Abstract
High-frequency stimulation of the subthalamic nucleus (STN-HFS) is a powerful approach for treating the motor symptoms of Parkinson’s disease. It results in clinical improvement in PD patients, further reducing L-3, 4-dihydroxyphenylalanine (L-DOPA) requirement and thus L-DOPA-induced dyskinesia. However, it remains unclear how STN-HFS modifies the response to L-DOPA. We investigated the effect of STN-HFS on striatal extracellular concentrations of dopamine and its metabolites following acute L-DOPA administration in intact or partially dopaminergic denervated (DA PL) rats. L-DOPA treatment significantly increased striatal dopamine levels in intact and DA PL animals, with the maximal effect observed 1 h after L-DOPA injection. This increase was more pronounced in DA PL rats (ipsilateral to the lesion) than in intact animals. It remained fairly stable 1 h after the maximal effect of L-DOPA and then decreased towards basal values. STN-HFS in intact rats had no effect on the maximal L-DOPA-induced increase in striatal extracellular dopamine concentration or the return to basal values, the profiles observed being similar to those for non-stimulated intact animals. Conversely, STN-HFS amplified the L-DOPA-induced increase in striatal dopamine levels during the stimulation period (1 h) in DA PL rats, and this increase was sustained throughout the post-stimulation period (2.5 h), without the return to basal levels observed in stimulated intact and non-stimulated rats. These new neurochemical data suggest that STN-HFS interferes with L-DOPA effects, probably synergically, by stabilising dopamine levels in the striatum, and shed light on the mechanisms of STN-HFS in PD.
Keywords: 3,4-Dihydroxyphenylacetic Acid; metabolism; Animals; Data Interpretation, Statistical; Denervation; Dihydroxyphenylalanine; pharmacology; Dopamine; metabolism; Dopamine Agents; pharmacology; Electric Stimulation; Extracellular Space; drug effects; metabolism; Homovanillic Acid; metabolism; Male; Microdialysis; Neostriatum; drug effects; metabolism; physiology; Oxidopamine; Rats; Rats, Sprague-Dawley; Subthalamic Nucleus; physiology; Synaptic Transmission; drug effects; physiology
Keywords: Parkinson's disease, high-frequency stimulation, subthalamic nucleus, dopamine, L-DOPA treatment
Introduction
L-3, 4-dihydroxyphenylalanine (L-DOPA) remains the most effective treatment for Parkinson’s disease (PD). However long-term L-DOPA therapy induces many motor fluctuations (Fahn, 1974; Marsden and Parkes, 1976). L-DOPA induces a short-duration response (SDR) and a long-duration response (LDR) in patients with PD (Muenter and Tyce, 1971; Nutt et al., 1992; 1995). The SDR is a clinical improvement that lasts several hours after a single dose of L-DOPA whereas the LDR is a sustained improvement in parkinsonian following chronic L-DOPA therapy, persisting for several days after the end of treatment. Many PD patients experience changes in the therapeutic response to L-DOPA and dopamine agonists, such as apomorphine (Marsden and Parkes, 1976; Marsden, 1982; Nutt et al., 1992; 1995). The duration of the SDR and LDR is proportional to disease severity in patients with mild, moderate and severe disease (Muenter and Tyce, 1971; Contin et al., 1990; Nutt et al., 1992; Quattrone and Zappia, 1993; Zappia et al., 1997; 1999; Parkinson Study Group, 2004). Fluctuations in motor responses, such as “wearing-off” and “on-off” fluctuations, as well as L-DOPA-induced dyskinesia, are commonly observed in patients in advanced stages of PD. As the LDR is thought to be due to the slow release of L-DOPA from residual presynaptic neurones (Quattrone and Zappia, 1993; Nutt and Holford, 1996), this response becomes less important with respect to SDR in advanced PD. It has therefore been suggested that the pulsatile administration of L-DOPA is the principal cause of motor fluctuations (Juncos et al., 1989), because of the induction of abnormal plasticity in the basal ganglia, leading to the induction of dyskinesia and a decrease in the “on” duration (Mouradian et al., 1990). For this reason, continuous dopaminergic stimulation occupies a particularly important position in therapeutic strategies (Obeso et al., 2000; Olanow and Obeso, 2000; Olanow et al., 2000; 2001).
