Abstract
Vertebrates vary in resistance and resilience to infectious diseases, and the mechanisms regulating the trade-off between these two often opposing protective processes are not well understood. Variability in the sensitivity of species to induction of damaging inflammation in response to equivalent pathogen loads (resilience) complicates the use of animal models that reflect human disease. We found that induction of pro-inflammatory cytokines from macrophages in response to inflammatory stimuli in vitro is regulated by proteins in the sera of species in inverse proportion to their in vivo resilience to lethal doses of bacterial lipopolysaccharide over a range of 10,000-fold. This finding suggests that proteins in serum rather than intrinsic cellular differences may play a role in regulating variations in resilience to microbe-associated molecular patterns between species. Involvement of circulating proteins as key molecules raises hope that the process might be manipulated to create better animal models and potentially new drug targets.
Keywords: Inflammation, species, cell activation, endotoxic shock, cytokines
INTRODUCTION
Two recent articles have proposed that animals, like plants, have evolved two different strategies to protect against infectious diseases: resistance and tolerance (called in this article “resilience” to distinguish it from previously described uses of the term “tolerance”) (1;2). The mechanisms underlying resistance to infection (the ability to limit pathogen burden) are reasonably well studied and are related to induction of inflammation by the host (1). In contrast, the mechanisms that underlie resilience (the ability to tolerate infections for an equivalent pathogen load by limiting harmful consequences of inflammation) are less well studied (1;2). Resistance and resilience are often inversely correlated, and there is a tradeoff between the two frequently opposing strategies (2). There is little known about the mechanisms that determine the balance of this trade-off between different vertebrate species.
Most animal models are utilized for the purpose of generating results that can be extrapolated to human diseases. For example, in drug development, rodent studies are part of routine pre-clinical studies that determine progression to clinical studies in humans. Mice are the most commonly utilized species in biomedical research. Advantages of mice as opposed to other species as the top choice of species for animal models include: cost, size, public acceptance, availability of reagents, rapid generation time, and ease of genetic manipulation. However, a problem with this approach for the study of inflammation is that rodents are highly resilient to most models of induced inflammation compared to humans. One of the most common assays used to assess novel pathways of inflammation by academic investigators and pharmaceutical companies alike consists of challenging mice with the Gram-negative bacterial cell wall molecule, lipopolysaccharide (LPS), which activates cells through Toll-like receptor 4 (TLR4). Most wild type mice are highly resilient to challenge with LPS. The dose of LPS utilized in most in vivo studies is 1–25 mg/kg, which leads to death in about half of the mice (3–6). This dose is about 1,000,000 times the 2–4 ng/kg dose of LPS utilized in human volunteer studies to induce fever and cytokines (7), and about 1000–10,000 times the dose required to induce severe disease with shock in humans (8;9). It is generally assumed that this marked difference in resilience to pro-inflammatory molecules such as LPS is related to cellular differences between species. The studies reported here were undertaken to better understand the mechanism(s) involved.
Direct comparison of the sensitivity of macrophage stimulating agonists between mice and humans to agents other than LPS is limited by the safety of challenging humans. However, the resilience of mice to challenge with inflammatory agonists is probably not limited to LPS. For example, mice are at least several orders of magnitude more resilient to the superantigen Staphylococcal enterotoxin B compared to rabbits (10;11), which are similar to humans in sensitivity to LPS (12).
Acute lethal toxicity from bacteria, LPS, or superantigens occurs through the release of cytokines that induce fever and shock prior to death. Tumor necrosis factor (TNF) is the primary cytokine involved because antibodies directed to TNF block lethality induced by LPS and bacteria (12–14). This process is believed to be macrophage dependent, because replacement of macrophages in mice unable to respond to LPS due to a mutation in TLR4 with macrophages from sensitive animals restores sensitivity (15;16). Many assays have been developed to mimic this concept in vitro by utilizing isolated macrophages in cell culture to which different agonists are added, followed by quantification of TNF. This type of in vitro assay often forms the basis of studies to assess inflammatory pathways or candidate anti-inflammatory drugs for further development.
The discrepancy of several orders of magnitude in the sensitivity of mice and humans to LPS in vivo is not paralleled by the response of macrophages from the two species when the macrophages are removed and tested in cell culture after 1 to 24 hours incubation. Low and roughly similar concentrations of LPS (pg/ml) or other TLR agonists readily stimulate TNF and other mediator production from primary mouse macrophages or human (blood) monocytes or cell culture lines from each species when grown in cell culture under identical conditions. This dichotomy in response suggested to us that cells of the macrophage lineage might behave differently in vitro than in vivo.
To explore potential mechanisms that would explain the difference in sensitivity between mice and humans to pro-inflammatory macrophage stimuli, we studied the production of cytokines from human and mouse macrophages in response to different agonists in whole blood and in the microenvironment of mouse and human serum. These studies indicated that proteins in mouse serum markedly suppress the induction of pro-inflammatory cytokines compared to human serum. We broadened these studies to include sera from additional species that vary markedly in sensitivity to LPS and found a striking inverse correlation between ex vivo macrophage suppression by sera from different species and the published lethal sensitivity to LPS in these same species. These data support the concept that one mechanism for the large difference in resilience between species to pro-inflammatory macrophage stimuli may be regulation by proteins in serum rather than intrinsic cellular differences.
MATERIALS AND METHODS
Reagents, cells, and cytokine assays
Bacterial products and macrophage agonists were obtained as follows: Zymosan (Sigma), CpG (Sigma), Staphylococcal Toxic Shock toxin-1 (TSST-1) (Toxin Technology), LPS O111:B4 (List Biological Laboratories, and Sigma), Pam3CysSK4 (P3C) (EMC Microcollections). Peptidoglycan associated lipoprotein (PAL) was prepared as described (17). Heat killed bacteria were prepared by growing E. coli O18 in broth followed by double boiling for 30 minutes and extensive washing by centrifugation prior to use.
