Abstract
Background
Etomidate acts at γ-Aminobutyric acid type A (GABAA) receptors containing β2 or β3, but not β1 subunits. Mutations at β residue 265 (Ser in β1; Asn in β2 or β3) profoundly affect etomidate sensitivity. Whether these mutations alter etomidate binding remains uncertain.
Methods
Heterologously expressed α1β2γ2L GABAA receptors and receptors with β2(N265S) or β2(N265M) mutations were studied electrophysiologically in both Xenopus oocytes and HEK293 cells. Experiments quantified the impact of β2N265 mutations or substituting β1 for β2 on basal channel activation, GABA EC50, maximal GABA efficacy, etomidate-induced leftward shift in GABA responses, etomidate direct activation, and rapid macrocurrent kinetics. Results were analyzed in the context of an established allosteric coagonist mechanism.
Results
Mutations produced only small changes in basal channel activity, GABA EC50, maximal GABA efficacy, and macrocurrent kinetics. Relative to wild-type, β2(N265S) reduced etomidate enhancement of apparent GABA affinity 6-fold, and reduced etomidate direct activation efficacy 14-fold. β2(N265M) totally eliminated both etomidate modulation of GABA responses and direct channel activation. Mechanism-based analysis showed that the function of both mutants remains consistent with the allosteric coagonist model, and that β2(N265S) reduced etomidate allosteric efficacy 5-fold, while etomidate-binding affinity dropped 3-fold. Experiments swapping β2 subunits for β1 indicated that etomidate efficacy is reduced 34-fold, while binding affinity drops less than 2-fold.
Conclusions
Mutations at β2N265 profoundly alter etomidate sensitivity with only small changes in basal and GABA-dependent channel activity. Mutations at the β2N265 residue, or replacement of β2 with β1, influence etomidate efficacy much more than binding to inactive receptors.
Introduction
Etomidate is a potent intravenous general anesthetic that produces its behavioral effects via ionotropic γ-Aminobutyric acid type A (GABAA) receptors, the major inhibitory postsynaptic ion channels in mammalian brain1,2. Etomidate slows decay of GABAergic inhibitory postsynaptic currents (IPSCs) in neurons and similarly slows deactivation of GABAA receptor-mediated macrocurrents elicited with brief agonist pulses3,4. Etomidate potentiates currents elicited by submaximal GABA, shifting GABA EC50 to lower concentrations. High concentrations of etomidate also directly activate GABAA receptors. In α1β2γ2L GABAA receptors, these etomidate actions are quantitatively described by an allosteric model with two equivalent coagonist sites linked to channel gating5.
GABAA receptors contain a central chloride ion channel surrounded by five homologous subunits, each with a large amino-terminal extracellular domain, four transmembrane domains (M1-M4), and a large intracellular domain between M3 and M46. The most abundant GABAA receptor subtype, α1β2γ2L, incorporates 2α, 2β, and 1γ arranged counterclockwise as γ-β-α-β-α when viewed from the synaptic cleft7-9. Photolabeling with an etomidate analog, [3H]-azi-etomidate,10,11 identified two GABAA receptor residues on adjacent subunits, M286 in the β subunit M3 domain and M236 in the α subunit M1 domain.
The amino acid at position 265 (15′) in the M2 domain of β subunits is also a determinant of etomidate sensitivity. Etomidate modulates mammalian GABAA receptors containing β2 or β3 subunits, which both have Asn (N) at position 265, while minimally affecting receptors containing β1 subunits, which have Ser (S) at position 26512. Ser substitutions for Asn265 in β2 and β3 reduce etomidate sensitivity, while receptors containing mutant β1(S265N) subunits become etomidate sensitive13-15. In addition, the homolog of β2/3(N265) in the anesthetic-insensitive drosophila rdl GABAA receptor is a Met (M), and mutation of β2/3N265 to Met also dramatically reduces etomidate modulation16,17. The β3(N265M) and β2(N265S) mutations have been used in transgenic animal studies probing the role of GABAA receptors in anesthetic actions in vivo1,2. Structural models of α1β2γ2L GABAA receptors,11,18,19 based on a 4 Å resolution structure of the homologous nicotinic acetylcholine receptor from Torpedo20, shows β2N265 at the periphery of the etomidate binding pocket.
The aims of this study were to quantitatively define the impact of β2N265 mutations on α1β2γ2L GABAA receptor function both in the absence and presence of etomidate, and also to determine whether mutations at β2N265 affect etomidate binding versus its allosteric efficacy. We assessed the effects of β2(N265S) and β2(N265M) mutations on spontaneous channel activity, GABA concentration responses, maximum GABA efficacy, etomidate modulation of GABA-dependent activation, and direct channel activation by etomidate in the absence of GABA. Macrocurrent activation, desensitization, and deactivation rates were also measured in wild-type and mutant receptors, using submillisecond GABA concentration jumps. Results were analyzed within the mechanistic framework of allosteric coagonism 5.
Materials and Methods
Animal use
Female Xenopus laevis were housed in a veterinary-supervised environment and used in accordance with local and federal guidelines and with approval from the Massachusetts General Hospital subcommittee on research and animal care (Boston, Massachusetts). Frogs were anesthetized by immersion in ice cold 0.2% tricaine (Sigma-Aldrich, St. Louis, MO) prior to mini-laparotomy for oocyte harvest.
Chemicals
R(+)-Etomidate was obtained from Bedford Laboratories (Bedford, OH). The clinical preparation in 35% propylene glycol was diluted directly into buffer. Previous studies have shown that propylene glycol at the dilutions used for these studies has no effect on GABAA receptor function 5. Picrotoxin was purchased from Sigma-Aldrich and dissolved in electrophysiology buffer (2 mM) by prolonged gentle shaking. Alphaxalone was purchased from MP Biomedical (Solon, OH) and prepared as a stock solution in dimethylsulfoxide. Salts and buffers were purchased from Sigma-Aldrich.
Molecular Biology
Complementary DNA sequences for human GABAA receptor α1, β2, β1, and γ2L subunits were cloned into pCDNA3.1 vectors (Invitrogen, Carlsbad, CA). To create expression plasmids for β2(N265S), β2(N265M) and α1(L264T) mutants, oligonucleotide-directed mutagenesis was performed on the appropriate wild-type clone using QuickChange kits (Stratagene, La Jolla, CA). Clones from each mutagenesis reaction were sequenced through the entire subunit gene to confirm the presence of the mutation and absence of stray mutations.
