Abstract
Background
Morbidity and mortality due to influenza could be reduced by improved vaccination.
Methods
To develop a novel skin delivery method for simple and self administration, we prepared microneedle patches with stabilized influenza vaccine and investigated their protective immune responses.
Results
Mice immunized by a single microneedle dose of trehalose-stabilized influenza vaccine developed strong antibody responses that were long-lived. Compared to traditional intramuscular immunization, stabilized microneedle vaccination was superior in inducing protective immunity as evidenced by efficient lung viral clearance and enhanced humoral and antibody secreting cell immune responses after lethal challenge. Vaccine stabilization was found to be important, because mice immunized with an unstabilized microneedle vaccine elicited weaker IgG2a antibody response and were only partially protected against viral challenge. Improved trafficking of dendritic cells to regional lymph nodes by microneedle delivery to the skin might play a role in contributing to improved protective immunity.
Conclusions
These findings suggest that vaccination in the skin using a microneedle patch can improve protective efficacy, induce long-term sustained immunogenicity, and may provide a simple method of administration to improve influenza vaccination.
Keywords: influenza virus, vaccine, microneedle, memory response, intradermal immunization
INTRODUCTION
Influenza remains a critical respiratory disease globally and is caused by viruses which are continuously undergoing antigenic change [1, 2]. Vaccination is the most cost-effective public health measure to prevent disease caused by this pathogen [3]. Most vaccines are administered to humans by systemic hypodermic needle injections including intramuscular (IM), subcutaneous, and in some case intradermal (ID) immunizations. Immunization using hypodermic needles requires trained medical personnel and thus there are limitations for mass vaccination. Also, generation of biohazardous needle waste and needle-associated injuries and diseases are additional problems related to lower vaccination coverage rates. Simpler vaccination methods that are less painful and easy for possible self-administration could significantly reduce morbidity and mortality due to vaccine-preventable diseases such as influenza [4–6].
Intradermal or transcutaneous vaccination in the skin has been suggested as an attractive method and has been carried out to improve vaccine efficacies [7–9]. Particularly, improved vaccine immunogenicity may be enabled by targeting skin’s antigen-presenting Langerhans and dermal dendritic cells (DCs) via intradermal delivery [9]. However, transdermal delivery is blocked by skin’s outermost barrier layer of stratum corneum [8] and intradermal injection is time consuming, painful, unreliable, and requires highly trained medical personnel [10].
Recently, we and others have fabricated micron-scale needles that pierce stratum corneum to administer drugs, proteins, and DNA vaccines into skin [11–13]. Microneedles can be assembled into patches suitable for self-administration using low-cost manufacturing [12] and have been reported as painless and well-tolerated by human subjects [14, 15]. Some work has addressed vaccine delivery via the ID route using single hollow microneedles involving delivery of a liquid vaccine formulation by clinical personnel [16]. More recent studies have examined deliveries of influenza vaccine to mice using coated microneedle patches with high dose vaccines [17, 18]. Additional studies have assessed ID immunization with influenza vaccines using hypodermic needles [7, 9]. However, the limitations on carrying out detailed immunologic studies in humans, especially, to assess memory responses after viral challenge, and the difficulty to make ID injections in thin mouse skin has resulted in limited study of memory responses to influenza vaccination in the skin.
In this study, we have used microneedles to target vaccine delivery to the skin of mice using a microneedle patch designed for simple administration with minimal training and studied the resulting immune responses before and after challenge. This study also examined the immunogenic effect of influenza antigen stabilization using trehalose during microneedle vaccine formulations.
MATERIALS AND METHODS
Preparation of inactivated influenza virus
Formalin-inactivated influenza H1N1 A/PR/8/34 virus was prepared as described previously [19]. For imaging experiments, inactivated whole virus was labeled by mixing 200 μL of inactivated virus (3 mg/ml) with 10 μL of octadecyl rhodamine B chloride (R18, Invitrogen) and incubating at 25°C for 1 h. Unbound R18 molecules were removed by ultracentrifugation (28,000 × g for 1h).
Fabrication and coating of microneedles, and measurement of hemagglutination (HA) activity
Stainless steel microneedles were fabricated using laser cutting and electropolishing [20]. To apply a vaccine coating, microneedles were dipped six times at 25°C into coating solution using a dip-coating device [20] and air dried. The coating solution was composed of 1% (w/v) carboxymethylcellulose (CMC) sodium salt (Carbo-Mer), 0.5% (w/v) Lutrol F-68 NF (BASF), with or without 15% (w/v) D-(+)-trehalose dihydrate (Sigma Aldrich) and 1 mg/ml inactivated virus in phosphate buffered saline (PBS).
Microneedles were imaged by bright-field and fluorescence microscopy (Olympus) with a CCD camera (Leica Microsystems and Diagnostic Instruments, respectively). To image delivery of vaccine into skin, microneedles coated with R18-labeld virus were inserted into human cadaver skin for 10 min and fixed by freezing in histology mounting compound (Tissue-Tek) for 10 min, after which microneedles were removed and skin was sectioned using a cryostat (Microm). This use of human skin was approved by the Georgia Tech Institutional Review Board.
To measure HA activity, vaccine coated microneedles were incubated in PBS for 12 h. To determine HA titers, 50 μl of dissolved coating in PBS was serially diluted in 50 μl of PBS mixed with an equal volume of a fresh 0.5% suspension of chicken red blood cells (Lampire) and incubated for 1 h at 25°C. The titers were determined as the endpoint dilutions inhibiting the precipitation of red blood cells [21].