The animal care for these studies was in accordance with the institutional guidelines. The preparation of elicited peritoneal and bone marrow derived macrophages (BMDM) in mice, cell based cytokine assays, and the Limulus lysate assays were performed as described in the presence of the reagents indicated (17–19). Mouse peripheral blood monocytes were prepared from blood obtained after intra-cardiac puncture by centrifugation in Lympholyte-Mammal (Cedarlane, Hornby, Canada).
Human monocytes were purified by adherence after isolation of peripheral blood mononuclear cells from human blood samples as previous described (18). Monocyte lysates were generated after three freezing-thawing cycles. Human neutrophils were purified and studied as described (20). The whole blood assays were performed using fresh human or fresh mouse blood diluted 1:4 as described (21). For the protease experiments, mouse sera were exposed to trypsin conjugated to beads (Pierce) at a concentration of 0.1ml beads/ml protein for 22 h at 37 °C before testing on cells. Mouse sera were obtained different mouse strains as described in results. Mouse sera from lipopolysaccharide binding protein (LBP) KO mice and CD14 KO mice and control mice were the kind gift of Centre Nationale de la Research, Orleans, France. Wild-derived mice sera were from the Institute Pasteur animal facility.
Murine TNF, IL-6, and IL-10 and human TNF, IL-1β, IL-1α, IL-6, IL-8, IL-1ra, and IL-10 were measured with R&D System kits according to the manufacturers’ instructions.
Electrophoretic Mobility Shift Assay (EMSA), Western Blot analysis of p38 and erk, and qPCR
EMSA was carried out on nuclear extracts of activated human monocytes as previously described (22). Western blot analysis was performed using anti–phospho-p38, anti-p38, anti-phospho-erk and anti-erk antibodies (Cell Signaling, Beverly, MA) on cytoplasmic extracts as previously described (23). Densitometry analysis was performed using the NIH Image software.
Total RNAs were extracted from monocytes by RNeasy miniprep kit (QIAGEN, USA) following the manufacturer’s protocol and cDNA was generated by reverse transcription. Quantitative real time polymerase chain reaction (qPCR) (Stratagen MX3005P®) was performed using Brilliant®II SYBR®Green QPCR Master mix (Invitrogen, Cergy Pontoise, France) and TNF and GADPH primers (Invitrogen, Cergy Pontoise, France). PCR program was 95°C for 10 min, followed by 40 cycles at 95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec. Specificity of the SYBR green-amplified product was confirmed by dissociation curve analysis. The transcript amount for TNF gene was normalized to the house keeping gene, GAPDH. MyD88s, SIGIRR, SOCS1, SOCS3, and Tollip primers were designed as previously described (24). The IRAK-M primer was obtained from SABiosciences Corporation (Frederick, MD).
Data for the sensitivity to LPS in different species
Data for the sensitivity to LPS for the different species in Table 2 and Figure 5 were obtained from the literature as follows: human (induction of shock in two reports of self administration) (8;9), rabbits (12;25), sheep (26;27), calves (induction of severe disease) (28), dogs (29–31), guinea pigs (4), hamsters (4), mice (3–5), rats(4;29), rhesus monkey (no deaths)(25), chickens (no deaths)(32;33), lizards (no deaths) (29). Human, mouse (Balb/C, C3H/HeN, C57Bl/6, CD-1), and rabbit sera were prepared in our laboratories and stored at – 80 °C in aliquots prior to testing. Sera from other species tested were obtained as follows: sheep (Sigma), calf (Sigma), dog (Sigma), guinea pig (Sigma), hamster (Sigma), rat (Sigma), rhesus monkey (kind gift of Alan Cross, University of Maryland), chicken (Sigma), turtle (Sigma). We were unable to find information regarding the lethal dose of LPS for turtles, and have utilized the information for lizards in its place as noted. We have used 15 µg/kg for the dose of LPS required to induce shock in humans in Figure 2 and Table 2 because the report with this dose is better documented (8).
Statistical Methods
Results are shown as the mean +/− s.e.m of two replicates per data point for a single experiment representative of the total number of experiments (n). Unless otherwise specified, results were analyzed by 2-way ANOVA with post-hoc Bonferroni-adjusted tests of the species effect at each dose level. Significance is indicated with stars, where P <0.05 = *, P <0.01 = **, P <0.001 = ***. Spearman's rank correlation coefficient was calculated for the scatter-plots shown in Figure 2.