High-frequency stimulation (HFS) of the subthalamic nucleus (STN) has been reported to improve all motor symptoms in PD patients, particularly in those who experience motor fluctuations, such as the wearing-off phenomenon (Limousin et al., 1998; Moro et al., 1999; Fraix et al., 2000; Krack et al., 2003), but the mechanisms underlying the improvement in symptoms remain unclear (Benabid et al., 2002; Dostrovsky and Lozano, 2002; McIntyre et al., 2004). It also remains unclear whether STN-HFS interferes with L-DOPA-induced changes in striatal dopamine content, by attenuating fluctuations in particular. De la Fuente-Fernandez et al. (2001) have shown that fluctuations in synaptic dopamine concentrations in the striatum precede the clinically apparent wearing-off phenomenon. They suggested that an increase in dopamine turnover might be involved in L-DOPA-related motor complications.
In this study, we compared the effects of acute L-DOPA treatment with and without STN-HFS (according to the procedures used for treating PD in humans) on the striatal extracellular content of dopamine (DA) and its metabolites, in normal and partially dopaminergic-lesioned rats, by means of in vivo intracerebral microdialysis. Our data provide insight into the interactions between the effects of L-DOPA treatment and STN-HFS on dopaminergic neurotransmission.
Materials and Methods
Animals
We used 55 adult male Sprague-Dawley rats (Janvier, Le Genest-St-Isle, France) weighing 200 to 380g, housed under standard laboratory conditions (12 h dark/light cycle), with food and water provided ad libitum. Protocols conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication 865-23) and French Ministry of Agriculture regulations (authorisation number 38-R 1001).
Lesion procedure
For partial SNc lesioning, all animals were anaesthetised with chloral hydrate (400 mg/kg i.p.) and secured in a Kopf stereotaxic apparatus (Phymep, Paris, France). We treated 25 animals with desipramine (25 mg/kg, s.c.), to protect noradrenergic neurons, and then injected 3 μg of 6-hydroxydopamine (6-OHDA)(Sigma, St Quentin-Fallavier, France) dissolved in 1 μl of sterile 0.9 % NaCl and 0.2% ascorbic acid into the left SNc of these animals, at a flow rate of 0.5 μl/min. The stereotaxic co-ordinates of the injection site were anteroposterior (AP), + 3 mm, lateral (L), + 2.4 mm, and dorsoventral (DV), −7.1 mm, with the incisor bar at +3.3 mm below the interaural plane. All stereotaxic co-ordinates cited here are according to the stereotaxic atlas of Paxinos and Watson (1982). Animals were kept warm after the injections and were allowed to recover from anaesthesia. They were returned to the animal facility for three weeks, to allow the degeneration of dopaminergic neurons induced by the neurotoxin to stabilise, and were then processed for microdialysis experiments.
Implantation of microdialysis probes and stimulating electrode
Normal (n=30) and partially 6-OHDA SNc lesioned (PL) (n=25) rats were anaesthetised by inhalation (1 l/min) of a 5% halothane/air (22% O2, 78% N2) mixture and mounted in a stereotaxic frame (David Kopf Instruments, Tajunga, CA). Anaesthesia was maintained with an inhaled 1% halothane/air mixture (1 l/min). The dorsal skull was exposed, and holes were drilled to facilitate the bilateral implantation of the dialysis probes into the striatum, and the unilateral (left side, i.e. ipsilateral to the 6-OHDA injection) implantation of the stimulation electrode in the STN. The stereotaxic co-ordinates used were (relative to bregma): (1) microdialysis probe (into striatum): AP, + 1 mm; L, +/− 3 mm; V, − 7 mm; (2) stimulation electrode (into STN): AP, −3.7 mm; L, 2.4 mm; V, −7.8 mm. During implantation and microdialysis experiments, body temperature was maintained at 37°C with a feedback-controlled heating pad (Harvard Apparatus, Edenbridge, UK).
Electrical stimulation of the STN
Concentric stimulating bipolar electrodes (outer diameter 250 μm, SNEX 100, Rhodes Medical Instruments, Woodland Hills, CA) were used. Stimuli were delivered over a one-hour period, with a World Precision Instrument (Stevenage, UK) acupulser and a stimulus isolation unit. Stimulation parameters corresponded to those used in clinical practice (frequency 130 Hz, 60 μs rectangular pulse width and 200 μA intensity). At the end of each experiment, an electrical lesion was created in the STN so that the position of the electrode could be checked post-mortem.