Immunization and viral challenge infection
BALB/c mice (n=10 per group, 8–10 week old, female) were anesthetized intramuscularly with 110 mg/kg ketamine (Abbott Laboratories) mixed with 11 mg/kg xylaxine (Phoenix Scientific). The skin on the back of the mouse was exposed by removing the hair with depilatory cream (Nair), washed with 70% ethanol, and dried. An in-plane five-needle array of microneedles coated with 0.4 μg of inactivated influenza virus was manually inserted into the skin and left for 10 min. For an IM control, 0.4 μg of inactivated influenza virus in 100 μl PBS was injected intramuscularly into the upper quadriceps muscles of mice (50 μl per leg). The mock control mice received similar microneedles with coating solution without influenza vaccine. To determine the amount of inactivated virus vaccine coated on microneedle, vaccine coated microneedles were soaked in PBS solution for 12 h at 4°C, and the amount of protein was measured by a BCA protein assay kit (Pierce Biotechnology).
For virus challenge, slightly anesthetized mice were intranasally infected with the mouse-adapted A/PR8 virus (50 μl of 20 LD50) five weeks after vaccination [19]. Mice were observed daily to monitor body weight changes and mortality rates. Animals with more than 25% body weight loss were sacrificed to minimize suffering. All animal studies were approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Antibody responses and antibody secreting cells
Influenza virus-specific antibodies of different isotypes (IgG, IgG1, IgG2a and IgG2b) were determined by following the standard protocol of enzyme-linked immunosorbent assay (ELISA) as described previously [19].
To determine recall immune responses, bone marrow and spleen cells harvested at day 4 post challenge were cultured in 96 well plates at 5 × 105 cells/well. Supernatants (4 × dilutions) were used to determine virus-specific antibody levels at days 1, 3, and 6 post culture [22].
Analysis of lung samples
Lung viral titers at day 4 post challenge were determined by counting the number of plaques formed after incubation with serially diluted lung extracts on the MDCK cells [19]. Inflammatory cytokines (IL-6, IFN-γ) in lungs collected at day 4 post challenge were analyzed by Ready-Set-Go cytokine kits (eBioscience, San Diego, CA) following the manufacturer’s procedure [23]. Lung antibodies were similarly determined by ELISA using serially diluted lung extracts [23].
Dendritic cell labeling and analysis
We applied an approach similar to one previously used to track skin dendritic cells draining to regional lymph nodes [24, 25]. Fluorescein isothiocyanate (FITC) (Sigma) was reconstituted in the coating buffer to a final concentration of 1 mg/ml. Microneedles were coated with FITC and inserted into mouse skin as described above. Equal amounts of FITC-coated microneedles dissolved into 100 μl PBS were IM immunized. After 24h, mice were sacrificed, lymph nodes collected, and DCs were prepared using collagenase and DNAse I [26]. Suspensions containing 5 × 105 single cells were then stained with DC phenotypic markers and analyzed on a FACScan flow cytometry (Becton Dickinson). Granular cells were gated and analyzed for CD11c+ and CD11c+FITC+ cell populations.
Statistical Analysis
Every assay was measured using at least three samples, from which the arithmetic mean and standard error of the mean were calculated and reported in the figures. A two-tailed paired Student’s t-test was performed when comparing two time points of the same set of animals (Table 1). When comparing three or more conditions, a one-way analysis of variance (ANOVA; α=0.05) was performed. In some cases, non-parametric methods expressing medians were compared to validate the results. In all cases, a value p<0.05 was considered statistically significant.
Table 1.
Hemagglutination (HA) activity A, virus specific serum antibody levels expressed in terms of end-point dilution titers before and after challenge(× 103) B, and ratios of virus specific IgG2a and IgG1 antibodies before and after challenge C.
| HA activity (%) | Before challengeD |
After challengeE |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | IgG | IgG1 | IgG2a | IgG2b | IgG2a/IgG1 | IgG | IgG1 | IgG2a | IgG2b | IgG2a/IgG1 | |
| MockF | - | 0.2±0.5 | 0.2±0.03 | 0.2±0.03 | 0.1 | - | 0.2±0.05 | 0.2±0.03 | 0.2±0.03 | 0.1±0.02 | - |
| MNG | 1.6±0.3 | 19.2±2.4 | 25.6±1.6 | 3.2±0.7 | 0.8±0.07 | 0.125 | 25.6±3.9 | 51.2±6.7 | 12.8±0.7 | 0.4±0.02 | 0.25 |
| MN+TreH | 63.7±7.9 | 51.2±2.9 | 12.8±2.1 | 102.4±5.8 | 9.6±2.1 | 8 | 102.4±5.6 | 38.4±8.9 | 153.6±16 | 9.6±0.9 | 4 |
| IMI | 100 | 51.2±6.1 | 6.4±0.5 | 102.4±4.3 | 6.4±1.7 | 16 | 19.2±2.1 | 3.2±0.2 | 25.6±2.8 | 1.6±0.2 | 8 |
HA activity was measured as an indicator of the functional integrity of hemagglutinin on the inactivated viral vaccine and is expressed as a percent of activity relative to the same mass of unprocesses antigen.
Titers are expressed as the highest dilution having a mean optical density at 450 nm greater than the mean value plus 3 standard deviations of naïve serum samples. Total IgG titers did not equal the sum of IgG isotype titers, probably due to differences in experimental measurement sensitivities. Such discrepancies have also been observed previously [32].
IgG2a/IgG1 ratios were determined based on antibody titers.
Serum samples collected at week 4 after immunization (n=10).
Serum samples collected at day 4 after challenge (n=4).
Microneedle immunization without influenza vaccine.
Microneedle immunization without trehalose formulation.
Microneedle immunization with trehalose formulation.
Intramuscular immunization with intact influenza vaccine.
The same animals were used to generate the data in Figure 3C and Table 1.