RESULTS
Comparison of cytokine production from ex vivo stimulation of mouse and human whole blood
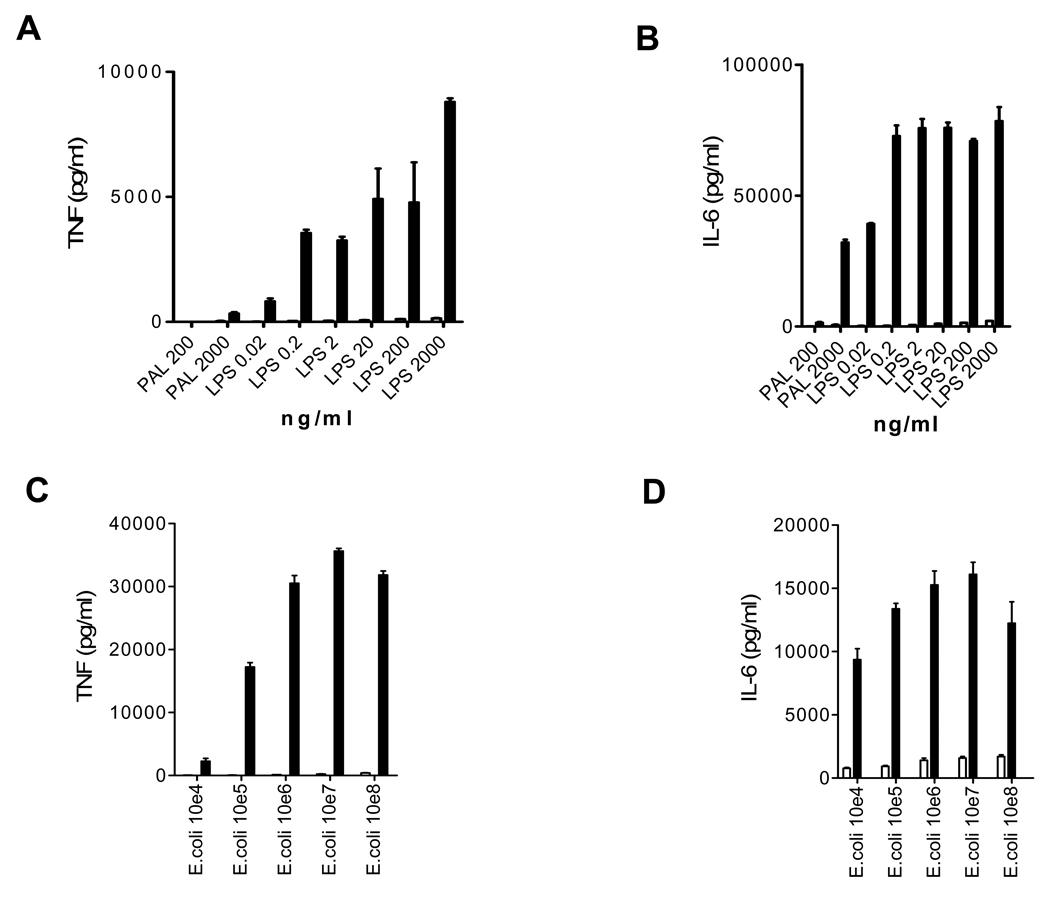
We first added LPS or the bacterial cell wall TLR2 agonist peptidoglycan-associated lipoprotein (PAL)(17) to fresh whole blood drawn from mice and humans, and measured TNF and IL-6 18 hours later (Fig 1A, 1B). As expected, in the human blood both agonists stimulated large amounts of TNF and IL-6. Remarkably and in very striking contrast, extremely low amounts of each cytokine were stimulated in mouse blood. These findings were confirmed using a biological assay to detect the TNF (data not shown). Similar findings were obtained using whole blood stimulated with heat killed E. coli bacteria (Fig 1C, 1D). Thus, the very large in vivo discrepancy between humans and mice in sensitivity to LPS and also to bacteria is reproduced in vitro in whole blood in an ex vivo system.
Figure 1. Cytokine induction in whole blood samples.
A and B: LPS and peptidoglycan associated lipoprotein (PAL) induce much more TNF and IL-6 in 20% human whole blood than in 20% mouse whole blood. Data are reflective of 5 experiments. C and D: Heat killed E. coli bacteria induce much more TNF and IL-6 in 20% human whole blood than in 20% mouse whole blood. Data are reflective of 2 experiments. In all panels, human blood is depicted by solid bars, and mouse blood is depicted by open bars. Data are shown for mouse whole blood in all panels but cytokine responses are so low that they are barely visible in panels A–C despite high stimuli concentrations.
Effect of mouse and human serum on isolated mouse and human mononuclear cells
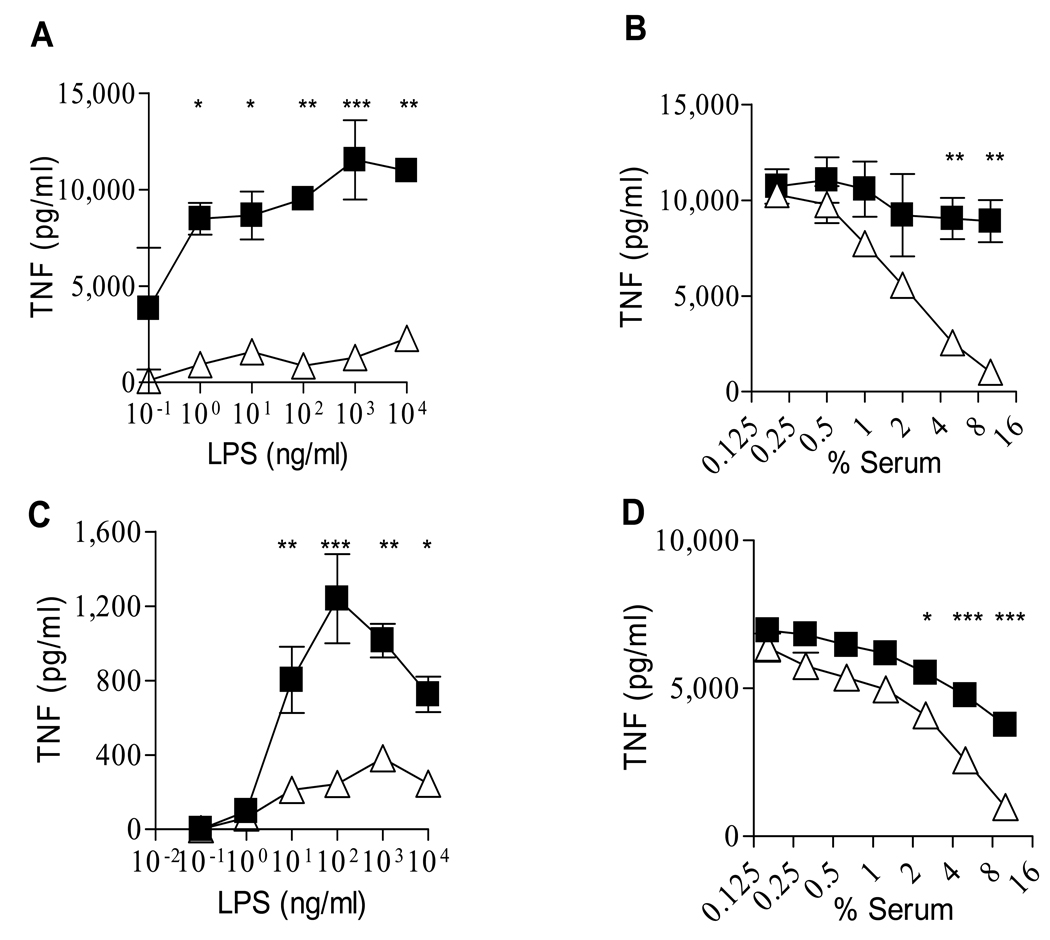
We next studied the effect of mouse and human serum on isolated human peripheral blood monocytes. Mouse serum suppressed LPS-induced cytokines from human monocytes to a much greater extent than human serum and in a dose-dependent manner (Fig 2A and 2B). The inhibitory effect of mouse serum was not observed on IL-10 production by activated human monocytes, and on IL-8 and IL-1 receptor antagonist production by isolated and LPS-activated human neutrophils (data not shown).