Expression of GABAA receptors
Messenger RNA was synthesized in vitro from linearized DNA templates and purified using commercial kits (Ambion Inc., Austin, TX). Subunit messenger RNAs were mixed at 1α:1β and at least two-fold excess γ to promote homogeneous receptor expression 21,22. Xenopus oocytes were microinjected with 25-50 nl (15-25 ng) of messenger RNA mixture and incubated at 18 °C in ND96 (in mM: 96 NaCl, 2 KCl, 0.8 MgCl2, 1.8 CaCl2, 5 HEPES, pH 7.5) supplemented with gentamicin (0.05 mg/ml) for 24-48 hr prior to electrophysiology. HEK293 cells were cultured on glass cover slips, maintained as previously described 23, and transfected with plasmids encoding GABAA receptor subunit mixtures (1α:1β:2γ) using lipofectamine (Invitrogen). A eukaryotic green fluorescent protein expression plasmid, pmaxGFP (Amaxa, Gaithersburg, MD), was mixed with the GABAA receptor subunit plasmids to aid in identification of transfected cells. Transfected cells were maintained in culture medium for 24-48 hr prior to electrophysiology experiments.
Oocyte Electrophysiology
GABAA receptor responses to GABA were assessed in Xenopus oocytes using two microelectrode voltage clamp electrophysiology, as previously described 24. GABA pulses were from 5 to 20 s, depending on the concentration of GABA used and the time to steady-state peak current. Normalizing GABA responses were recorded at maximal GABA (1-10 mM). Picrotoxin-sensitive leak currents were measured by superfusion with 2 mM picrotoxin, followed by washout for at least 5 minutes before testing maximal GABA response. Alphaxalone (2 μM) was used as a gating enhancer in combination with 10 mM GABA, to provide estimates of GABA efficacy. Oocyte currents were low-pass filtered at 1 kHz (Model OC-725B, Warner Instruments, Hamden, CT) and digitized at 1-2 kHz using commercial digitizer hardware (Digidata 1200, Molecular Devices, Sunnyvale, CA) and software (pClamp 7. Molecular Devices).
Electrophysiology in HEK293 cell membrane patches
Excised outside-out membrane patches were voltage-clamped at −50 mV and current recordings were performed at room temperature (21-23 °C) as previously described 23. Bath and superfusion solutions contained (in mM) 145 NaCl, 5 KCl, 10 HEPES, 2 CaCl2, and 1 MgCl2 at pH 7.4 (pH adjusted with N-methyl glucosamine). The intracellular (pipette) fluid contained (in mM) 140 KCl, 10 HEPES, 1 EGTA, and 2 MgCl2 at pH 7.3 (pH adjusted with KOH). Currents were stimulated using brief (0.5 – 1.0 s) pulses of GABA delivered via a multi-channel superfusion pipette coupled to piezo-electric elements that switched superfusion solutions in under 1 ms. Currents were filtered at 5 kHz and digitized at 10 kHz for off-line analysis.
Data Analysis
Leak-correction and measurement of peak currents was performed off-line using Clampfit8.0 software (Molecular Devices). Peak GABA-activated or etomidate-activated oocyte currents were normalized to maximal GABA-activated currents measured in the same cell. Concentration-response curves were assembled from pooled normalized data from multiple oocytes. Pooled data sets were fitted with logistic (Hill) functions using non-linear least squares in Origin 6.1 (OriginLab, Northampton, MA) and Prism 5.02, (GraphPad Software, San Diego, CA):
| Eq. 1 |
where A is amplitude, EC50 is the half-maximal activating concentration, and nH is Hill slope.
Etomidate potentiation of GABA responses was quantified as the ratio of the GABA EC50 values in the absence of anesthetic to that in the presence of 3.2 μM etomidate. GABA concentration-response curves shift leftward (i.e., to a lower GABA EC50) in the presence of etomidate; thus large EC50 ratios indicate strong modulation, while a ratio of 1.0 or less indicates lack of positive modulation25.
Picrotoxin-sensitive leak currents (IPTX) were normalized to , providing estimates of basal open probability (P0). Incorporation of the α1(L264T) mutation was used to enhance basal gating for comparison of the effects of β2N265 mutations. Maximal GABA efficacy was assessed by first activating oocyte-expressed channels with 10 mM GABA. After full current activation and partial desensitization, superfusate was switched to 10 mM GABA plus 2 μM alphaxalone, a strong positive modulator of wild-type and both mutant receptors. Maximal GABA efficacy was calculated as the ratio of current immediately before the addition of alphaxalone to the secondary current peak after the addition of alphaxalone (IGABA+alphax).
Estimated open probability , defined as the fraction of activatable receptors in the open state, was calculated by explicitly adding spontaneous current and renormalizing to the full range of open probability, assuming picrotoxin-blocked leak represents no activation (Popen = 0) and maximal GABA plus alphaxalone activates all nondesensitized channels (Popen = 1.0). 26 In practice the elements used to calculate are all normalized to :
| Eq. 2 |
Quantitative analysis based on Monod-Wyman-Changeux coagonism5 was performed as follows: Average values calculated from GABA concentration-responses (with and without etomidate) and etomidate direct activation data were pooled. With both [GABA] and [etomidate] specified as independent variables, these data were globally fitted to Eq. 3 using nonlinear least squares:
| Eq. 3 |
This equation describes a two-state equilibrium allosteric mechanism with two classes of agonist sites (one for GABA and one for etomidate), each with two equivalent sites. L0 in Eq. 3 is a dimensionless basal equilibrium gating variable, approximately P0-1. KG and KE are equilibrium dissociation constants for GABA and etomidate binding to inactive states, and c and d are dimensionless parameters representing the respective ratios of binding constants in active versus inactive states. The agonist efficacies of GABA and etomidate are inversely related to c and d, respectively.
To analyze membrane patch macrocurrents for activation, desensitization, and deactivation kinetics, data windows were specified in each trace for different phases of the waveform. Activation windows were from 10% above the baseline trace to a point where desensitization had reduced the peak current by 3-5%. Desensitization windows were from the current peak to the end of GABA application. Deactivation windows were from the end of GABA application to the end of the sweep. Windowed data were fitted to multiple exponential functions using nonlinear least squares:
| Eq. 4 |
The number of components for each fit was determined by comparison of single-, double-, and triple-exponential fits, using an F-test to choose the best exponential fit model with a confidence value of P = 0.99 (Clampfit8.0; Molecular Devices).
Statistical analysis
Results are reported as mean ± standard deviation unless otherwise indicated. Nonlinear regression errors are those from fits in Origin 6.1 (OriginLab). Statistical comparison of fitted parameters was performed using Prism 5.02 (GraphPad Software). Single parameter group comparisons were performed using either a two-tailed Student t-test (with independent variances) or ANOVA with Tukey's post-hoc multiple comparisons test in Microsft Excel (Microsoft Corporation, Remond, WA) with an add-on statistical toolkit (StatistiXL; Nedlands, Australia). Statistical significance was inferred at p < 0.05.