Microdialysis
Home-made microdialysis probes were prepared and used as previously described (Windels et al., 2000; Bruet et al., 2003). They consisted of a concentric arrangement of a stainless steel tube (outer diameter, 0.4 mm, (Phymep, Paris, France), and polyethylene tubing (outer diameter, 1.09 mm; inner diameter, 0.38 mm) (Phymep) into which we inserted a piece of silica tubing (outer diameter, 150 μm; inner diameter, 75 μm) (Phymep). The silica tubing extended beyond the distal end of the steel tube and was covered with a cuprophan tubular dialysis membrane (Hospal, Lyons, France), sealed at the bottom with epoxy glue. The length of the dialysis membrane was adapted for the rat brain nucleus studied (3 mm for the striatum).
The perfusion liquid flowed out of the distal end of the steel tube, passing proximally between the tube and the membrane (Tossman and Ungerstedt, 1986). The probes were perfused with artificial CSF (149 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2 and 5.4 mM glucose, pH=7.3) at a flow rate of 1 μL/min. Before implantation, each probe was tested in vitro in a standard catecholamine solution, to determine DA, DOPAC and HVA recovery (Tossman and Ungerstedt, 1986).
For microdialysis experiments, two experimental animal groups were used: non-stimulated (intact, n= 15; DA PL, n= 13) and stimulated (intact+STN-HFS, n= 15; DA PL+STN-HFS, n= 12). The dialysis probes were implanted in each animal and dialysates were collected at 15-minute intervals, for seven hours. The first eight fractions (2 h) were discarded to prevent effects due to parenchymal disturbance and to ensure that a steady-state was reached. The next four fractions (1 h) were collected for basal value determination. All animals then received an intraperitoneal injection of benserazide (12.5 mg/kg, Sigma, St Quentin-Fallavier, France) + L-DOPA (50 mg/kg, Sigma).
The non-stimulated rats were first used to evaluate the duration of the L-DOPA effect on extracellular DA content, in intact control and DA PL rats. The first eight fractions were discarded to prevent effects due to parenchymal disturbance and to ensure that a steady-state values were obtained. The next four fractions (1 h) were collected for basal value determination, and sixteen consecutive fractions (4 h) were then collected after L-DOPA injection: 6 fractions were used to determine the maximal effect of L-DOPA, and the remaining 10 fractions were collected to estimate the effect of L-DOPA over a period of 2.5 h.
We then carried out a similar experiment in stimulated rats. As for the non-stimulated animals, the first eight fractions were discarded to prevent effects due to parenchymal disturbance and to ensure that the values obtained corresponded to an approximate steady state. Four fractions (1 h) were then collected for basal value determination, followed by six fractions (1.5 hours) collected after L-DOPA injection to assess the maximal effect of this drug on striatal DA content. The next four fractions were collected during STN-HFS for 1 h. A further six fractions were then collected to estimate post-stimulation effects over a period of 1.5 hours. All dialysates were collected automatically with a refrigerated autosampler (Univentor, Zejton, Malta) and stored at −80°C until analysis.
DA, DOPAC and HVA assays
DA, DOPAC and HVA concentrations in the dialysates were determined by high-performance liquid chromatography (HPLC), with electrochemical detection. The system consisted of a pump (Shimadzu model LC-10 AD, Shimadzu Europe, Munich, Germany), a refrigerated automatic injector (Famos model, Dionex, France), a reverse-phase Hypersil RP 18 analytical column (Aquasil 150×1 mm, particle size 3 μm; ThermoHypersil, les Ulis, France) and an electrochemical detector (Decade, Antec, The Netherlands) equipped with an analytical cell (type VT-03, Antec). Chromatograms were collected and treated with integration software (CLAS VP, Shimadzu, France).
The mobile phase consisted of sodium dihydrogen phosphate buffer (NaH2PO4, 50 mM; Merck), octane sulfonic-1 acid sodium salt (1.7 mM; Merck), disodium ethylenediamine tetra-acetic acid (Na2-EDTA, 200 μM; Merck). The pH was adjusted to 3 with concentrated phosphoric acid (H3PO4), and 5% acetonitrile (ACN, Sigma) was added to the final solution. All solvents were filtered through Millipore filters with 0.22 μm pores (Millipore, France) before use.
The mobile phase was delivered by a pump with a flow rate of 60 μl/min. The working electrode potential was +750 mV, which represented the best compromise between the optimum oxidation potentials of DA, DOPAC and HVA. Detector sensitivity was 1 nA for DA, 50 nA for DOPAC and 10 nA for HVA. The running time for each determination was 15 minutes.