Figure 2. Effect of serum on LPS-induced TNF production by isolated mononuclear phagocytes.
A. 10% mouse serum (open triangles) suppresses LPS-induced TNF production from human monocytes relative to 10% human serum (closed squares); reflective of 4 experiments. B. Dose dependent suppression of mouse serum (open triangle) on TNF induced from human monocytes by 1 ng/ml LPS in comparison with human serum (closed squares); reflective of 3 experiments. C. Mouse serum (10%) (open triangles) suppresses TNF from murine elicited peritoneal macrophages more than human serum (10%)(closed squares); reflective of 5 experiments. D. Dose effect of suppression of mouse (open triangles) and human (closed squares) serum on TNF induced from murine elicited peritoneal macrophages by 200 ng/ml LPS; reflective of 5 experiments.
We also compared the effect of mouse and human serum on mouse elicited peritoneal macrophages. Mouse serum suppressed LPS-induced TNF from peritoneal macrophages to a greater extent than human serum at high LPS doses (Fig 2C and 2D). Similar findings were obtained with mouse bone marrow derived macrophages and to a lesser extent on mouse monocytes and alveolar macrophages (data not shown).
The decrease in TNF when monocytes were exposed to mouse compared to human serum was present for other TLR agonists, superantigenic toxin (TSST-1), whole bacteria (E. coli) and a complex fungal structure (Zymosan) (Table 1). Furthermore, the inhibitory property of mouse serum was also revealed when measuring released IL-1β or cell-associated IL-1α. These findings provided further confirmation that the suppressive effect of mouse serum occurred to a wide variety of microbial stimuli when tested on cells derived from both mice and humans and was observed on different key inflammatory cytokines with different release process and mechanisms of action during the inflammatory process. The effect was not caused by toxicity to the cells, because mouse sera did not cause toxicity to cells as assessed by the tetrazolium salt assay (MTT test) (34) or by staining the cells with crystal violet. In addition, mouse sera would not be expected to be toxic to mouse cells.
Table 1.
Percent change in cytokine production by human monocytes in the presence of 10% mouse serum as compared to 10% human serum
| TNF | released IL-1β | IntracellularIL-1α | |||||
|---|---|---|---|---|---|---|---|
| Stimulus | exp. I | exp. II | exp. I | exp. II | exp. I | exp. II | |
| E. coli LPS | 1 ng/ml | −93 | −98 | −84 | −88 | −58 | −55 |
| Peptidoglycan | 10 µg/ml | −98 | −87 | −84 | −57 | −46 | −13 |
| Zymosan | 100 µg/ml | −97 | −95 | −67 | −32 | −17 | −17 |
| TSST-11 | 10 µg/ml | −97 | −81 | nd3 | −77 | −53 | −34 |
| CpG | 6 µg/ml | −88 | −85 | nd | −67 | −99 | −96 |
| PAL2 | 4 µg/ml | −83 | −74 | −100 | −53 | −28 | −64 |
Toxic shock syndrome toxin-1 from Staphylococcus aureus
Peptidoglycan associated lipoprotein
not done
Effect of sera from different mouse strains on monocytes
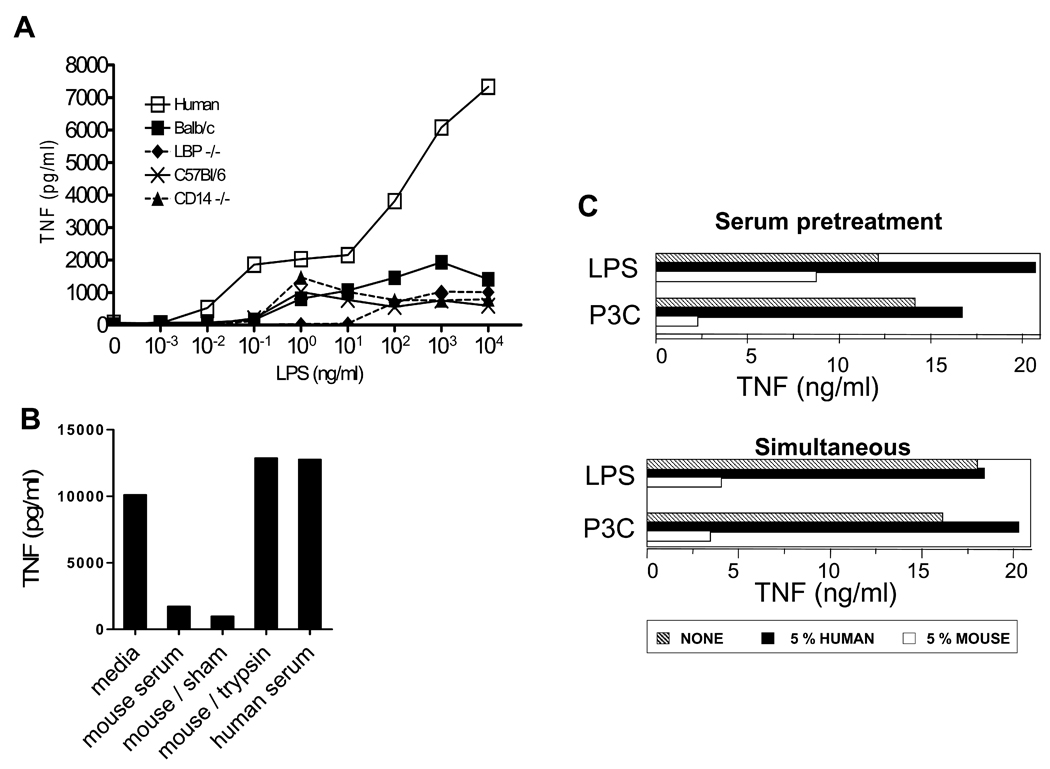
There were only slight differences in the suppression caused by sera from laboratory mice (C3H/HeN, Balb/C, C57Bl/6, and CD-1) and wild-derived mouse strains (not shown), and there was no difference between sera from aged or young mice (not shown) or with sera from galactosamine injected mice (data not shown). The effect was independent of two plasma proteins known to be important in some TLR agonist-cell interactions, CD14 and LPS binding protein, because mouse sera from KO mice lacking each of these proteins still suppressed TNF production from human monocytes similarly to sera from wild type mice (Fig 3A).
