Clinical Research Center grant (M01-RR-14467), sponsored by the National Center for Research Resources from the National Institutes of Health.
REFERENCES
- 1.Grey E, Bratteli C, Glasser SP, et al. Reduced small artery but not large artery elasticity is an independent risk marker for cardiovascular events. Am J Hypertens. 2003;16:265–269. doi: 10.1016/s0895-7061(02)03271-5. [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 3.Cohn JN, Finkelstein SM. Abnormalities of vascular compliance in hypertension, aging and heart failure. J Hypertens Suppl. 1992;10:S61–S64. [PubMed] [Google Scholar]
- 4.Prisant LM, Resnick LM, Hollenberg SM. Arterial elasticity among normotensive subjects and treated and untreated hypertensive subjects. Blood Press Monit. 2001;6:233–237. doi: 10.1097/00126097-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 5.McVeigh G, Brennan G, Hayes R, Cohn J, Finkelstein S, Johnston D. Vascular abnormalities in non-insulin-dependent diabetes mellitus identified by arterial waveform analysis. Am J Med. 1993;95:424–430. doi: 10.1016/0002-9343(93)90313-e. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol. 1994;266:H693–H701. doi: 10.1152/ajpheart.1994.266.2.H693. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Desouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, Desouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 9.Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN. Vascular wall function as a risk marker for cardiovascular disease. J Hypertens Suppl. 1999;17:S41–S44. [PubMed] [Google Scholar]
- 11.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140:669–682. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- 12.Hodes RJ, Lakatta EG, McNeil CT. Another modifiable risk factor for cardiovascular disease? Some evidence points to arterial stiffness. J Am Geriatr Soc. 1995;43:581–582. doi: 10.1111/j.1532-5415.1995.tb06111.x. [DOI] [PubMed] [Google Scholar]
- 13.Fjeldstad AS, Montgomery PS, Gardner AW. Age-related differences in arterial compliance are independent of body mass index. Angiology. 2008;59:454–458. doi: 10.1177/0003319707306455. [DOI] [PubMed] [Google Scholar]
- 14.Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (Lond) 2002;103:371–377. doi: 10.1042/cs1030371. [DOI] [PubMed] [Google Scholar]
- 15.Avolio A, Jones D, Tafazzoli-Shadpour M. Quantification of alterations in structure and function of elastin in the arterial media. Hypertension. 1998;32:170–175. doi: 10.1161/01.hyp.32.1.170. [DOI] [PubMed] [Google Scholar]
- 16.Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7:29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- 17.Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 18.Acree LS, Montgomery PS, Gardner AW. The influence of obesity on arterial compliance in adult men and women. Vasc Med. 2007;12:183–188. doi: 10.1177/1358863X07079323. [DOI] [PubMed] [Google Scholar]
- 19.Fjeldstad AS, Fjeldstad C, Acree LS, et al. The relationship between arterial elasticity and metabolic syndrome features. Angiology. 2007;58:5–10. doi: 10.1177/0003319706297911. [DOI] [PubMed] [Google Scholar]
- 20.Nickel KJ, Acree LS, Montgomery PS, Gardner AW. Association between lower-extremity function and arterial compliance in older adults. Angiology. 2008;59:203–208. doi: 10.1177/0003319707306143. [DOI] [PubMed] [Google Scholar]
- 21.Duprez DA, Somasundaram PE, Sigurdsson G, Hoke L, Florea N, Cohn JN. Relationship between C-reactive protein and arterial stiffness in an asymptomatic population. J Hum Hypertens. 2005;19:515–519. doi: 10.1038/sj.jhh.1001860. [DOI] [PubMed] [Google Scholar]
- 22.Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging. 2008;28:287–293. doi: 10.1111/j.1475-097X.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 23.Singhal A, Farooqi IS, Cole TJ, et al. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–1924. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 24.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 25.Cohn JN, Finkelstein S, McVeigh G, et al. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26:503–508. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein SM, Cohn JN. First- and third-order models for determining arterial compliance. J Hypertens Suppl. 1992;10:S11–S14. [PubMed] [Google Scholar]