Results
GABA concentration-response relationships and etomidate modulation
GABA concentration-responses with and without etomidate were measured in Xenopus oocytes and normalized to without etomidate (fig. 1; table 1). A logistic fit to pooled wild-type α1β2γ2L receptor data revealed a GABA EC50 of 26 μM and a Hill slope of 1.2 ± 0.09. Addition of 3.2 μM etomidate dramatically enhanced currents elicited with low GABA, causing a 14-fold decrease in the wild-type GABA EC50 to 1.9 μM and a small increase in the maximal GABA-activated current. Currents from α1β2(N265S)γ2L receptors expressed in oocytes were characterized by GABA EC50 = 27 μM, which was not significantly different from that of wild-type (p = 0.67). Addition of etomidate weakly enhanced currents elicited with low GABA and produced a 2.3-fold reduction in GABA EC50 to 12 μM. This shift was significantly smaller than that observed in wild-type (p < 0.0001). GABA-activated currents from α1β2(N265M)γ2L receptors were characterized by an EC50 value of 32 μM, significantly different from wild-type (p = 0.011). Etomidate did not enhance GABA-activated currents from this mutant, and GABA EC50 in the presence of 3.2 μM etomidate was 34 μM, not significantly different from GABA EC50 without etomidate (p = 0.64).
Figure 1. GABA concentration-response curves and etomidate left shifts.
Each panel displays mean ± sd data for peak GABA-activated oocyte currents normalized to the maximum response. Solid symbols represent control data (no etomidate). Open symbols represent responses measured in the presence of 3.2 μM etomidate. Lines through data represent non-linear least squares fits of data to logistic functions. Fitted parameters are reported in table 1. Insets in the lower right of each panel display examples of current sweeps elicited with either 10 or 1000 μM GABA in a single oocyte (bars above sweeps indicate application). Insets in the upper left of each panel display examples of current sweeps recorded from the same oocyte in the presence of etomidate. Panel A: Wild-type α1β2γ2L (squares). Etomidate strongly potentiates currents at 10 μM GABA and induces a large leftward shift in GABA concentration-responses (14-fold reduction in EC50); Panel B: α1β2(N265S)γ2L (circles). Etomidate weakly potentiates currents elicited with 10 μM GABA and induces a small leftward shift in GABA concentration-response (2.3-fold reduction in EC50); Panel C: α1β2(N265M)γ2L (triangles). Etomidate does not enhance GABA-activated currents and does not significantly shift the GABA concentration-response curve.
Table 1.
β2N265 Mutation Impact on GABA Responses, Etomidate Modulation, and Direct Etomidate Activation of GABAA Receptors
| Receptor | ||||
|---|---|---|---|---|
| Fitted Parameter a | α1β2γ2L | α1β2(N265S)γ2L | α1β2(N265M)γ2L | |
| GABA Responses (0 Etomidate) | GABA EC50 (μM) | 26 ± 1.8 | 27 ± 1.5 | 32 ± 0.9* |
| GABA Hill Slope | 1.2 ± 0.18 | 1.4 ± 0.15 | 1.6 ± 0.07* | |
| Etomidate Modulation of GABA Responses | GABA EC50 with 3.2 μM | 1.9 ± 0.36 | 12 ± 0.71** | 34 ± 2.1** |
| Etomidate (μM) | ||||
| EC50 ratio (ctl/Eto) b | 14 | 2.3** | 0.95** | |
| Etomidate Direct Activation (0 GABA) | Etomidate EC50 (μM) | 36 ± 1.1 | 78 ± 25 | na |
| Etomidate Hill slope | 1.3 ± 0.54 | 1.7 ± 0.48 | na | |
| Etomidate efficacy (Fraction GABA max) |
0.43 ± 0.17 | 0.03 ± 0.011** | < 0.001** | |
Errors are calculated in Origin6.1 (OriginLab, Northampton, MA). P values for fit comparisons were assessed using GraphPad Prism 5.02 (GraphPad Software, San Diego, CA).
Differs from wild-type at p < 0.05
Differs from wild-type at p < 0.005.
Etomidate direct activation
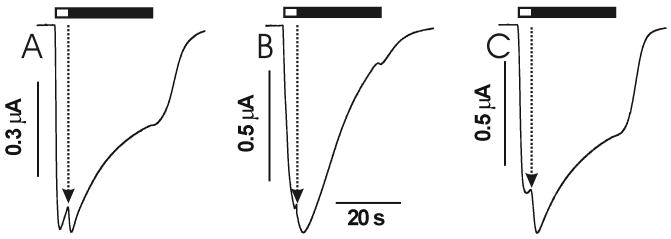
Etomidate, in the absence of GABA, directly activated chloride currents in oocytes expressing wild-type α1β2γ2L GABAA receptors (fig. 2, table 1). Maximal direct activation averaged 43% of the maximal GABA-activated current , and was observed at 0.3 mM etomidate. Half-maximal direct activation was at 36 μM etomidate. In α1β2(N265S)γ2L receptors, etomidate elicited small currents with maximum amplitude only 3% of that elicited by high GABA. Half-maximal direct activation in α1β2(N265S)γ2L receptors was at about 80 μM etomidate, but it was not significantly different from wild-type because of large uncertainty (p =0.24). Etomidate at up to 1 mM did not elicit any currents in oocytes expressing α1β2(N265M)γ2L receptors.
Figure 2. Direct activation of GABAA receptor currents by etomidate.
Panels A-C display voltage-clamp currents elicited first with 300 μM etomidate and then with 1000 μM GABA in oocytes. Small etomidate responses are magnified ten times (dotted sweeps). A: Wild-type α1β2γ2L receptors. B: α1β2(N265S)γ2L. C: α1β2(N265M)γ2L. Panel D shows mean (± sd) data from at least 5 oocytes for each type of receptor. Etomidate-activated currents are normalized to maximal GABA (1-3 mM) currents measured in the same oocyte. Wild type (solid squares); α1β2(N265S)γ2L (solid circles); α1β2(N265S)γ2L 10 × responses (open circles); α1β2(N265M)γ2L (solid triangles). Lines through wild-type and β2(N265S) data represent nonlinear least squares fits to logistic functions. Fitted parameters are reported in table 1.
Maximal GABA efficacy
Maximal GABA efficacy was estimated by first activating receptors with a saturating concentration of GABA (10 mM ≈ 380 × EC50), then adding 2 μM alphaxalone (fig. 3). Alphaxalone more than doubled currents elicited with GABA concentrations below EC50 showing that it is a strong positive modulator of all three receptors (not shown). In oocytes expressing wild-type α1β2γ2L receptors, alphaxalone enhanced maximal GABA currents on average by 9 ± 3.6% (n = 8). Alphaxalone enhanced maximal currents in receptors containing β2(N265S) and β2(N265M) mutations by 7 ± 3.2% (n = 10) and 19 ± 6.1% (n = 7), respectively. Assuming that 10 mM GABA plus alphaxalone activates all nondesensitized channels, we infer that GABA alone has an efficacy of 0.92 ± 0.030 in wild-type, 0.93 ± 0.030 in α1β2(N265S)γ2L, and 0.84 ± 0.043 in α1β2(N265M)γ2L. The results for the β2(N265M) mutant demonstrate significantly lower GABA efficacy (ANOVA p < 0.001) than either of the other two receptors. Wild-type and β2(N265S) mutant results were not significantly different (p = 0.74).