Figure 3. Comparison of sera from different mouse strains, effect of trypsin treatment and of timing.
A. TNF production by human monocytes in response to increasing amounts of LPS in the presence of 5% human serum (open squares) or 5% sera from different mouse strains: Balb/c (close squares), C57Bl/6 (crosses), LPS binding protein (LBP) deficient mice (diamonds), and CD14 deficient mice (closed triangles). B. LPS-induced TNF by human monocytes in the presence of 5% native mouse serum after exposure of mouse serum to trypsin-conjugated beads or sham beads (n=2 for monocytes; similar results were obtained using mouse macrophages, n=2). C. The inhibitory effect of mouse serum on human monocytes does not require their simultaneous presence with the TLR agonists. The sera (5%) were either added for 3h and then cells were extensively washed before activation for 18h with LPS or Pam3CysSK4 (P3C), (labeled Serum pretreatment); or cells were left without serum for 3h and then cultured for 18h in the presence of human or mouse serum (5%) and LPS or Pam3CysSK4 (P3C), (labeled Simultaneous); representative of 2 experiments. Similar results were obtained with BMDM, n = 2.
Effect of heating and trypsin on suppressive activity
The suppressive effect of the mouse serum on the human monocytes and mouse macrophages was not changed by heating the serum at 56 °C for 30 minutes to inactivate complement (data not shown), but was sensitive to exposure of the serum to trypsin conjugated to beads (fig 3B).
Mouse serum acts on cellular signaling pathways of cells rather than binding and neutralization of LPS
In contrast to the results on cells, after LPS was incubated in mouse or human serum for 3 hours, there was no difference in the activation of Limulus Amoebocyte lysate (data not shown). This result suggests that the suppression was not simply a direct neutralization of LPS by substances in the mouse serum, at least as assessed by activation of the Limulus Amoebocyte lysate cascade.
We next studied if the simultaneous presence of mouse serum and TLR2 or TLR4 agonists was required to observe a reduced production of TNF by human monocytes. When cells were incubated for three hours with human or mouse serum, extensively washed and further incubated for 18h in the presence of the TLR agonists in the absence of serum, the production was far lower after the pre-culture in the presence of mouse serum than in the presence of human serum (fig. 3C). Similar findings were obtained after pre-treatment of murine BMDM with mouse serum before LPS activation (data not shown).
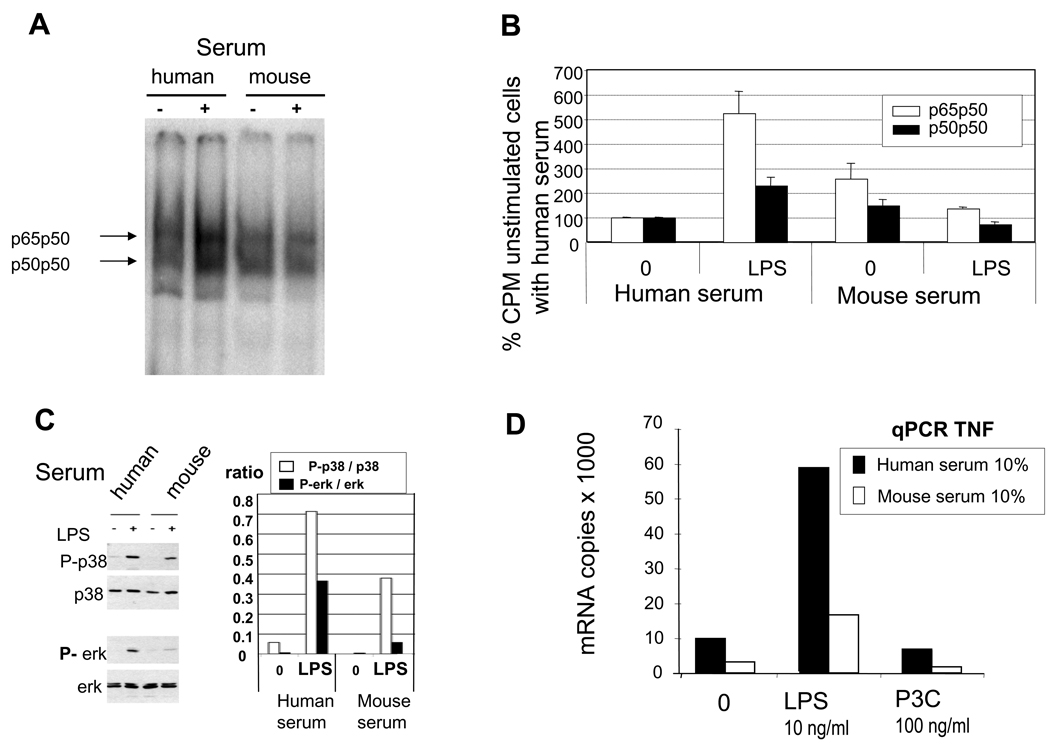
We studied whether the inhibitory effect of mouse serum was observed in cell signaling pathways downstream of TLR4. As shown in figure 4A and 4B, both forms of NF-κB (p50p50 and p65p50) were less present in the nuclei of human monocytes activated with LPS in the presence of mouse serum as compared to human serum as revealed by EMSA analysis. Similarly, the phosphorylation of p38 and erk mitogen-activated protein (MAP) kinase was less pronounced when cells were cultured in the presence of mouse serum rather than in human serum (fig 4C). These events were associated with a marked reduction of mRNA coding for TNF in the presence of mouse serum (Fig.4D). No up-regulation of mRNA expression was observed for MyD88s, SIGIRR, SOCS1, SOCS3, Tollip and IRAK-M, all of which have been described to limit the TLR4 signaling cascade (35), (data not shown).