RESULTS
Fabrication of microneedle patches and delivery into skin
Microneedles fabricated by laser-cutting stainless steel sheets (figure 1A) were designed to be long enough to penetrate through stratum corneum and viable epidermis and into superficial dermis by gentle manual insertion, but short enough to avoid pain [15]. Our delivery strategy involved dip coating solid microneedles with formulations of influenza vaccine (A/PR/8/34) that dry onto the microneedles and then rapidly dissolve in skin. Dip coating produced thick, uniform coatings localized to microneedle shafts (figure 1B, 1C). Insertion of microneedles into skin led to dissolution (figure 1D, 1E) and deposition (figure 1F, 1G) in skin within minutes.
Figure 1.

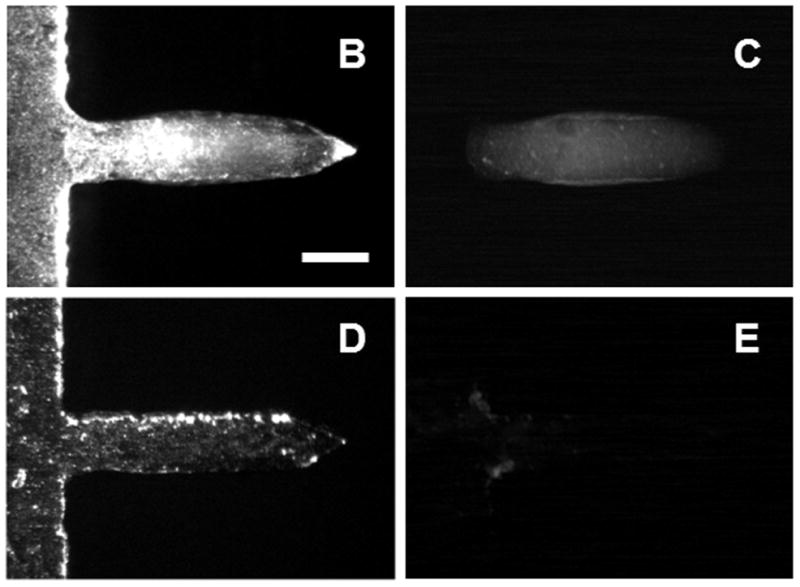
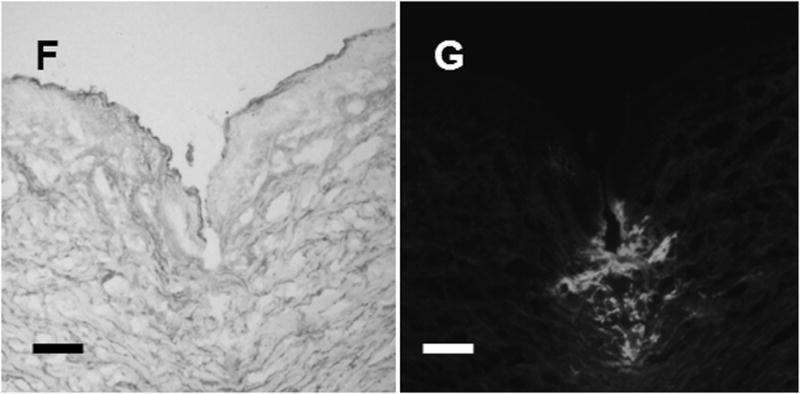
Microneedle coated with influenza vaccine. (A) Image of a 5-microneedle array (scale bar = 500 μm). Bright-field (B, D) and fluorescence (C, E) micrographs of a microneedle coated with red-fluorescent inactivated influenza virus before (B, C) and 10 min after (D, E) insertion into human cadaver skin (scale bar = 200 μm). Histologic section of human cadaver skin fixed after insertion of a vaccine-coated microneedle imaged by (F) bright-field microscopy showing skin deformation and needle track across epidermis and into superficial dermis and (G) fluorescence microscopy showing deposition of red-fluorescent vaccine coating in skin (scale bar = 200 μm).
Addition of trehalose disaccharide to the microneedle coating formulation significantly improved retention of HA activity of influenza vaccine antigen after drying on microneedles (Table 1). The optimized formulation retained 64% of HA activity after coating. This indicated an increase in antigen stability by trehalose, at least over the 24 h timeframe of the stabilization study.
Antibody responses after delivery of stabilized microneedle influenza vaccine
We next evaluated immune responses induced by stabilized microneedle vaccine delivery and compared with IM injection in mice. Groups of mice (n=10, BALB/c mice) received a single dose immunization via skin delivery using a microneedle array coated with 0.4 μg of inactivated whole virus with trehalose (MN+Tre) or without trehalose (MN). Considering that the thickness of mouse skin is approximatley 500–600 μm [24] and the length of the microneedles is 700 μm, most vaccine was probably deposited within the mouse skin. The IM control group was intramuscularly immunized with 0.4 μg of unprocessed inactivated virus vaccine. An additional group was mock-treated using microneedles without vaccine.
Total IgG and IgG2a levels after stabilized microneedle vaccination were similar to IM vaccination (p>0.05, table 1). Interestingly, both types of microneedle delivery (MN and MN+Tre) induced higher levels of IgG1 antibodies than IM immunization (p<0.01). For stabilized MN and IM deliveries, IgG2a was the dominant isotype (IgG2a/IgG1 = 8 for MN+Tre; IgG2a/IgG1 = 16 for IM) (table 1). In contrast, microneedle delivery without trehalose (MN) showed lower levels of antibodies, as well as a shift in the isotype profile such that IgG1 was the dominant antibody isotype (IgG2a/IgG1 = 0.125 for MN) with significantly lower IgG2a and IgG2b antibody responses (table 1).
Overall, these results suggest that the stabilized microneedle (MN+Tre) vaccine can induce higher (in case of IgG1) or comparable antibody responses to IM immunization. Moreover, vaccine stabilization with trehalose during microneedle coating was critical for maintaining the isotype profile in favor of IgG2a, whereas unstabilized vaccine shifted to IgG1.