Figure 3. The impact of β2N265 mutations on maximal GABA efficacy.
Each panel displays a voltage-clamp current sweep from an oocyte expressing different types of GABAA receptors. Currents were elicited first with 10 mM GABA for 4 seconds (open bar), then with 10 mM GABA plus 2 μM alphaxalone (solid bar). Maximal GABA currents were enhanced by alphaxalone in all three receptors. The arrows point to the GABA current immediately before alphaxalone enhancement, which was used to calculate GABA efficacy. Panel A: Wild-type α1β2γ2L; Panel B: α1β2(N265S)γ2L; Panel C: α1β2(N265M)γ2L. Alphaxalone enhances α1β2(N265M)γ2L more than wild-type or α1β2(N265S)γ2L receptors, indicating that GABA efficacy is lower in α1β2(265M)γ2L than in the other channels.
Spontaneous channel activity
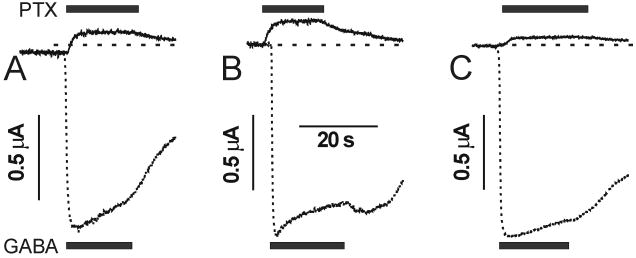
Basal gating of all three receptors was assessed using picrotoxin, a potent GABAA receptor inhibitor. Picrotoxin did not significantly reduce resting oocyte leak currents in cells expressing any of the three receptors (n ≥ 3; data not shown). Given that maximal GABA currents in oocytes were as high as 7 μA and our equipment can detect changes as small as 5 nA, we infer that the basal open probability of all three receptors is less than 0.1%. To more precisely quantify the effects of mutations on basal gating, we substituted wild-type α1 subunits with α1(L264T), containing a mutation that confers measurable basal gating activity (fig. 4). Picrotoxin (2 mM) produced an apparent outward current in α1(L264T)β2γ2L receptors that averaged 12 ± 4.5% (n = 7) of maximal GABA response. In oocytes expressing α1(L264T)β2(N265S)γ2L and α1(L264T)β2(N265M)γ2L receptors, picrotoxin produced apparent outward currents that averaged respectively, 15 ± 7.0% (n = 11) and 7 ± 2.4% (n = 7) of maximal GABA currents. The α1(L264T)β2γ2L and α1(L264T)β2(N265S)γ2L results were not significantly different (ANOVA p = 0.15), while α1(L264T)β2(N265M)γ2L results differed significantly from the other two receptors (p = 0.0004 vs. wt and p < 0.0001 vs. N265M). These results indicate that the β2(N265S) mutation causes little or no change in basal gating, while the β2(N265M) mutation reduces basal gating probability approximately two-fold.
Figure 4. Estimating the influence of β2N265 mutations on basal channel gating.
A mutation in the α1 subunit (L264T) was used to produce GABAA channels that open in the absence of GABA. A high concentration of picrotoxin (PTX; bars above sweeps indicate application) was applied to block spontaneously open channels, producing an apparent outward current, which was normalized to maximal GABA-activated current (GABA; bars below sweeps indicate application) for each type of receptor. Panel A: Wild-type α1(L264T)β2γ2L; Panel B: α1(L264T)β2(N265S)γ2L; Panel C: α1(L264T)β2(N265M)γ2L. IPTX/IGABA is smaller for α1(L264T)β2(N265M)γ2L than for α1(L264T)β2γ2L or α1(L264T)β2(N265S)γ2L receptors.
Allosteric coagonist modeling
Global mechanism-based analysis of oocyte data was performed as previously described 26. An initial fit with all parameters free to vary was used to determine the L0 value for wild-type (25,000 ± 9,000). Because our experimental results indicated that the β2(N265S) mutation does not alter basal gating, the L0 value was used in fitting α1β2(N265S)γ2L receptor data was constrained to equal this wild-type value (Table 2). Because our data indicated that the β2(N265M) mutation reduced basal gating probability two-fold relative to wild-type channels, the L0 value for fitting α1β2(N265M)γ2L data was fixed at twice that used for wild-type. Fits to calculated data sets for both wild-type and α1β2(N265S)γ2L converged for all four remaining free parameters in equation 3 (Methods). Because α1β2(N265M)γ2L receptors are totally insensitive to etomidate, fits to data for this receptor did not converge on values for either KE and d. These parameters were removed from equation 3 for subsequent fit to α1β2(N265M)γ2L data, resulting in well-determined parameters for both KG and c. Results of the nonlinear least squares fits are reported in table 2 and displayed in figure 5.
Table 2.
Allosteric Coagonist Models for Wild-Type and β2N265 Mutant GABAA Receptors
| Receptor | |||
|---|---|---|---|
| Model Parameter a | α1β2γ2L | α1β2(N265S)γ2L | α1β2(N265M)γ2L |
| L0 b (Basal Gating) |
25,000 | 25,000 | 50,000 |
| KG (μM) (GABA Dissociation Constant) |
70 ± 22 | 68 ± 7.4 | 59 ± 3.6 |
| c-1 (GABA Efficacy)c |
530 ± 105 | 560 ± 37 | 530 ± 28 |
| KE (μM) (Etomidate Dissociation Constant) |
40 ± 9.6 | 88 ± 42 | n.a. |
| d-1 (Etomidate Efficacy)d |
130 ± 12 | 26 ± 10.8** | n.a. |
Errors are calculated in Origin6.1 (OriginLab, Northampton, MA). P values for fit comparisons were assessed using GraphPad Prism 5.02 (GraphPad Software, San Diego, CA).
Differs from wild-type at p < 0.005.
Model parameters (equation 3, methods) were fitted to estimated Popen data (equation 2, methods) using non-linear least squares.
L0 values were fixed in the fits. The L0 value for fits to both wild-type and α1β2(N265S)γ2L data was based on analysis of wild-type data where L0 was allowed to vary (L0 = 25,000 ± 9,000). For fits to α1β2(N265M)γ2L data, the value for L0 was set at twice the value used for wild-type, and KE and d were removed from the fitting equation.
GABA efficacy is inversely related to c.
Etomidate efficacy is inversely related to d. Data and results are graphically displayed in figure 5.
Figure 5. Allosteric coagonist models for GABA and etomidate-dependent receptor activation.