Histology
At the end of the microdialysis experiments, unlesioned rats (n=30) were killed by decapitation under deep anaesthesia. Their brains were quickly removed from the skull and frozen in isopentane at −30°C. Lesioned animals (n=25) were perfused transcardially, under halothane anaesthesia, with 200 mL of 0.9 % NaCl, pH 7.2, followed by 300 mL of 4% paraformaldehyde in 0.1 M PBS, pH 7.4 (2.6 mM KCl, 1.4 mM KH2PO4, 136 mM NaCl, and 83 mM NaH2PO4). Brains were quickly removed and immersed overnight in 20% sucrose in 0.1 M phosphate buffer, pH 7.4, frozen in cooled (−40°C) isopentane, and stored at −30°C. Serial frontal sections (20 μm) were cut on a cryostat (Microm HM 500; Microm, Francheville, France). The correct locations of the microdialysis probes and stimulation electrode were checked by collecting several striatal and subthalamic tissue sections (Paxinos and Watson, 1982) and by counterstaining with cresyl violet. These histological controls were systematically carried out for all the animals in each experimental group. All animals presenting internal bleeding around the microdialysis probes or electrodes were excluded to prevent microdialysate contamination (n=4), as were animals with incorrectly positioned stimulation electrodes (n=8). We assessed the dopaminergic denervation induced by nigral 6-OHDA injection, by tyrosine hydroxylase (TH) immunostaining of striatal and nigral sections from the fixed brains of lesioned animals. Free-floating sections were thoroughly washed with TBS and incubated for 1 hour in 0.3% Triton X-100 in TBS (TBST) and 3 % normal goat serum (Sigma-Aldrich, St Quentin Fallavier, France). They were then incubated with primary antisera diluted in TBST containing 1 % normal goat serum for 24 h, with shaking, at 4°C. The antiserum was diluted 1:500 for TH staining (mouse monoclonal antibody; Chemicon, Temecula, CA). Antibody binding was detected by incubation with avidin-biotin-peroxidase conjugate (Vectastain ABC Elite, Vector Laboratories, Burlingame, CA), using 3,3′-diaminobenzidine as the chromagen. This substrate was applied to the sections for 2 to 5 min, as previously described (Guesdon et al., 1979). Sections were dehydrated through a graded series of ethanol solutions, cleared in xylene, mounted in DPX (DBH Laboratories Supplies, Poole, UK) and covered with a coverslip for microscopy.
The area of the SNc displaying cell loss and the area of the striatum displaying dopaminergic terminal loss were estimated by comparison with the unlesioned side or control values. These areas were determined by anatomical densitometry of TH immunolabelling, using a camera, and the mean optical density was calculated with Autoradio V4.03 software (SAMBA Technologies, Meylan, France). These data were then compared with data previously obtained in similar conditions (Blanchard et al., 1995; Chritin et al., 1996; Bruet et al., 2001).
Data analysis
The basal levels of the measured substances are expressed as concentrations in dialysates. Basal DA, DOPAC and HVA levels in the striatum were analysed, for the various experimental groups, with a Mann-Whitney U test. For data expressed as a percentage of the basal value, the mean concentration of the four samples preceding L-DOPA injection was set at 100%. The effects of L-DOPA treatment and of STN-HFS on extracellular DA, DOPAC and HVA levels were analysed by one-way repeated measures ANOVA over time (Windels et al., 2005; Boulet et al., 2006). Dunnett’s or Games-Howell tests were used for comparisons with prestimulation and/or baseline levels for L-DOPA effects. Values of p<0.05 were considered statistically significant.
Results
Control of the extent of the DA lesion and of the location of the electrode and microdialysis probes
One month after the unilateral injection of 6-OHDA, DA PL animals (n=25) showed weaker than normal TH immunolabelling in the lateral part of the ipsilateral SNc. In most DA PL rats, the total number of SNc TH+ neurons was about 50% lower than that on the unlesioned side (Fig. 1A). In the striatum of the same rats, DA nerve terminals, detected by TH immunolabelling, were preferentially lost from the dorsolateral (denervated) part of the striatum (Fig. 1B). In this area, TH immunolabelling (detected by optical density measurements) was a mean of 70% (p<0.001) lower than normal, whereas it was only 10 to 25% (p<0.01) lower in the medial region of the striatum (non denervated part). About 40% of the total striatal surface showed a loss of TH immunostaining.
Figure 1.