Protection against lethal challenge infection
Vaccinated mice were challenged with a lethal dose of influenza A/PR8 virus (20 × LD50) at five weeks after a single dose immunization (figure 2). Mice either immunized with a stabilized microneedle vaccine (MN+Tre) or IM were completely protected without body weight loss. However, the microneedle group without trehalose (MN) showed body weight losses ranging from 15% to over 25%, which is statistically significant (p<0.05). The survival rate of mice in the unstabilized group (MN) was decreased to 67%. All mice in the mock control died or had to be euthanized by day 6. Thus, stabilized microneedles provided protection equal to IM immunization and formulating microneedles to maintain influenza vaccine stability was critically important in providing protective immunity against lethal challenge infection.
Figure 2.
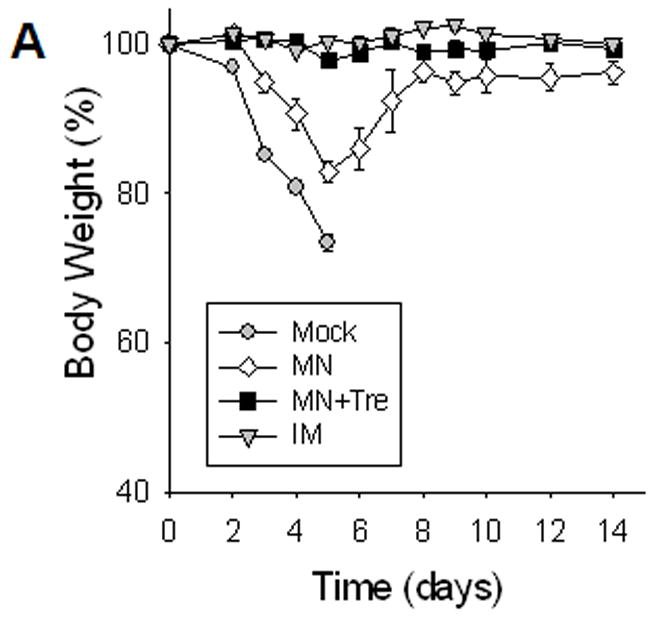
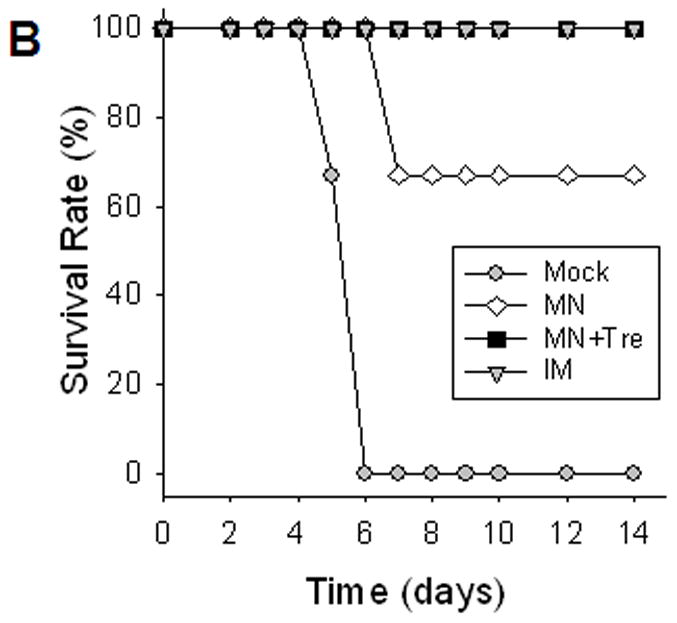
Protection against lethal challenge infection. Immunized mice were challenged with a lethal dose (20 LD50) of a highly pathogenic A/PR8 influenza virus 5 weeks after a single vaccination (n=10). (A) Body weight change. (B) Survival rates were monitored daily for 14 days (n=6). Similar survival rates were obtained in two independent experiments indicating reproducible results. Mock, microneedle immunization without vaccine; MN, microneedle immunization with influenza vaccine formulated in the absence of trehalose; MN+Tre, microneedle immunization with influenza vaccine formulated in the presence of trehalose (15%); IM, intramuscular immunization with unprocessed influenza vaccine. Dead animals were removed and only live animals were counted for the body weight analysis, reflecting the rebound in body weight as a result of recovery. For the analysis at day 4 post challenge, 4 out of 10 mice were sacrificed and the remaining 6 mice were monitored.
Protective efficacy of microneedle vaccination
Determining the lung viral titers could be informative to indicate the strength of the host protective immune capacity to control the challenge virus replication. We found that at day 4 post challenge, the IM or microneedle (MN) immunized mice had significantly reduced lung viral titers compared to mock control mice (figure 3A, p<0.01). Interestingly, viral titers from mice immunized with stabilized microneedle vaccine (MN+Tre) were below the detection limit, indicating drastically improved clearance of challenge virus by day 4, compared to unstabilized MN and IM. (p<0.05)
Figure 3.
