Abstract
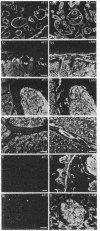
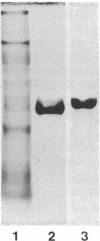
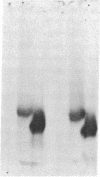
We have identified a tissue-specific basement membrane-associated protein by using monoclonal antibodies prepared against a protein fraction of human placenta. In immunofluorescence, the monoclonal antibodies stained basement membranes of Schwann cells, striated muscle, and trophoblast, whereas no reaction was seen with any other basement membrane or tissue structure. In antibody-affinity chromatography of proteolytic digests of human placenta, a 65-kDa polypeptide was bound by these monoclonal antibodies. Rabbit antisera and monoclonal antibodies raised against the isolated 65-kDa polypeptide stained human and monkey tissues identically to the original monoclonal antibodies and reacted with an 80-kDa polypeptide in tissue extracts prepared without proteolysis. The 65-kDa and 80-kDa polypeptides were shown to be immunologically distinct from laminin, type IV collagen, fibronectin, and major serum proteins. They presumably represent a novel basement membrane-associated protein, which we have named merosin. No merosin immunoreactivity could be detected in cultures of any of 28 established cell lines. In developing mouse tissues, merosin staining first appeared at the newborn stage. The restricted tissue distribution and late developmental appearance of merosin suggest that the protein has a tissue-specific function associated with a high level of differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen R., Nielsen M., Wewer U., Engvall E., Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981 Dec;41(12 Pt 1):5076–5081. [PubMed] [Google Scholar]
- Carlin B., Jaffe R., Bender B., Chung A. E. Entactin, a novel basal lamina-associated sulfated glycoprotein. J Biol Chem. 1981 May 25;256(10):5209–5214. [PubMed] [Google Scholar]
- Chiu A. Y., Matthew W. D., Patterson P. H. A monoclonal antibody that blocks the activity of a neurite regeneration-promoting factor: studies on the binding site and its localization in vivo. J Cell Biol. 1986 Oct;103(4):1383–1398. doi: 10.1083/jcb.103.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu A. Y., Sanes J. R. Development of basal lamina in synaptic and extrasynaptic portions of embryonic rat muscle. Dev Biol. 1984 Jun;103(2):456–467. doi: 10.1016/0012-1606(84)90333-6. [DOI] [PubMed] [Google Scholar]
- Chung A. E., Jaffe R., Freeman I. L., Vergnes J. P., Braginski J. E., Carlin B. Properties of a basement membrane-related glycoprotein synthesized in culture by a mouse embryonal carcinoma-derived cell line. Cell. 1979 Feb;16(2):277–287. doi: 10.1016/0092-8674(79)90005-9. [DOI] [PubMed] [Google Scholar]
- Dehm P., Kefalides N. A. The collagenous component of lens basement membrane. The isolation and characterization of an alpha chain size collagenous peptide and its relationship to newly synthesized lens components. J Biol Chem. 1978 Oct 10;253(19):6680–6686. [PubMed] [Google Scholar]
- Ekblom P., Miettinen M., Rapola J., Foidart J. M. Demonstration of laminin, a basement membrane glycoprotein, in routinely processed formalin-fixed human tissues. Histochemistry. 1982;75(3):301–307. doi: 10.1007/BF00496733. [DOI] [PubMed] [Google Scholar]
- Engvall E., Davis G. E., Dickerson K., Ruoslahti E., Varon S., Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986 Dec;103(6 Pt 1):2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Engvall E., Krusius T., Wewer U., Ruoslahti E. Laminin from rat yolk sac tumor: isolation, partial characterization, and comparison with mouse laminin. Arch Biochem Biophys. 1983 Apr 15;222(2):649–656. doi: 10.1016/0003-9861(83)90562-3. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fitch J. M., Linsenmayer T. F. Monoclonal antibody analysis of ocular basement membranes during development. Dev Biol. 1983 Jan;95(1):137–153. doi: 10.1016/0012-1606(83)90013-1. [DOI] [PubMed] [Google Scholar]