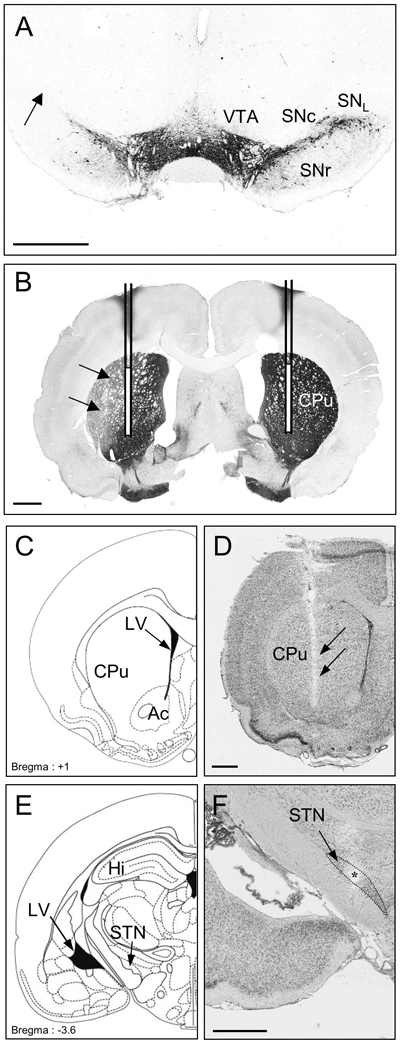
Photographs of TH-immunostained coronal rat brain sections at the nigral (A) and striatal (B) levels and of cresyl violet-stained coronal rat brain sections at the striatal (D) and subthalamic (F) levels in 6-OHDA-lesioned rats. C and E: Schematic diagrams adapted from the stereotaxic atlas of Paxinos and Watson (1982). Note, on the lesioned side (left), the loss of dopaminergic cells in the lateral part of the SNc (A) and the loss of dopaminergic terminals in the dorsolateral part of the striatum (B). The location of microdialysis probes in the striatum is schematically represented in B and visualized in D on the lesioned side. Note also the correct implantation of the stimulation electrode within the STN (F). F, The asterisk indicates the point stimulated. Scale bar, 1 mm.
Ac: Nucleus accumbens; CPu: Caudate putamen; Hi: Hippocampus; LV: Lateral ventricle; SNc: Substantia nigra pars compacta; SNL: Substantia nigra pars lateralis; SNR: Substantia nigra pars reticulata; STN: Subthalamic nucleus; VTA: Ventral tegmental area.
However, some DA PL animals displayed severe (>80%, n=2) or moderate (<30%, n=3) lesions of the SNc and were discarded to ensure that the lesioned rat group was homogeneous.
Dense TH immunolabelling was detected throughout the SNc, the ventral tegmental area, the striatum, the nucleus accumbens, and the olfactory tubercles, on the intact side, in 6-OHDA-injected rats (Fig. 1A, B). Figures 1D and F illustrate the correct implantation of the microdialysis probe in the parenchyma of the striatum (Fig. 1D) and of the stimulation electrode in the STN (Fig. 1F). In DA PL animals, the microdialysis probe was located between the denervated and non-denervated parts of the striatum. Figure 1F shows the small electrical lesion (asterisk) created at the end of the experiment, indicating the point stimulated.
Basal extracellular DA, DOPAC and HVA levels in the striatum are increased by acute L-DOPA injection in intact and DA PL rats
The concentrations of DA, DOPAC and HVA were determined in the striatum of intact control (n=25) and DA PL (n=13) rats from the two experimental groups. Mean concentrations were 0.011 ± 0.0001 μM for DA, 1.7 ± 0.08 μM for DOPAC and 1.697 ± 0.085 μM for HVA in intact animals, and 0.0124 ± 0.0018 μM for DA, 1.137 ± 0.097 μM for DOPAC and 1.145 ± 0.085 μM for HVA in DA PL rats (Table 1). In DA PL rats, the concentration of striatal DA ipsilateral to the lesion tended to increase, as shown by comparisons with the unlesioned side and control values (0.0124 ± 0.0018 versus 0.011 ± 0.0019 μM). Partial DA denervation significantly decreased the levels of DOPAC (−33%, p<0.001) and of HVA (−32.5%, p<0.001), as shown by comparison with control values.
Table 1.