Figure 4. Effect of mouse serum on intracellular signaling and TNF mRNA production.
A. Electrophoretic mobility shift assay (EMSA) to determine the presence of NF-κB in nuclei of human monocytes activated for 45 minutes by LPS (10 ng/ml) in the presence of 5% mouse or human serum. B. Quantification of NF-κB complexes identified by EMSA, n=2. C. Activation of p38 MAPK and erk MAPK in human monocytes cultured in the presence of 5% human or mouse serum and in the absence or presence of LPS (1 ng/ml), and quantification of phosphorylated forms as compare to native form. D. Assessment of TNF mRNA by qPCR in human monocytes cultured for 3 h in the presence of human or mouse serum (10%) and either LPS (10 ng/ml) or Pam3CysSK4 (P3C),(100 ng/ml), n = 3.
Correlation of suppression of cytokine production by sera from different species with reported sensitivity to LPS in vivo
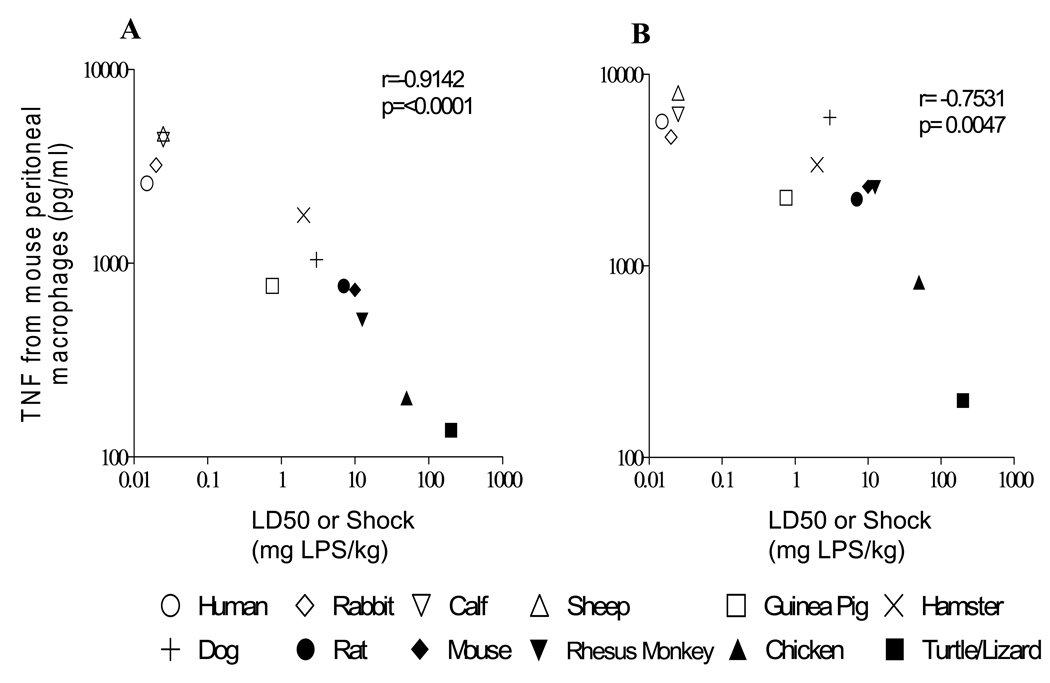
Lethal toxicity to LPS varies almost 10,000-fold in different species (Table 2). Although mice fall towards the resilient end of the reported dose response, some species such as chickens and lizards are considerably more resilient to LPS than mice. To evaluate whether lethal toxicity to LPS in widely diverse species could be predicted by the suppressive activity of sera obtained from these species in vitro, we measured the LPS-induced TNF production from mouse elicited peritoneal macrophages in the presence of 5% and 20% serum from each of the species in Table 2. TNF production from mouse peritoneal macrophages in the species sera was inversely proportional to the published lethal dose at which 50% of the animals expire (the LD50) for each species (r=−0.75 to −0.91, p<0.005) (Figure 5).
Table 2.
Dose response of different animal species to lipopolysaccharide1
| Animal | Response | LPS dose (mg/kg) |
|---|---|---|
| Human | shock | 0.015 |
| Rabbits | LD100 | 0.02 |
| Sheep | LD100 | 0.025 |
| Calves | Shock | 0.025 |
| Guinea Pigs | LD50 | 0.75 |
| Hamsters | LD50 | 2 |
| Dogs | LD50 | 3 |
| Rats | LD50 | 7 |
| Mice | LD50 | 10 |
| Rhesus Monkey | no deaths | 12.5 |
| Chicken | no deaths | 50 |
| Turtles/Lizards | no deaths | 200 |
Assembled from published reports as described in Methods and modified from Clark et al (29).
Figure 5. LPS-induced TNF from mouse macrophages in the presence of species sera is inversely proportional to the lethal sensitivity of each species to LPS.
Data are shown for concentration of TNF in culture supernatants from elicited mouse peritoneal macrophages incubated with 20ng/ml LPS for 18h in the presence of 20% serum (A) or 5% serum (B) from each species. LD50 or LD100 or shock for each species is listed in Table 2, and was derived from prior publications, as described in Methods. For rhesus monkey, chicken and turtles, symbols represent published doses of LPS at which there were no effect. In each plot, turtle serum was tested but information regarding lethality was obtained for lizards, as stated in Methods. For the data shown, the correlation remained significant when this point was removed from analysis. Open symbols were chosen to represent species with LD50 or shock < 1 mg LPS/kg and closed symbols were chosen to represent species with LD50 or shock > 5 mg LPS/kg.