- Harrisson F., Van Hoof J., Vanroelen C., Foidart J. M. Masking of antigenic sites of fibronectin by glycosaminoglycans in ethanol-fixed embryonic tissue. Histochemistry. 1985;82(2):169–174. doi: 10.1007/BF00708202. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessle H., Engvall E. Type VI collagen. Studies on its localization, structure, and biosynthetic form with monoclonal antibodies. J Biol Chem. 1984 Mar 25;259(6):3955–3961. [PubMed] [Google Scholar]
- Hessle H., Sakai L. Y., Hollister D. W., Burgeson R. E., Engvall E. Basement membrane diversity detected by monoclonal antibodies. Differentiation. 1984;26(1):49–54. doi: 10.1111/j.1432-0436.1984.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Jacque C., Delassalle A., Raoul M., Baumann N. Myelin basic protein deposition in the optic and sciatic nerves of dysmyelinating mutants quaking, jimpy, Trembler, mld, and shiverer during development. J Neurochem. 1983 Nov;41(5):1335–1340. doi: 10.1111/j.1471-4159.1983.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Krusius T., Gehlsen K. R., Ruoslahti E. A fibroblast chondroitin sulfate proteoglycan core protein contains lectin-like and growth factor-like sequences. J Biol Chem. 1987 Sep 25;262(27):13120–13125. [PubMed] [Google Scholar]
- Leivo I., Engvall E. C3d fragment of complement interacts with laminin and binds to basement membranes of glomerulus and trophoblast. J Cell Biol. 1986 Sep;103(3):1091–1100. doi: 10.1083/jcb.103.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo I., Vaheri A., Timpl R., Wartiovaara J. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol. 1980 Apr;76(1):100–114. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- Leu F. J., Engvall E., Damjanov I. Heterogeneity of basement membranes of the human genitourinary tract revealed by sequential immunofluorescence staining with monoclonal antibodies to laminin. J Histochem Cytochem. 1986 Apr;34(4):483–489. doi: 10.1177/34.4.3512698. [DOI] [PubMed] [Google Scholar]
- MIDGLEY A. R., Jr, PIERCE G. B., Jr IMMUNOHISTOCHEMICAL ANALYSIS OF BASEMENT MEMBRANES OF THE MOUSE. Am J Pathol. 1963 Dec;43:929–943. [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Sanes J. R., Marshall L. M. Cholinesterase is associated with the basal lamina at the neuromuscular junction. Nature. 1978 Jan 12;271(5641):172–174. doi: 10.1038/271172a0. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Morris N. P., Burgeson R. E. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol. 1986 Oct;103(4):1577–1586. doi: 10.1083/jcb.103.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A., Stockdale F. E. Identification of sarcolemma-associated antigens with differential distributions on fast and slow skeletal muscle fibers. J Cell Biol. 1987 Apr;104(4):967–979. doi: 10.1083/jcb.104.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- WIRSEN C., LARSSON K. S. HISTOCHEMICAL DIFFERENTIATION OF SKELETAL MUSCLE IN FOETAL AND NEWBORN MICE. J Embryol Exp Morphol. 1964 Dec;12:759–767. [PubMed] [Google Scholar]
- Wan Y. J., Wu T. C., Chung A. E., Damjanov I. Monoclonal antibodies to laminin reveal the heterogeneity of basement membranes in the developing and adult mouse tissues. J Cell Biol. 1984 Mar;98(3):971–979. doi: 10.1083/jcb.98.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer U., Albrechtsen R., Manthorpe M., Varon S., Engvall E., Ruoslahti E. Human laminin isolated in a nearly intact, biologically active form from placenta by limited proteolysis. J Biol Chem. 1983 Oct 25;258(20):12654–12660. [PubMed] [Google Scholar]