Extracellular levels of DA, DOPAC and HVA (μM) measured in the striatum on the left side of intact and partially SNc-lesioned rats (DA PL) in basal conditions and after acute L-DOPA administration, before (L-DOPA effect), during (post L-DOPA + 1 h STN-HFS) and after (post L-DOPA + post STN-HFS) STN-HFS.
| INTACT |
DA PL |
|||||||
|---|---|---|---|---|---|---|---|---|
| Basal levels | L-DOPA effect | Post L-DOPA +STN HFS (t=1h) | Post L-DOPA +STN HFS (t=2.5h) | Basal levels | L-DOPA effect | Post L-DOPA +STN HFS (t=1h) | Post L-DOPA +STN HFS (t=2.5h) | |
|
Dopamine (μM) |
0.011 ± 0.0001 |
0.021 ± 0.0019 (+ 92% vs basal levels; p<0.001) |
0.0191 ±0.0017 (+ 74% vs basal levels; p<0.001) (−9% vs L-DOPA effect; ns) |
0.0154 ± 0.0014 (+ 40% vs basal levels; p<0.05) (− 52% vs L-DOPA effect; p<0.05) |
0.0124 ± 0.0018 (+12% vs Intact; ns) |
0.047 ± 0.0069 (+ 279% vs basal levels; p<0.001) |
0.0587 ± 0.0086 (+ 373% vs basal levels; p<0.001) (+94% vs L-DOPA effect; ns) |
0.052 ± 0.0076 (+319% vs basal levels; p<0.05) (+40% vs L-DOPA effect; ns) |
|
DOPAC (μM) |
1.7 ± 0.08 |
3.638 ± 0.17 (+ 114% vs basal levels; p<0.001) |
4.182 ± 0.19 (+ 146% vs basal levels; p<0.001) (+15% vs L-DOPA effect; ns) |
3.383 ± 0.16 (+ 99% vs basal levels; p<0.001) (−7% vs L-DOPA effect; ns) |
1.137 ± 0.097 (−33% vs Intact; p<0.001) |
3.821 ± 0.326 (+236% vs basal levels; p<0.001) |
5.732 ± 0.488 (+404% vs basal levels; p<0.001) (+ 168% vs LDOPA effect; p<0.05) |
4.242 ± 0.361 (+273% vs basal levels; p<0.001) (+11%vs L-DOPA effect; ns) |
|
HVA (μM) |
1.697 ± 0.085 |
2.885 ± 0.145 (+ 70% vs basal levels; p<0.001) |
3.292 ± 0.165 (+ 94% vs basal levels; p<0.001) (+ 24% vs L-DOPA effect; p<0.001) |
3.767 ± 0.19 (+ 122% vs basal levels; p<0.001) (+ 52% vs L-DOPA effect; p<0.001) |
1.145 ± 0.069 (−32.5% vs Intact; p<0.001) |
2.622 ± 0.158 (+ 129% vs basal levels; p<0.001) |
3.928 ± 0.237 (+ 243% vs basal levels; p<0.001) (+ 114% vs LDOPA effect; p<0.001) |
4.328 ± 0.262 (+ 278% vs basal levels; p<0.001) (+ 149% vs LDOPA effect; p<0.001) |
The values presented for basal levels and L-DOPA effect periods are averages of the corresponding measures in intact rats, and in DA PL rats. Values corresponding to the “post L-DOPA+ 1 h STN HFS” and the “post L-DOPA+ post STN HFS (t=2.5 h)” periods correspond to values for intact rats subjected to STN-HFS in the part of the table headed “intact”, and DA PL rats subjected to STN-HFS in part of the table headed “DA PL”. The results are expressed as means ± SEM. ns: not significant.
L-DOPA treatment significantly increased DA, DOPAC and HVA levels in the striatum of intact control and DA PL rats. These increases were more pronounced in DA PL animals (ipsilateral to the lesion) than in intact control rats and the mean variation for the two experimental groups was: +279% (p<0.001) versus +92% (p<0.05) for DA; +236% (p<0.001) versus +114% (p<0.001) for DOPAC, and +129% (p<0.001) versus +70% (p<0.001) for HVA (Table 1).
The DA turnover index was calculated as the concentration of DA metabolites (DOPAC+HVA) divided by the concentration of extracellular DA. It reflects the relationship between DA metabolism and DA release. This DA turnover index was not significantly affected by L-DOPA injection in either intact or DA PL rats, but it did increase over the 1 h and 2.5 h post-injection periods studied (Table 2).
Table 2.