DISCUSSION
Taken together, these experiments suggest that species contain proteins in serum that suppress macrophage production of inflammatory cytokines such as TNF, IL-1β, IL-1α and IL-6 to markedly different extents. The close inverse correlation of ex vivo macrophage inhibition with the published lethal dose of LPS in each species and the dependency of LPS-induced death on TNF make it tempting to hypothesize that these proteins are in some way related to differential LPS-induced cytokine production in each species. This difference is particularly striking between mice and humans, and seems especially important to understand given the frequent use of mice to study infectious diseases in humans.
Our experiments call attention to a paradox that has received little attention. Evidence that the innate immune response to microbe-associated pattern molecules is important in the protection of mice against infection comes from decades old studies reporting that mice which have defective TLR4 signalling are less resistant to Gram-negative bacterial infection than wild type mice (36), as well as from a more recent study in which mice were shown to have increased resistance to Y. pestis that was engineered to contain LPS with increased TLR4 activation (37). If early immune cell activation through TLR agonist ligands such as LPS is important for resistance to infection, how can mice which have such a markedly decreased response to LPS limit infection in tissues? One possibility is that there could be differential responses to inflammatory challenge in different compartments in mice so that there is adequate inflammation induced locally in tissues, but that inflammation is controlled in blood to limit potentially dangerous systemic inflammation (38).
Our findings support and extend the ideas presented in two recent articles that discuss the trade-off between resistance and resilience as defense mechanisms for surviving infection (1;2). The large difference in resilience between species to LPS, and likely to other TLR agonists, indicate that vertebrates have evolved with substantially different set points for this balance, at least in blood. Such a concept has important implications. First, this large difference in set point seems relevant to the study of human illnesses in which macrophage activation is believed to be important. These conditions include sequelae caused by TLR ligand-induced inflammation during host protection from infection, and also could extend to some non-infectious illnesses in which inflammation is believed to relevant, such as atherosclerosis, Alzheimer’s disease, and some forms of cancer. The translation of results in mouse models to humans is problematic in some settings for all of these illnesses. This concept might explain why inflammatory conditions such as sepsis and many autoimmune diseases occur or are easily induced in man, who are at one extreme end of a spectrum of sensitivity, but are hard to induce in more resilient animals such as rodents without genetic manipulation to alter host response and secondary inflammation. Second, the data presented here suggest a unifying hypothesis for the marked difference in sensitivity of species to LPS, and provide a potential means of determining which species may be more or less helpful for investigators in choosing appropriate animal models that reflect human diseases that involve activation of macrophages. Third, the markedly different set points to TLR agonist challenge suggest that species must have evolved different host response strategies to handle equal microbial challenges. Fourth, a better knowledge of the different inflammatory set points in species and the underlying mechanisms may be helpful for and treating inflammatory diseases in veterinary practice.
The inhibitory properties of mouse serum on TNF production appeared as a direct effect on mononuclear phagocytes, since the observation was maintained in pre-treated cells and did not require a simultaneous presence of mouse serum with TLR agonists. The reduced production of TNF induced by mouse serum parallels a reduced production of TNF mRNA. The latter was itself the consequence of a reduced activation of the signalling cascade by mouse serum as compared to human serum, as illustrated by a reduced phosphorylation of p38 and erk MAP kinases, leading to a reduced translocation of NF-kB to the nucleus, a prerequisite for TNF mRNA production. Much more work will be needed to understand which proteins in mouse serum are responsible for the suppression and to obtain a better understanding of the signalling mechanisms involved.
Tremendous resources are invested worldwide in the use of animal models for the development of new drugs and to explore the pathophysiology of inflammatory diseases. Our data suggest that the large differences in resilience to pro-inflammatory stimuli between species, with humans towards one end and rodents towards the other end of a spectrum, may not be due to an intrinsic cellular process, but rather may be related to mechanisms involving and perhaps regulated by serum proteins. This finding provides hope that these proteins and their receptors can be identified to develop better animal models and possibly new therapeutic approaches.
Acknowledgements
This work was funded by the National Institute of Health (AI059010, GM59694), Shriners Hospital for Crippled Children (8720), and Institutional funding from the Institut Pasteur.
Footnotes
Conflicts of interest: no author has conflicts of interest
Reference List
- 1.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8(11):889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318(5851):812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- 3.Glode LM, Mergenhagen SE, Rosenstreich DL. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect Immun. 1976;14(3):626–630. doi: 10.1128/iai.14.3.626-630.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCuskey RS, McCuskey PA, Urbaschek R, Urbaschek B. Species differences in Kupffer cells and endotoxin sensitivity. Infect Immun. 1984;45(1):278–280. doi: 10.1128/iai.45.1.278-280.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaedler R, Dubos RJ. The susceptibility of mice to bacterial endotoxins. J Exp Med. 1961;113:559–570. doi: 10.1084/jem.113.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds K, Novosad B, Hoffhines A, Gipson J, Johnson J, Peters J, et al. Pretreatment with troglitazone decreases lethality during endotoxemia in mice. J Endotoxin Res. 2002;8(4):307–314. doi: 10.1179/096805102125000515. [DOI] [PubMed] [Google Scholar]