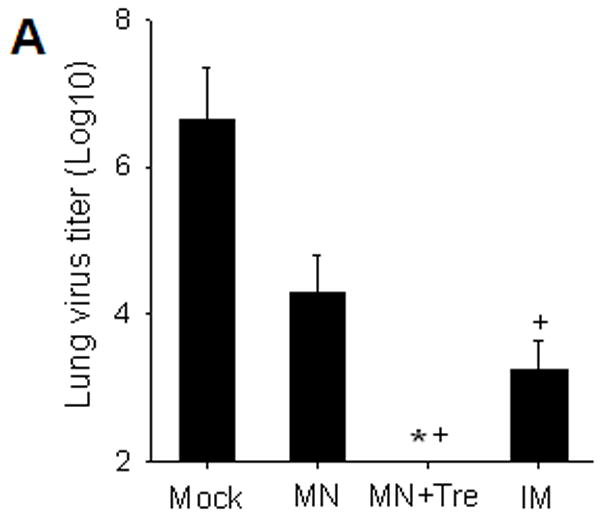
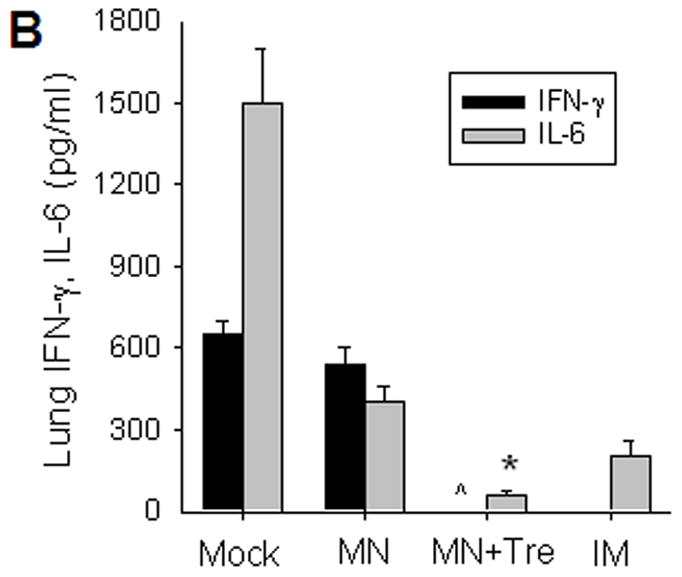
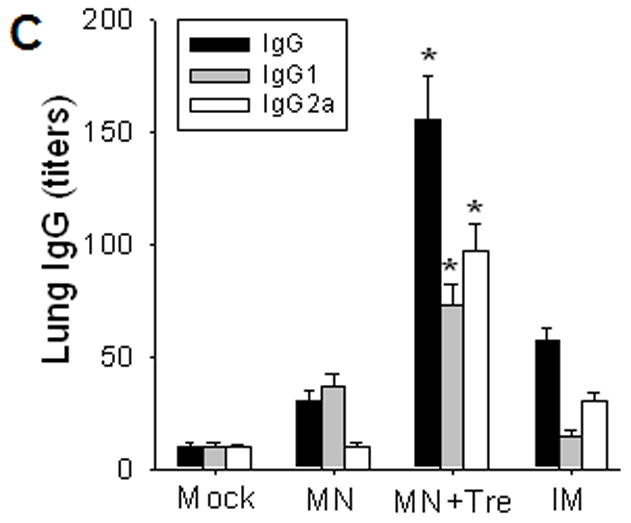
Protective efficacy of microneedle vaccination. (A) Lung virus titers. Lungs from individual mice were extracted (1 ml media per mouse lung, n=4, *p<0.05, + p<0.01). The detection limit for lung viral titers was 50 pfu per 1 ml lung extracts of individual mice. (B) Lung inflammatory IL-6 and IFN-γ cytokines (n=4, *p<0.05, ^p<0.05). (C) Virus specific antibodies in lungs (n=4, *p<0.05). Lungs were collected from individual mice at day 4 post challenge and antibody levels determined by ELISA were expressed end-point dilution titers. Groups of mice are as described in the legend of Figure 2. *: MN+Tre compared with Mock, MN, and IM. ^: MN+Tre compared with Mock, MN. +: MN+Tre compared with Mock.
We also measured production of lung proinflammatory cytokines known to cause tissue damage and increased mortality [25]. Challenged mice in the mock exhibited high levels of inflammatory cytokines, whereas no IFN-γ and significantly lower levels of IL-6 were detected in the lungs of stabilized MN immunized mice compared to those from the unstabilized MN group (figure 3B, p<0.05). Notably, mice immunized with stabilized microneedle vaccine showed lower levels of IL-6 than IM-immunized mice (p<0.05). Overall, these results indicate that microneedle delivery of stabilized vaccine was superior to IM immunization in inducing protective immunity to control challenge virus replication in the lung.
Rapid recall humoral immune responses induced by microneedle
To better understand the improved protection observed by microneedle vaccination, we compared influenza virus specific antibodies in sera and lungs at day 4 post challenge between the stabilized microneedle and IM immunizations. Both microneedle vaccine groups (MN, MN+Tre) showed significantly higher levels of virus specific total IgG and isotype antibodies including IgG1 and IgG2a isotypes at day 4 after challenge than those prior to challenge (table 1, p<0.05). In contrast, antibody levels in IM immunized mice were lower at day 4 post challenge than those before challenge (table 1, p<0.05). IgG2a was still the dominant isotype in both stabilized microneedle and IM immunization groups after challenge, which exhibited IgG2a/IgG1 ratios ranging between 4 and 8. The unstabilized microneedle (MN) group showed IgG1 as a dominant isotype.
Importantly, virus-specific antibodies including IgG, IgG1, and IgG2a in lungs were considerably higher in the stabilized microneedle group (MN+Tre) than those in the IM immunized and unstabilized microneedle (IM, MN) groups (figure 3C). It is interesting to note that total IgG from the IM group was higher in the lung but slightly lower in the serum samples compared to those corresponding samples from the MN (unstabilized) group (Table 1, figure 3C). This might be due to the 16 fold higher levels of IgG1 isotype antibody in the MN serum samples post challenge (51.2 ×103 IgG1 for MN and 3.2 × 103 for IM, Table 1), which contributed to higher total IgG in the MN group. These results indicate that anamnestically enhanced lung and serum antibodies might have contributed to improving protective immunity enabled by stabilized microneedle vaccination.