Estimated open probabilities were calculated from mean normalized concentration-response data (fig. 1 and 2) using equation 2. Solid symbols represent control GABA concentration-responses, open symbols represent GABA responses in the presence of 3.2 μM etomidate, and cross-hatched symbols represent etomidate direct activation responses. Combined data for each channel type were fitted with equation 3 (methods) and results plotted as solid lines in the panels. Fitted parameters are reported in table 2. Panel A: Wild-type α1β2γ2L (square symbols); Panel B: α1β2(N265S)γ2L (circles); Panel C: α1β2(N265M)γ2L (triangles).
Macrocurrent kinetics
GABA-activated currents in outside-out patches excised from transfected HEK293 cells were used to study macrocurrent kinetics of GABAA receptors (fig. 6, table 3). As previously reported 23, α1β2γ2L wild-type currents elicited with high GABA concentrations display rapid activation, multiphasic desensitization, and biphasic deactivation after discontinuation of agonist. Currents elicited from patches expressing mutant receptors showed similar kinetics, which were analyzed in detail. For wild-type and both mutants, the best fit (F-test; P = 0.99) number of exponential deactivation phases was two in all patches and the best-fit number of desensitization phases was two in the majority (18 of 23) of patches. Thus, for comparison, deactivation and desensitization phases of all patches were re-analyzed using double-exponential functions. Statistical comparison using ANOVA indicates that fitted time constants for activation, desensitization, and deactivation are indistinguishable for all three types of receptor channels (table 3). Fast deactivation rate analysis suggested that this phase in α1β2(N265M)γ2L may be faster than in wild-type receptors (59 s-1 vs. 42 s-1), although this difference was not statistically significant (p = 0.15).
Figure 6. GABAA receptor current kinetics from rapidly superfused patch-clamp studies.
Each panel displays a single current sweep recorded from a patch exposed to 1 mM GABA for 1 second (indicated by the bars over the current traces). Currents display three distinct phases that were analyzed separately: activation is the rapid evolution of GABA-activated inward current; desensitization is the drop in current during GABA application; and deactivation is the return to baseline after termination of GABA application. Inset sweeps display activation phases at an expanded scale. Results of kinetic analyses are summarized in table 3. Panel A: Wild-type α1β2γ2L; Panel B: α1β2(N265S)γ2L; Panel C: α1β2(N265M)γ2L.
Table 3.
β2N265 Mutation Impact on GABA-stimulated Activation, Desensitization, and Deactivation Time Constants
| Receptor | |||
|---|---|---|---|
| Current Phase | α1β2γ2L | α1β2(N265S)γ2L | α1β2(N265M)γ2L |
| Activation τ (ms) (n) | 0.61 ± 0.34 (7) | 0.72 ± 0.28 (6) | 0.63 ± 0.18 (6) |
| Fast Desensitization τ (ms) | 33 ± 9.7 | 26 ± 11 | 23 ± 12 |
| Fraction Fast (n) | 0.4 ± 0.09 (5) | 0.2 ± 0.17 (5) | 0.2 ± 0.09 (5) |
| Slow Desensitization τ (ms) | 1,300 ± 430 | 1,100 ± 180 | 1,300 ± 560 |
| Fraction Slow (n) | 0.6 ± 0.09 (5) | 0.8 ± 0.17 (5) | 0.8 ± 0.09 (5) |
| Fast Deactivation τ (ms) | 24 ± 7.6 | 21 ± 3.9 | 17 ± 8.7 |
| Fraction Slow (n) | 0.6 ± 0.35 (7) | 0.5 ± 0.30 (6) | 0.5 ± 0.13 (6) |
| Slow Deactivation τ (ms) | 210 ± 140 | 190 ± 150 | 140 ± 100 |
| Fraction Fast (n) | 0.4 ± 0.35 (7) | 0.5 ± 0.30 (6) | 0.5 ± 0.13 (6) |
Results are mean ± sd time constants (τ) for different phases of macrocurrents recorded using rapid patch superfusion (n indicates number of patches analyzed). The activation phase (current growth during GABA application) was fitted to single exponential functions using non-linear least squares regression. Desensitization (current decline during GABA application) and deactivation (current decline after GABA washout) phases were fitted to double exponential functions. Statistical comparisons performed using ANOVA identified no differences that were significant at p < 0.05.
Etomidate activation of spontaneously gating receptors with β1 versus β2
A final set of experiments addressed the efficacy and potency of etomidate direct activation in spontaneously active receptors containing the α1(L264T) mutation combined with γ2L and either β2 or β1 subunits (fig. 7). Oocyte-expressed α1β1γ2L receptors displayed no detectable (< 0.1%; n = 5) picrotoxin-sensitive spontaneous activity and are characterized by GABA EC50 = 190 μM (fig. 7A, solid diamonds). Oocyte-expressed α1(L264T)β1γ2L receptors display 11 ± 2.1% (n = 5) spontaneous activity and GABA EC50 = 3.8 μM (fig. 7A, open diamonds). High GABA responses are not significantly enhanced by alphaxalone in either α1(L264T)β1γ2L or α1(L264T)β2γ2L (not shown), indicating that maximal GABA efficacy in both gating mutant channels is near 1.0. Etomidate potently (EC50 = 0.8 μM) activates α1(L264T)β2γ2L receptors, with efficacy that is similar to GABA in these channels (fig. 7B, solid triangles). Using a value of 8 for L0 (calculated from Iptx/IGABA results; fig. 4), along with KE = 40 μM and d = 0.0076 from the wild-type α1β2γ2L global model fit (table 2), equation 3 closely predicts etomidate direct activation in α1(L264T)β2γ2L receptors. In contrast, the etomidate EC50 for direct activation of α1(L264T)β1γ2L receptors is 43 μM, and maximal etomidate activation reaches about 60% of the maximal GABA response. The α1(L264T)β1γ2L etomidate direct activation data was used to calculate using equation 2 and then fitted with equation 3, resulting in L0 = 9, KE = 58 μM, and d = 0.26. Thus, our estimated parameters for etomidate binding to closed receptors containing β1 versus β2 subunits differ by less than 50% (KE = 58 μM with β1 vs. 40 μM with β2), whereas etomidate efficacy (inversely related to d) is 34 times larger in β2-containing receptors versus β1-containing receptors (d-1 = 3.85 with β1 vs. 132 with β2).
Figure 7. The α1(L264T) gating mutation used to interpret etomidate interactions with GABAA receptors containing β1 or β2 subunits.