(DOPAC+HVA)/dopamine ratios for each experimental group.
| No STN-HFS |
STN-HFS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Basal levels | L-DOPA effect | Post L-DOPA (t=1h) | Post L-DOPA (t=2.5h) | Basal levels | L-DOPA effect | Post L-DOPA (t=1h) | Post L-DOPA (t=2.5h) | |
| Intact rats | 478.2 ± 36.9 |
560.1 ± 61 (+ 17% vs basal levels; ns) |
789.1 ± 130 (+ 65% vs basal levels; ns) (+ 41 % vs L-DOPA effect; ns) |
1296.9 ± 213 (+ 171 % vs basal levels; p<0.001) (+ 131 % vs L-DOPA effect; p<0.05) |
478.2 ± 36.9 |
560.1 ± 61 (+ 17% vs basal levels; ns) |
779.9 + 87.75 (+ 63% vs basal levels; p<0.001) (+ 39 % vs L-DOPA effect; ns) |
1037.5 ± 120.9 (+ 117% vs basal levels; p<0.001) (+ 85 % vs L-DOPA effect; p<0.001) |
| DA PL rats |
391.4 ± 70.5 (− 18% vs Intact; p<0.05) |
370.15 ± 67.6 (− 5% vs basal levels; ns) |
1178.1 ± 216.1 (+ 200% vs basal levels; p<0.001) (+218 % vs L-DOPA effect; p<0.001) |
1555.8 ± 187.6 (+ 297% vs basal levels; p<0.001) (+ 320% vs L-DOPA effect; p<0.001) |
391.4 ± 70.5 (− 18% vs Intact; p<0.05) |
370.15 ± 67.6 (− 5% vs basal levels; ns) |
488.7 ± 96.4 (+ 25% vs basal levels; ns) (+ 32% vs L-DOPA effect; ns) |
454.8 ± 63.3 (+ 16%vs basal levels; ns) (+ 22% vs L-DOPA effect; ns) |
The results are expressed as means ± SEM. ns: not significant.
Figure 2A illustrates the variations of striatal DA, DOPAC and HVA levels in intact (n=12) and DA PL (n=5) rats after acute L-DOPA injection only. In these non-stimulated animals, L-DOPA injection triggered a gradual increase in extracellular striatal DA levels during the period of 1.5 hours following the injection (data not shown). This increase was maximal in the last two of the six fractions collected during this period and the mean variation observed in these two fractions was used as a reference for L-DOPA effect. In intact rats, striatal DA levels remained high during the first hour of the post-L-DOPA effect period, decreasing towards basal values 2.5 h after the maximal effect of L-DOPA (Fig. 2A). The increase in striatal DA levels induced by L-DOPA injection was more pronounced in DA PL rats than in intact rats (+279% versus +92%, p<0.001) and remained fairly stable during the first hour of the post-L-DOPA effect period. As in intact animals, DA levels returned towards basal values 2.5 h after the maximal effect of L-DOPA (Fig. 2A), but values remained significantly above these basal levels (Fig. 2A).
Figure 2. Extracellular DA, DOPAC and HVA levels in the striatum measured in intact and DA PL rats (ipsilateral to the lesion and/or to STN stimulation) following an acute injection of L-DOPA without (A) or with (B) STN-HFS.
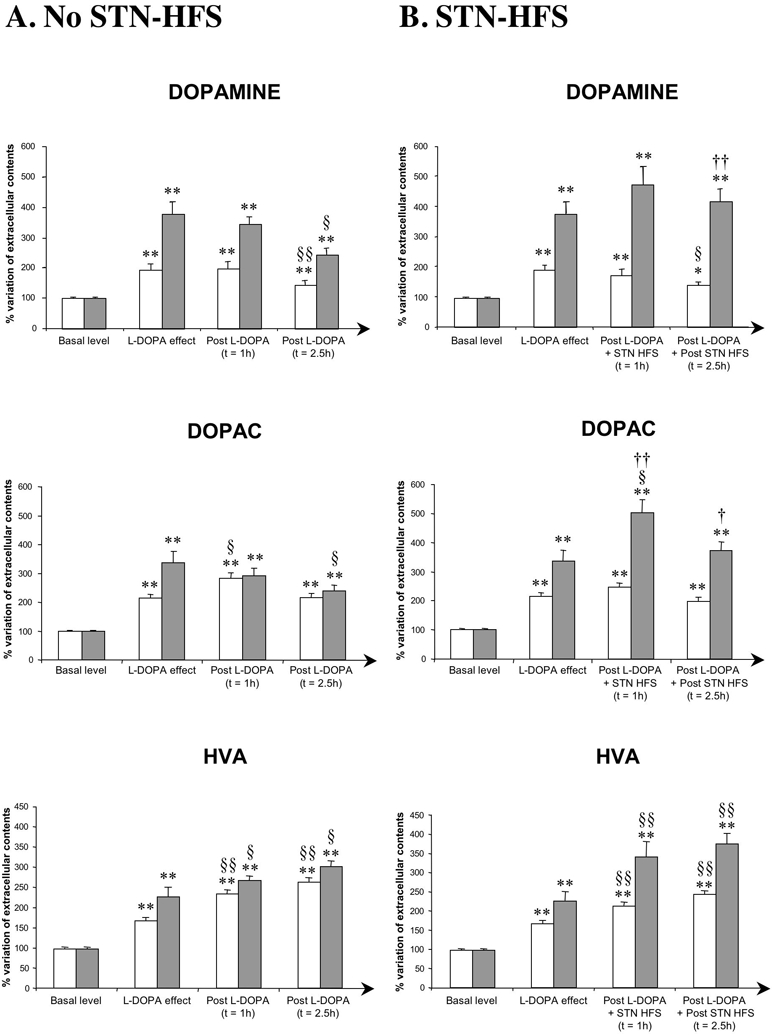
The mean concentration ± SEM of the four samples preceding L-DOPA injection was used to determine baseline levels and was set at 100%. The L-DOPA effect represents the mean concentration ± SEM of the last two of the six samples collected following L-DOPA and corresponding to its maximal effect. In A, the post-L-DOPA values presented correspond to the mean concentration ± SEM of the four (1 h) or six (2.5 h) fractions collected after L-DOPA injection.