- 7.van der Poll T, Lowry SF. Biological responses to endotoxin in humans. In: Tellado JM, Forse RA, Solomkin Basel JS, editors. Modulation of the Inflammatory Responsein Severe Sepsis. Switzerland: Karger; 1995. pp. 18–32. 1995; 20: 18–32. [Google Scholar]
- 8.Taveira da Silva AM, Kaulbach HC, Chuidian FS, Lambert DR, Suffredini AF, Danner RL. Brief report: Shock and multiple-organ dysfunction after self-administration of Salmonella endotoxin. N Engl J Med. 1993;328:1457–1460. doi: 10.1056/NEJM199305203282005. [DOI] [PubMed] [Google Scholar]
- 9.Sauter C, Wolfensberger C. Interferon in human serum after injection of endotoxin. Lancet. 1980;2(8199):852–853. doi: 10.1016/s0140-6736(80)90189-0. [DOI] [PubMed] [Google Scholar]
- 10.Liu CT, Sanders RP. Modification of lethality induced by staphylococcal enterotoxin B in Dutch rabbits. Am J Vet Res. 1980;41(3):399–404. [PubMed] [Google Scholar]
- 11.Chen JY, Qiao Y, Komisar JL, Baze WB, Hsu IC, Tseng J. Increased susceptibility to staphylococcal enterotoxin B intoxication in mice primed with actinomycin D. Infect Immun. 1994;62(10):4626–4631. doi: 10.1128/iai.62.10.4626-4631.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathison JC, Wolfson E, Ulevitch RJ. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988;81:1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 14.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 15.Freudenberg MA, Keppler D, Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalek SM, Moore RN, McGhee JR, Rosenstreich DL, Mergenhagen SE. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980;141(1):55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- 17.Hellman J, Roberts JDJ, Tehan MM, Allaire JE, Warren HS. Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J Biol Chem. 2002;277(16):14274–14280. doi: 10.1074/jbc.M109696200. [DOI] [PubMed] [Google Scholar]
- 18.Warren HS, Amato SF, Fitting C, Black KM, Loiselle PM, Pasternack MS, et al. Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J Exp Med. 1993;177:89–97. doi: 10.1084/jem.177.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, et al. MyD88-Dependent and MyD88-Independent Pathways in Synergy, Priming, and Tolerance between TLR Agonists. J Immunol. 2007;178(2):1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 20.Marie C, Pitton C, Fitting C, Cavaillon JM. IL-10 and IL-4 synergize with TNF-alpha to induce IL-1ra production by human neutrophils. Cytokine. 1996;8(2):147–151. doi: 10.1006/cyto.1996.0021. [DOI] [PubMed] [Google Scholar]
- 21.Wilson BMG, Severn A, Rapson NT, Chana J, Hopkins P. A convenient human whole blood culture system for studying the regulation of tumour necrosis factor release by bacterial lipopolysaccharide. J Immunol Methods. 1991;139:233–240. doi: 10.1016/0022-1759(91)90193-j. [DOI] [PubMed] [Google Scholar]
- 22.Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, et al. NF-κB expression in mononuclear cells of septic patients resembles that observed in LPS-tolerance. Am J Respir Crit Care Med. 2000;162:1877–1883. doi: 10.1164/ajrccm.162.5.2003058. [DOI] [PubMed] [Google Scholar]
- 23.Adib-Conquy M, Moine P, Asehnoune K, Edouard A, Espevik T, Miyake K, et al. Toll-like receptors-mediated TNF and IL-10 production differ during systemic inflammation. Am J Respir Crit Care Med. 2003;168(2):158–164. doi: 10.1164/rccm.200209-1077OC. [DOI] [PubMed] [Google Scholar]
- 24.Adib-Conquy M, Adrie C, Fitting C, Gattolliat O, Beyaert R, Cavaillon JM. Up-regulation of MyD88s and SIGIRR, molecules inhibiting Toll-like receptor signaling, in monocytes from septic patients. Crit Care Med. 2006;34(9):2377–2385. doi: 10.1097/01.CCM.0000233875.93866.88. [DOI] [PubMed] [Google Scholar]
- 25.Sheagren JN, Wolff SM, Shulman NR. Febrile and hematologic responses of rhesus monkeys to bacterial endotoxin. Am J Physiol. 1967;212(4):884–890. doi: 10.1152/ajplegacy.1967.212.4.884. [DOI] [PubMed] [Google Scholar]
- 26.Golenbock DT, Will JA, Raetz CR, Proctor RA. Lipid X ameliorates pulmonary hypertension and protects sheep from death due to endotoxin. Infect Immun. 1987;55(10):2471–2476. doi: 10.1128/iai.55.10.2471-2476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiffer ER, Reber G, De Moerloose P, Morel DR. Evaluation of unfractionated heparin and recombinant hirudin on survival in a sustained ovine endotoxin shock model. Crit Care Med. 2002;30(12):2689–2699. doi: 10.1097/00003246-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Ohtsuka H, Higuchi T, Matsuzawa H, Sato H, Takahashi K, Takahashi J, et al. Inhibitory effect on LPS-induced tumor necrosis factor in calves treated with chlorpromazine or pentoxifylline. J Vet Med Sci. 1997;59(11):1075–1077. doi: 10.1292/jvms.59.1075. [DOI] [PubMed] [Google Scholar]
- 29.Clark IA. Correlation between susceptibility to malaria and babesia parasites and to endotoxicity. Trans R Soc Trop Med Hyg. 1982;76(1):4–7. doi: 10.1016/0035-9203(82)90003-7. [DOI] [PubMed] [Google Scholar]
- 30.Goldfarb RD, Tambolini W, Wiener SM, Weber PB. Canine left ventricular performance during LD50 endotoxemia. Am J Physiol. 1983;244(3):H370–H377. doi: 10.1152/ajpheart.1983.244.3.H370. [DOI] [PubMed] [Google Scholar]
- 31.Cobb JP, Natanson C, Quezado ZM, Hoffman WD, Koev CA, Banks S, et al. Differential hemodynamic effects of L-NMMA in endotoxemic and normal dogs. Am J Physiol. 1995;268(4 Pt 2):H1634–H1642. doi: 10.1152/ajpheart.1995.268.4.H1634. [DOI] [PubMed] [Google Scholar]
- 32.Adler HE, DaMassa AJ. Toxicity of endotoxin to chicks. Avian Dis. 1979;23(1):174–178. [PubMed] [Google Scholar]
- 33.Berczi I, Bertok L, Bereznai T. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Can J Microbiol. 1966;12(5):1070–1071. doi: 10.1139/m66-143. [DOI] [PubMed] [Google Scholar]
- 34.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 35.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10(5):233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien AD, Rosenstreich DL, Scher I, Campbell GH, MacDermott RP, Formal SB. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980;124(1):20–24. [PubMed] [Google Scholar]
- 37.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7(10):1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 38.Munford RS. Detoxifying endotoxin: time, place and person. J Endotoxin Res. 2005;11(2):69–84. doi: 10.1179/096805105X35161. [DOI] [PubMed] [Google Scholar]