Panel A depicts GABA concentration-response data (mean ± SD) for oocytes expressing α1β1γ2L (solid diamonds) and α1(L264T)β1γ2L receptors (open diamonds). Lines represent fits to logistic functions: α1β1γ2L GABA EC50 = 190 ± 32 μM, Hill slope = 0.61 ± 0.064; α1(L264T)β1γ2L GABA EC50 = 3.8 ± 0.43 μM, Hill slope = 0.55 ± 0.073. Panel B depicts estimated Popen values (mean ± SD) from etomidate concentration-response studies of both α1(L264T)β1γ2L (crossed diamonds) and α1(L264T)β2γ2L (solid triangles). Lines through α1(L264T)β1γ2L points represent a nonlinear least squares fit to equation 3 with [GABA] = 0: L0 = 9.1 ± 0.84, KE = 58 ± 6.4 μM, d = 0.26 ± 0.038. The line through α1(L264T)β2γ2L data is equation 3 with L0 = 8 and other values set at those derived for α1β2γ2L receptors(KE = 40 μM, d = 0.0076).
Discussion
Our aim in this study was to better define the role of the β subunit residue at position 265 in GABAA receptor function both in the absence and presence of etomidate. Previous studies have reported diminished sensitivity to etomidate in heterologously expressed GABAA receptors containing either β2 or β3 subunits with N265S15 or N265M13,16,17,27,28 mutations. Unlike prior reports, our experiments quantitatively assessed etomidate modulation of GABA activation as the ratio of GABA EC50s in the absence and presence of a standard etomidate concentration, a robust measure of positive allosteric modulation.25,29 This approach demonstrates that in α1β2γ2L GABAA receptors, the β2(N265S) mutation reduces etomidate modulation by 6-fold (2.3-fold shift vs. 14-fold for wild-type), while β2(N265M) eliminates this shift entirely. We further tested the impact of the β2N265 mutations on direct receptor activation by etomidate. We found that etomidate elicits over 40% of maximal GABA current in wild-type, about 3% in α1β2(N265S)γ2L receptors, and no detectable current in α1β2(N265M)γ2L receptors. Thus, the effect of the β2N265 mutations on etomidate direct activation parallels that on modulation of GABA responses. Previous studies have also noted that both direct etomidate activation and modulation of GABA responses are reduced in receptors containing β1 versus β212 or those containing β2/3(N265M) mutations13.
We also evaluated whether β2N265 mutations alter GABAA receptor gating in the absence of etomidate, including experiments to measure spontaneous (0 GABA) gating activity, GABA EC50, maximal GABA efficacy, and the macroscopic rates of transitions among major functional states. We found that none of these parameters was significantly altered by the β2(N265S) mutations, while the β2(N265M) mutation produced a 23% increase in GABA EC50 (table 1), and also significantly reduced maximal GABA efficacy from 0.92 to 0.84. While we could detect no spontaneous gating in wild-type, α1β2(N265S)γ2L or α1β2(N265M)γ2 channels, novel experiments exploiting the spontaneously gating α1(L264T)β2γ2L mutant channel background reveal that substituting β2(N265M) for β2 reduces basal gating activity by 50%, while substitution with β2(N265S) causes no significant change. Analysis of GABA-activated current kinetics recorded using sub-millisecond concentration jumps shows that activation, desensitization, and deactivation rates are not significantly altered by either β2(N265S) or β2(N265M), although rapid deactivation tended to be faster in α1β2(N265M)γ2L receptors than in wild-type (table 3).
A number of previous studies have reported that β2(N265M) or β3(N265M) mutations increase GABA EC50 16,17,28,30. Nishikawa et al30 studied a series β2N265 mutations in α1β2γ2L receptors in HEK293 cells, and found that GABA EC50 approximately doubled with β2(N265M), and increased about 50% with β2(N265S). One previous study by Miko et al31 investigated spontaneous gating in homomeric β1 GABAA receptors and the effects of β1(S265) mutations. In that study, mutant β3(N265S) subunits did not induce spontaneous gating in homomeric or heteromeric channels. No previous studies have assessed the influence of β2N265 or β3N265 mutations on macrocurrent kinetics in heterologously expressed channels. However, because GABA reuptake from synapses occurs in under a millisecond32, GABAergic inhibitory postsynaptic current decay is largely dependent on channel deactivation. Reynolds et al2 reported that GABAergic miniature inhibitory postsynaptic current decays were similar in cerebellar Purkinje neurons from both wild-type and β2(N265S) knock-in mice. Drexler et al33 found that IPSC decays in neocortical neurons of wild-type and β3(N265M) knock-in mice were not significantly different. The subunit compositions of channels mediating these neuronal IPSCs is uncertain, but inhibitory postsynaptic current prolongation by etomidate was dramatically reduced in currents recorded from knock-in versus wild-type cells, demonstrating that GABAA receptors containing mutant β subunits were present. These results therefore indicate that in vivo neuronal GABAA channel kinetics are minimally altered by N265S and N265M mutations.
Allosteric gating models have proven useful for interpretation of how mutations alter the function of ligand-gated ion channels34,35. We have used allosteric coagonist models to interpret both etomidate actions on GABAA receptors5 and the effects of mutations on etomidate sensitivity26, and have applied this approach here to analyze the impact of the β2N265 mutants we studied (fig. 5, table 2). Importantly, allosteric coagonist models appear to fit our wild-type and mutant data well, reinforcing the hypothesis that both etomidate modulation of GABA activation and direct etomidate activation of GABAA receptors are manifestations of interactions at a single class of etomidate sites observed under different experimental conditions.5 After correcting L0 values according to our basal gating results, GABA binding (KG) and efficacy (c-1) parameters in all three fitted models (table 2) are all quite similar and not statistically different, suggesting that these mutations have little or no impact on GABA interactions. The total lack of etomidate effects in α1β2(N265M)γ2L receptors makes it impossible to fit etomidate binding and efficacy parameters in the allosteric model. However, comparison of coagonist model parameters for wild-type versus α1β2(N265S)γ2L receptors indicates that the β2(N265S) mutation reduces etomidate efficacy (d-1) five-fold from wild-type, while etomidate binding (1/KE) affinity is reduced about two-fold.
Direct activation experiments using the gating mutant α1(L264T) expressed together with β1 vs. β2 also addressed whether etomidate binding versus efficacy is affected by changing β subunit subtype. Combining the gating mutant L0 with fitted values for etomidate binding (KE) and efficacy (d) from the α1β2γ2L model accurately predicts etomidate direct activation of α1(L264T)β2γ2L channels. This result suggests that the α1(L264T) mutation does not affect etomidate binding or efficacy, but simply sensitizes receptors to etomidate gating by increasing their tendency to open. Most importantly, α1(L264T)β1γ2L and α1(L264T)β2γ2L channels display similar spontaneous activity, but those with β1 subunits display less etomidate direct activation and much lower apparent affinity (etomidate EC50) than those with β2 subunits. Allosteric mechanism analysis indicates that these changes are largely due to etomidate efficacy (d-1) that is 34-fold lower in α1(L264T)β1γ2L vs. α1(L264T)β2γ2L.