Long-term antibody responses by microneedle vaccination
Because long-term antibody levels are considered to be maintained by constitutively antibody-secreting plasma cells in bone marrow [22], we measured virus-specific antibody secretion from bone marrow cells collected at day 4 post-challenge (figure 4A). After one day of in vitro culture without antigen stimulation, the stabilized microneedle group showed significantly enhanced levels of virus-specific antibodies compared to IM group. Later, antibody levels in the microneedle group continued to rise (p<0.005) and remained higher than IM. To assess antibody-secreting B cell, spleen cells collected on day 4 post-challenge were incubated with inactivated viral antigen (figure 4B). Day 3 antibody levels in the stabilized microneedle group (MN+Tre) were higher than those in IM, indicating the generation of memory B cells more effectively by micorneedle vaccination. After 6 days in vitro culture, differences in secreted antibody levels between microneedle and IM immunization groups were not statistically significant (p>0.05).
Figure 4.
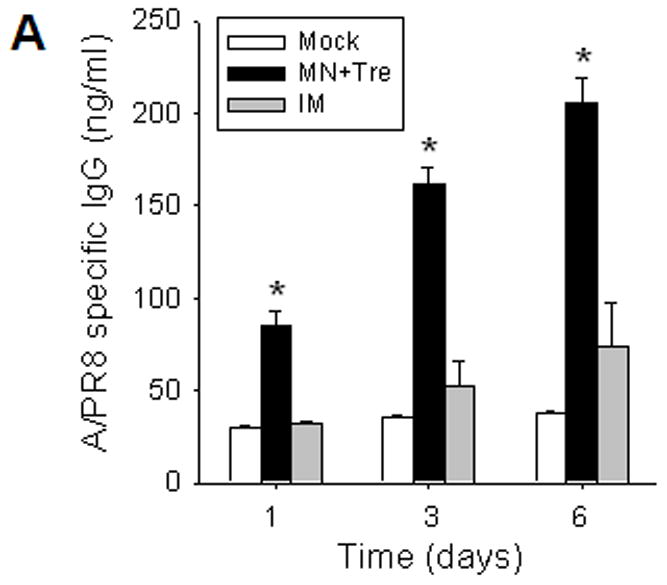
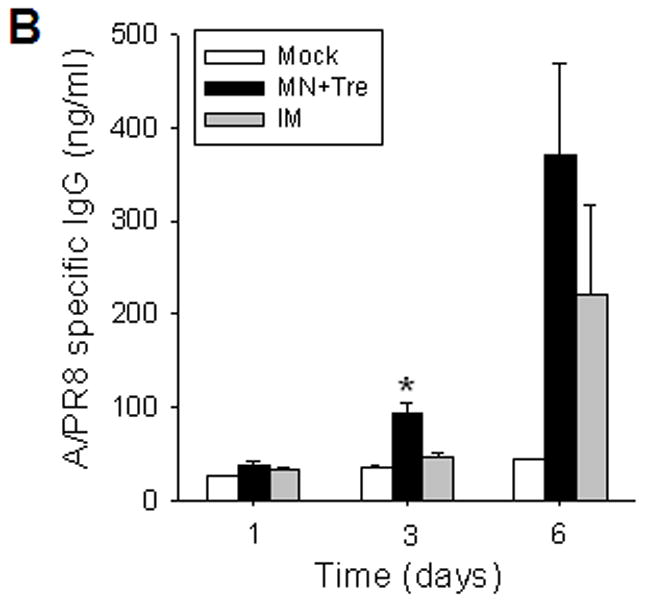
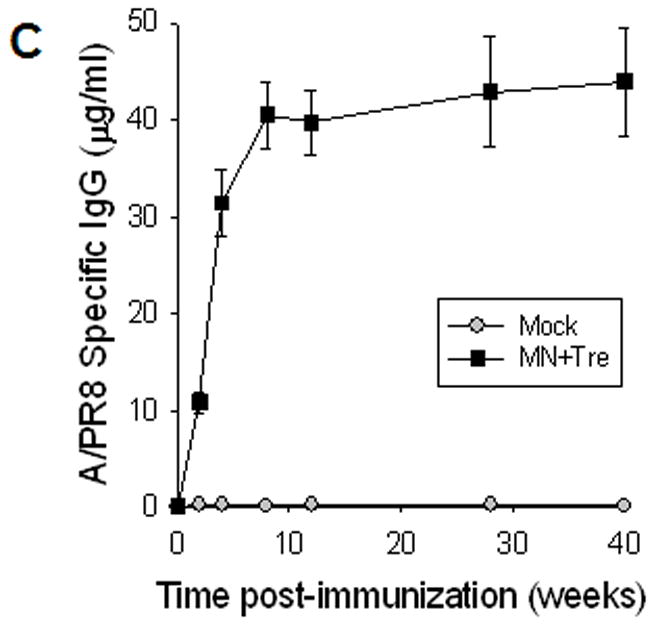
Rapid recall and long-term immune responses. Bone marrow and spleen cells were harvested at day 4 post challenge (n=4), and kinetics of virus-specific IgG antibody production were determined. Antibody levels in the fourfold diluted in vitro culture supernatants were determined by ELISA (OD at 450 nm) after 1 to 6 days of incubation, and were expressed in concentrations (ng/ml) using standard mouse antibodies. (A) Bone marrow cell cultures (5 × 105 cells/well) in the absence of influenza virus antigen stimulation (n=4, *p<0.05). (B) Spleen cell cultures (5 × 105 cells/well) in the plate coated with inactivated influenza viral antigen (n=4, *p<0.005). Groups of mice (n=4, *p<0.05) are as described in the legend of Figure 2. (C) Long-term maintenance of antibody levels by microneedle vaccination. In an independent experiment for long-term antibody responses, virus specific antibody responses were determined over a 9 month period in mice (n=6) immunized in the skin with trehalose-formulated microneedle vaccine (0.7 μg inactivated influenza virus). Time 0 is the IgG value from the serum samples obtained before immunization of mice with microneedle vaccine. Serial diluted serum samples were used for ELISA and antibody levels were expressed in concentrations (μg/ml) from a mouse antibody standard curve. *: MN+Tre compared with Mock and IM.