The analysis of β2N265 mutants contrasts with our recent study of tryptophan mutations at α1M236 and β2M286 26, and is most consistent with the conclusion that β2N265 is not a contact point for etomidate binding to GABAA receptors. Instead this residue may act as a transduction element between the etomidate sites and the ion channel. Other evidence also supports the conclusion that β2N265 does not contribute to etomidate binding. Recent structural models of the GABAA receptor transmembrane domains11,18,19, based on disulfide cross-linking and photolabeling data, depict βN265 located outside the intersubunit cleft where both etomidate and propofol bind. Furthermore, propofol does not protect cysteine substitutions at β2N265 from covalent modification, whereas it does protect cysteine modification at β2M286 36, a site where there is abundant evidence for contact with etomidate 11,26. An alternative interpretation of our findings is based on the concept of efficacy in allosteric gating models. Agonist (or coagonist) efficacy is the ratio of equilibrium binding dissociation constants in the two canonical states: active (open channel) versus inactive (closed channel). Thus, reduced etomidate efficacy in β2N265 mutants implies reduced etomidate affinity for the transient open GABAA receptor state. This introduces the possibility that while β2N265 does not contribute to etomidate binding to inactive GABAA receptors, it might make contact with etomidate during channel opening. This interpretation also points out the importance of defining anesthetic binding determinants in more than one receptor state, a task that may be achieved using time-resolved photolabeling 37,38.
Mutations that alter GABAA receptor anesthetic sensitivity in vitro represent potential tools for knock-in animal studies linking subunits to the various actions of anesthetics 39. In this regard, the β2(N265S) mutation is remarkable for reducing etomidate sensitivity without affecting basal or GABA-activated receptor activity. Indeed, Reynolds et al 2 reported that β2(N265S) knock-in mice have normal receptor expression, normal baseline and sleeping electroencephalographic activity, and normal baseline behavior. In comparison with β2(N265S), the β2(N265M) mutation produces two molecular effects: a small negative gating effect in the absence of etomidate combined with abolition of etomidate sensitivity. However, while the viability and general behavior of β3(N265M) knock-in mice is apparently normal, no data has been published on their awake or sleeping electroencephalographic patterns or susceptibility to seizures. One behavioral study 40 reported that baseline freezing in response to a learned fear context was significantly lower in β3(N265M) knock-ins versus wild-type mice.
GABAA receptor mutations with negative gating effects in vitro, such as γ2(K289M), are associated with increased GABA EC50 and with epilepsy in vivo 41, while gain-of-function mutations such as α1(S270H) reduce GABA EC50 and have been associated with grossly abnormal anatomic and behavioral phenotypes in knock-in animals 42. Allosteric models illustrate that in vitro GABA EC50 is a function of baseline channel open probability (L0), GABA binding affinity (KG), and efficacy (c). A single mutation could simultaneously alter both L0 and GABA efficacy, resulting in a near-normal GABA EC50, but significantly abnormal channel activity. Therefore, GABA EC50 alone may be a misleading predictor of channel activity and in vivo phenotype. Assessment of basal gating changes and maximal GABA efficacy, together with GABA EC50, will provide a stronger basis for this prediction.
Acknowledgments
The authors thank Aiping Liu, M.S., Senior Technician, Dept. of Anesthesia & Critical Care, Massachusetts General Hospital, Boston, Massachusetts for technical assistance. We also thank Uwe Rudolph, M.D., Director of the Laboratory for Genetic Pharmacology, McLean Hospital, Belmont, Massachusetts, for his insights into knock-in animal phenotypes.
Funding: This research was supported by grants (P01GM58448 and R01GM66724) from the National Institutes of Health, Bethesda, Maryland, and the Department of Anesthesia & Critical Care, Massachusetts General Hospital, Boston, Massachusetts
Footnotes
Attribution: Department of Anesthesia & Critical Care, Massachusetts General Hospital, Boston, Massachusetts
Contributor Information
Rooma Desai, Dept. of Anesthesia & Critical Care, Massachusetts General Hospital, Boston, Massachusetts.
Dirk Ruesch, Dept. of Anesthesia & Critical Care, University Hospital Giessen-Marburg, Marburg, Germany.
Stuart A. Forman, Dept. of Anesthesia & Critical Care, Massachusetts General Hospital, Boston, Massachusetts.
References
- 1.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds DS, Rosahl TW, Cirone J, O'Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA. Sedation and anesthesia mediated by distinct GABA(A) receptor isoforms. J Neurosci. 2003;23:8608–17. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Uchida I. Mechanisms of etomidate potentiation of GABAA receptor-gated currents in cultured postnatal hippocampal neurons. Neuroscience. 1996;73:69–78. doi: 10.1016/0306-4522(96)00018-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhong H, Rusch D, Forman SA. Photo-activated azi-etomidate, a general anesthetic photolabel, irreversibly enhances gating and desensitization of gamma-aminobutyric acid type A receptors. Anesthesiology. 2008;108:103–12. doi: 10.1097/01.anes.0000296074.33999.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rusch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on alpha1beta2gamma2L GABA-A receptors accounts for both direct activation and agonist modulation. J Biol Chem. 2004;279:20982–92. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- 6.Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol. 2006;54:231–63. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- 7.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Pharmacol Rev. 2008;60:243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–24. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–5. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 10.Husain SS, Ziebell MR, Ruesch D, Hong F, Arevalo E, Kosterlitz JA, Olsen RW, Forman SA, Cohen JB, Miller KW. 2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: A derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. J Med Chem. 2003;46:1257–65. doi: 10.1021/jm020465v. [DOI] [PubMed] [Google Scholar]
- 11.Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill-Venning C, Belelli D, Peters JA, Lambert JJ. Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type A receptor. Br J Pharmacol. 1997;120:749–56. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci USA. 1997;94:11031–6. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moody EJ, Knauer C, Granja R, Strakhova M, Skolnick P. Distinct loci mediate the direct and indirect actions of the anesthetic etomidate at GABA-A receptors. J Neurochem. 1997;69:1310–3. doi: 10.1046/j.1471-4159.1997.69031310.x. [DOI] [PubMed] [Google Scholar]
- 15.Cestari IN, Min KT, Kulli JC, Yang J. Identification of an amino acid defining the distinct properties of murine beta1 and beta3 subunit-containing GABA(A) receptors. J Neurochem. 2000;74:827–38. doi: 10.1046/j.1471-4159.2000.740827.x. [DOI] [PubMed] [Google Scholar]
- 16.Pistis M, Belelli D, McGurk K, Peters JA, Lambert JJ. Complementary regulation of anaesthetic activation of human (alpha6beta3gamma2L) and Drosophila (RDL) GABA receptors by a single amino acid residue. J Physiol (Lond) 1999;515:3–18. doi: 10.1111/j.1469-7793.1999.003ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegwart R, Jurd R, Rudolph U. Molecular determinants for the action of general anesthetics at recombinant alpha(2)beta(3)gamma(2)gamma-aminobutyric acid(A) receptors. J Neurochem. 2002;80:140–8. doi: 10.1046/j.0022-3042.2001.00682.x. [DOI] [PubMed] [Google Scholar]
- 18.Jansen M, Akabas MH. State-dependent cross-linking of the M2 and M3 segments: Functional basis for the alignment of GABAA and acetylcholine receptor M3 segments. J Neurosci. 2006;26:4492–9. doi: 10.1523/JNEUROSCI.0224-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bali M, Jansen M, Akabas MH. GABA-induced intersubunit conformational movement in the GABAA receptor alpha1M1-beta2M3 transmembrane subunit interface: Experimental basis for homology modeling of an intravenous anesthetic binding site. J Neurosci. 2009;29:3083–92. doi: 10.1523/JNEUROSCI.6090-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–89. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Boileau AJ, Baur R, Sharkey LM, Sigel E, Czajkowski C. The relative amount of cRNA coding for gamma2 subunits affects stimulation by benzodiazepines in GABA(A) receptors expressed in Xenopus oocytes. Neuropharmacology. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 22.Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA. Effect of γ2S subunit incorporation on GABAA receptor macroscopic kinetics. Neuropharmacology. 2003;44:1003–12. doi: 10.1016/s0028-3908(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 23.Scheller M, Forman SA. Coupled and uncoupled gating and desensitization effects by pore domain mutations in GABA(A) receptors. J Neurosci. 2002;22:8411–21. doi: 10.1523/JNEUROSCI.22-19-08411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusch D, Forman SA. Classic benzodiazepines modulate the open-close equilibrium in alpha1beta2gamma2L gamma-aminobutyric acid type A receptors. Anesthesiology. 2005;102:783–92. doi: 10.1097/00000542-200504000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Ehlert FJ. Analysis of allosterism in functional assays. J Pharmacol Exp Ther. 2005;315:740–54. doi: 10.1124/jpet.105.090886. [DOI] [PubMed] [Google Scholar]
- 26.Stewart DS, Desai R, Cheng Q, Liu A, Forman SA. Tryptophan mutations at azi-etomidate photo-incorporation sites on α1 or β2 subunits enhance GABAA receptor gating and reduce etomidate modulation. Mol Pharmacol. 2008;74:1687–95. doi: 10.1124/mol.108.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGurk KA, Pistis M, Belelli D, Hope AG, Lambert JJ. The effect of a transmembrane amino acid on etomidate sensitivity of an invertebrate GABA receptor. Br J Pharmacol. 1998;124:13–20. doi: 10.1038/sj.bjp.0701787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegwart R, Krahenbuhl K, Lambert S, Rudolph U. Mutational analysis of molecular requirements for the actions of general anaesthetics at the gamma-aminobutyric acidA receptor subtype, alpha1beta2gamma2. BMC Pharmacol. 2003;3:13. doi: 10.1186/1471-2210-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall D. Modeling the functional effects of allosteric modulators at pharmacological receptors: An extension of the two-state model of receptor activation. Mol Pharmacol. 2000;58:1412–23. doi: 10.1124/mol.58.6.1412. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa K, Jenkins A, Paraskevakis I, Harrison NL. Volatile anesthetic actions on the GABAA receptors: Contrasting effects of alpha 1(S270) and beta 2(N265) point mutations. Neuropharmacology. 2002;42:337–45. doi: 10.1016/s0028-3908(01)00189-7. [DOI] [PubMed] [Google Scholar]
- 31.Miko A, Werby E, Sun H, Healey J, Zhang L. A TM2 residue in the beta1 subunit determines spontaneous opening of homomeric and heteromeric gamma-aminobutyric acid-gated ion channels. J Biol Chem. 2004;279:22833–40. doi: 10.1074/jbc.M402577200. [DOI] [PubMed] [Google Scholar]
- 32.Overstreet LS, Westbrook GL, Jones MV. Measuring and Modeling the Spatiotemporal Profile of GABA at the Synapse. In: Quick MW, editor. Transmembrane Transporters. Hoboken, New Jersey: Wiley-Liss; 2002. pp. 259–75. [Google Scholar]
- 33.Drexler B, Jurd R, Rudolph U, Antkowiak B. Dual actions of enflurane on postsynaptic currents abolished by the gamma-aminobutyric acid type A receptor beta3(N265M) point mutation. Anesthesiology. 2006;105:297–304. doi: 10.1097/00000542-200608000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Changeux J, Edelstein SJ. Allosteric mechanisms in normal and pathological nicotinic acetylcholine receptors. Curr Opin Neurobiol. 2001;11:369–77. doi: 10.1016/s0959-4388(00)00221-x. [DOI] [PubMed] [Google Scholar]
- 35.Galzi JL, Edelstein SJ, Changeux J. The multiple phenotypes of allosteric receptor mutants. Proc Natl Acad Sci USA. 1996;93:1853–8. doi: 10.1073/pnas.93.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bali M, Akabas MH. Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol. 2004;65:68–76. doi: 10.1124/mol.65.1.68. [DOI] [PubMed] [Google Scholar]
- 37.Addona GH, Kloczewiak MA, Miller KW. Time-resolved photolabeling of membrane proteins: Application to the nicotinic acetylcholine receptor. Anal Biochem. 1999;267:135–40. doi: 10.1006/abio.1998.2959. [DOI] [PubMed] [Google Scholar]
- 38.Arevalo E, Chiara DC, Forman SA, Cohen JB, Miller KW. Gating-enhanced accessibility of hydrophobic sites within the transmembrane region of the nicotinic acetylcholine receptor's {delta}-subunit. A time-resolved photolabeling study. J Biol Chem. 2005;280:13631–40. doi: 10.1074/jbc.M413911200. [DOI] [PubMed] [Google Scholar]
- 39.Zeller A, Arras M, Jurd R, Rudolph U. Mapping the contribution of beta3-containing GABAA receptors to volatile and intravenous general anesthetic actions. BMC Pharmacol. 2007;7:2. doi: 10.1186/1471-2210-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao M, Sonner JM, Jurd R, Rudolph U, Borghese CM, Harris RA, Laster MJ, Eger EI., 2nd Beta3-containing gamma-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesth Analg. 2005;101:412–8. doi: 10.1213/01.ANE.0000154196.86587.35. [DOI] [PubMed] [Google Scholar]
- 41.Bianchi MT, Song L, Zhang H, Macdonald RL. Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. J Neurosci. 2002;22:5321–7. doi: 10.1523/JNEUROSCI.22-13-05321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Homanics GE, Elsen FP, Ying SW, Jenkins A, Ferguson C, Sloat B, Yuditskaya S, Goldstein PA, Kralic JE, Morrow AL, Harrison NL. A gain-of-function mutation in the GABA receptor produces synaptic and behavioral abnormalities in the mouse. Genes Brain Behav. 2005;4:10–9. doi: 10.1111/j.1601-183X.2004.00090.x. [DOI] [PubMed] [Google Scholar]