To determine long-term antibody responses induced by microneedle vaccination, we monitored the virus-specific antibody levels for the 40-week period for the 0.7 μg stabilized microneedle vaccination group. High levels of antibodies were found to be maintained for the 40-week period (figure 4C). These results suggest that microneedle delivery was highly effective in sustaining long-lived antibodies and in generating antibody-secreting B cells with properties to rapidly produce antibodies.
DC trafficking to the lymph nodes
These improved anamnestic responses could be explained by vaccine targeting to skin’s Langerhans and dermal dermal DCs via delivery to the skin [26]. To assess this possibility, we compared the levels of CD11c+ DCs in lymph nodes (figure 5) [27]. Mice receiving microneedle delivery showed higher populations of DCs trafficked to lymph nodes than the IM group. Importantly, microneedle delivery induced CD11c+FITC+ DC populations in inguinal lymph nodes approximately twice as large as those after IM delivery one day after delivery. These results suggest that microneedle delivery to the skin led to more effective migration of DCs capturing antigens to the lymph nodes compared to IM immunization. Because DCs are known to be professional antigen-presenting cells capable of stimulating naïve T and B cells [28], effectively targeting an influenza antigen to dermal DCs might explain efficient induction of recall immune responses after deliveryto the skin using microneedles.
Figure 5.
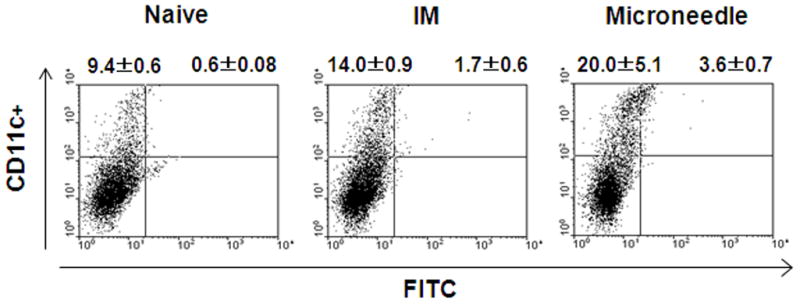
DC migration to the draining lymph nodes. After one day treatment of mice (n=5) with microneedle or IM delivery of FITC, the inquinal lymph nodes were harvested, and CD11c+ and CD11c+FITC+ DC populations were analyzed by flow cytometry. Percentages of gated populations in the upper quadrants of each dot plot are shown. The plots are representative from two independent experiments.
DISCUSSION
The findings reported here show that stabilized microneedle vaccination to the skin induced better protective immunity, as measured by lung virus titer, recall B cell responses, and long-lived plasma cells compared to unstabilized microneedle or IM immunization. In addition, virus specific post-challenge serum total IgG and isotype antibodies (IgG2a, IgG2b) were significantly higher in the stabilized microneedle vaccine group than those of unstabilized microneedle and IM immunization.
The IgG2a isotype antibody is known to promote a cascade of complement activation and to be more efficient in clearing viral and bacterial infections [29–31]. A high level of IgG2a induced by the microneedle immunization might have contributed to effective clearing of the challenge virus compared to IM immunization. The total levels of binding antibodies, and protective efficacies were much lower in the unstabilized microneedle group than those in the trehalose-stabilized microneedle group.
Our mechanistic analysis suggests that improved immunogenicity starts with more efficient antigen delivery to DCs in the skin, which migrated to the regional lymph node after immunization using microneedles. This, in turn, enables more efficient activation of naïve B cells to generate long-lived plasma cells and memory B cells, which rapidly elevate lung and serum antibodies after challenge. As evidence for long-lived immunity, we observed the long-term maintenance of virus-specific antibodies for over 9 months after a single vaccine dose using microneedles. In contrast, a previous study demonstrated that IM immunization with split influenza vaccines showed a decreasing trend of virus specific antibody levels 21 days after immunization [32]. The combination of these elevated humoral and cellular anamnestic responses can explain the effective control of viral replication in lungs resulting in lower lung inflammatory cytokines and the associated excellent protection against viral challenge.
Whole inactivated influenza vaccines contain a lipid-bilayer membrane containing hemagglutinin as a major glycoprotein. Hemagglutinin is the most important antigenic target for inducing protective immunity. A recent study demonstrated that solid microneedle vaccination with high doses of inactivated influenza virus induced similar protection as IM immunization although the stability of microneedle vaccines has not been investigated [17, 18]. The stability of vaccine is assumed to be important for its immunogenic efficacy. However, there was no comprehensive study on potential correlations between immunogenicity and vaccine integrity. We believe it is notable that a relatively simple formulation of trehalose was able to maintain immunogenicity of whole virus vaccine after drying onto microneedles and rehydration in the skin.
The immune responses induced by unstabilized microneedle vaccine were different from those induced by the trehalose-stabilized microneedle vaccine in terms of quantity and quality. It is interesting to note that the pattern of antibody isotypes was strikingly opposite between the unstabilized and stabilized groups of mice. We speculate that the functional integrity of hemagglutinin in the influenza vaccine might be important for effective interactions with receptors to recognize pathogen associated molecular patterns such as Toll-like receptors that are expressed on antigen presenting cells including Langerhans cells and dermal dendritic cells [33] or B cells [34]. Interactions between the hemagglutinin of influenza vaccines and receptors on antigen presenting cells are likely to induce T helper type 1 (Th1) cytokines, which would significantly influence the pattern of antibody isotypes. In contrast, macrophages engulfing particulate antigens without engaging receptor interactions were shown to induce Th2 type immune responses associated with IgG1 isotype antibodies [35].
Apart from immunologic merits, microneedles have potential logistic advantages too. Vaccination using microneedles has a reduced probability of blood-borne pathogen transmission due to inaccessibility to the blood stream. In future work, microneedles can be developed as a patch suitable for self-administration with a design engineered to prevent re-use by using dissolvable or retractable microneedles. In addition, microneedles are expected to be cost efficient for mass vaccination at a manufacturing price similar to a needle and syringe [12, 36]. These features of microneedles could increase coverage of seasonal and pandemic influenza vaccination by facilitating school-based vaccination of children and easy access to vaccination in elder-care facilities to minimize risks of cross-contamination and long delays associated with injection-based vaccination at centralized clinics [4]. Self-administration should be carefully monitored for potential concerns regarding any side effects by medical personnel. Also, vaccine distribution and self-administration are recommended to be carried out in the same on-site locations under the presence of clinicians in response to any reactogenicity responses during clinical trials.
In conclusion, we found that microneedle vaccination in the skin generated better control of viral replication and reduced inflammatory responses in lungs probably due to skin-derived dendritic cell activation and antibody secreting plasma cell responses in bone marrow compared to those after IM injection. These immunologic advantages, combined with logistic benefits, indicate that microneedle delivery to the skin may offer a strategy for improved influenza vaccination, which might also be applicable to delivery of other vaccines.
Acknowledgments
Financial support: NIH grants EB006369 and AI0680003.
This work was carried out at the Emory Vaccine Center and the Georgia Tech Center for Drug Design, Development and Delivery and Institute for Bioengineering and Biosciences. It was supported in part by NIH grants EB006369 (M.R.P.) and AI0680003 (R.W.C.), and SERCEB (R.W.C) and the Korea Ginseng Society (S.M.K). We thank Dr. Thuc-Vy Le for assistance in preparing lymph nodes, Dr. Huan Nguyen for mouse-adapted influenza virus A/PR8/34 strain, and Dr. Mark Allen for use of his laser microfabrication facilities.
Footnotes
Potential conflict of interests: M.P. is an inventor on patents (one of which has been licensed) and a consultant and advisor to companies working on microneedles. Although there are currently no products based on microneedles, and this study does not involve any commercial products, the results of this research could indirectly influence the success of possible future commercial activities in which M.P. has an interest.
Presented in part: Microneedle-based immunization against influenza, AIChE meeting, Philadelphia, PA, USA, November 2008 and Microneedle patches for improved influenza vaccination, Controlled release society meeting, Copenhagen, Denmark 2009.
References
- 1.Cox RJ, Brokstad KA, Ogra P. Influenza virus: Immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scandinavian J Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 2.Palese P. Influenza: Old and new threats. Nat Med. 2004;10:S82–7. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Prevention and control of influenza, recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 2008;57:1–60. [PubMed] [Google Scholar]
- 4.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19:95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 5.Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005;5:905–16. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 6.Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003;9:99–103. doi: 10.1038/nm0103-99. [DOI] [PubMed] [Google Scholar]
- 7.Belshe RB, Newman FK, Cannon J, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–94. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 8.Glenn GM, Taylor DN, Li XR, Frankel S, Montemarano A, Alving CR. Transcutaneous immunization: A human vaccine delivery strategy using a patch. Nat Med. 2000;6:1403–6. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 9.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 10.Laurent PE, Bonnet S, Alchas P, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–42. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–9. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 12.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Del Rev. 2004;56:581–7. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Widera G, Johnson J, Kim L, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–64. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 14.Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci. 2008;35:193–202. doi: 10.1016/j.ejps.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–94. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–9. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 17.Zhu QY, Zarnitsyn VG, Ye L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106:7968–73. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koutsonanos DG, Martin MdP, Zarnitsyn VG, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS ONE. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82:1350–9. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–37. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol. 2009;83:4489–97. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slifka MK, Matloubian M, Ahmed R. Bone-marrow is a major site of long-term antibody-production after acute viral-infection. J Virol. 1995;69:1895–902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan FS, Huang CZ, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–24. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azzi L, El-Alfy M, Martel C, Labrie F. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J Invest Dermatol. 2005;124:22–7. doi: 10.1111/j.0022-202X.2004.23545.x. [DOI] [PubMed] [Google Scholar]
- 25.Van Reeth K. Cytokines in the pathogenesis of influenza. Vet Microbiol. 2000;74:109–16. doi: 10.1016/s0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 26.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guebre-Xabier M, Hammond SA, Epperson DE, Yu JM, Ellingsworth L, Glenn GM. Immunostimulant patch containing heat-labile enterotoxin from Escherichia coli enhances immune responses to injected influenza virus vaccine through activation of skin dendritic cells. J Virol. 2003;77:5218–25. doi: 10.1128/JVI.77.9.5218-5225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 29.Coutelier JP, van der Logt JT, Heessen FW, Warnier G, Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987;165:64–9. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klaus GG, Pepys MB, Kitajima K, Askonas BA. Activation of mouse complement by different classes of mouse antibody. Immunology. 1979;38:687–95. [PMC free article] [PubMed] [Google Scholar]
- 31.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–2. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 32.Hauge S, Madhun A, Cox RJ, Haaheim LR. Quality and kinetics of the antibody response in mice after three different low-dose influenza virus vaccination strategies. Clin Vaccine Immunol. 2007;14:978–83. doi: 10.1128/CVI.00033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–50. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 34.Richards S, Watanabe C, Santos L, Craxton A, Clark EA. Regulation of B-cell entry into the cell cycle. Immunol Rev. 2008;224:183–200. doi: 10.1111/j.1600-065X.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debecker G, Sornasse T, Nabavi N, et al. Immunoglobulin isotype regulation by antigen-presenting cells in-vivo. Eur J Immunol. 1994;24:1523–8. doi: 10.1002/eji.1830240710. [DOI] [PubMed] [Google Scholar]
- 36.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. In: Compans RW, Orenstein WA, editors. Current topics in microbiology and immunology: vaccines for pandemic influenza. Berlin/Heidelberg, Germany: Springer-Verlag; 2009. [Google Scholar]