In B, post-L-DOPA+STN-HFS values correspond to the mean concentration ± SEM of the four samples collected during 1 h of STN-HFS and after the L-DOPA effect had reached its maximum. Post L-DOPA+Post STN-HFS values correspond to the mean concentration ± SEM of the subsequent 6 samples (2.5 h), collected after the end of STN-HFS.
Note that, in DA PL animals only, STN-HFS stabilised the increase in striatal DA levels induced by L-DOPA and increased levels of DOPAC and HVA during the stimulation and post-stimulation periods. Results are expressed as a percentage of variation of the baseline value. Each bar corresponds to the mean variation ± SEM calculated from all collected fractions from each animal of each group. White bars = intact rats (A, n=12; B, n=13) and grey bars = DA PL rats (A, n=5; B, n=8).
*p<0.05, **p<0.001: versus basal values; §: versus L-DOPA effect; †: versus no STN-HFS DA PL rats.
The L-DOPA-induced increase in striatal DOPAC and HVA levels followed a pattern similar to that for DA content, in both intact and DA PL rats, except that no pronounced return to basal levels was observed (Fig. 2A). In contrast, HVA levels remained high or increased still further during the microdialysis experiment.
Effect of STN-HFS on L-DOPA-induced increases of extracellular DA, DOPAC and HVA levels in the striatum in intact control and DA PL rats
The mean L-DOPA-induced increases in striatal DA, DOPAC and HVA levels under STN-HFS were 0.0191 ± 0.0017 μM, 4.182 ± 0.19 μM and 3.292 ±0.165 μM, respectively, for intact animals, and 0.0587 ± 0.0086 μM, 5.732 ± 0.488 μM and 3.928 ± 0.237 μM, respectively, for DA PL rats (Table 1). In intact stimulated animals (n=13), STN-HFS did not significantly affect the L-DOPA-induced increase in striatal extracellular concentrations of DA and DOPAC, whereas it increased those of HVA (+24%, p<0.001), during the one-hour stimulation period (Table 1, Fig. 2B). During the post-stimulation period, DA levels significantly decreased (−52%, p<0.05 with respect to L-DOPA-induced levels) whereas HVA levels increased (+52%, p<0.05 with respect to L-DOPA-induced levels). DOPAC levels were not significantly affected. However, DA, DOPAC and HVA levels remained above basal levels 2.5 h after the maximal effect of L-DOPA in stimulated intact animals. The profile of change in levels of DA and its metabolites was similar to that in intact animals treated with L-DOPA, without STN-HFS. However, DA turnover index increased in intact animals during the stimulation (+63%, p<0.05 vs basal values) and post-stimulation (+117%, p<0.001 vs basal levels or +85%, p<0.001 vs L-DOPA effect) periods (Table 2).
In stimulated DA PL animals (n=8), STN-HFS significantly amplified the L-DOPA-induced increases in striatal extracellular DA, DOPAC and HVA levels. These levels increased by +94% (non-significant), + 168% (p<0.05) and + 114% (p<0.05) for DA, DOPAC and HVA, respectively, during the one-hour stimulation period (Table 1, Fig. 2B). In contrast to what was observed in non-stimulated DA PL (Fig 2A) and stimulated intact animals (Fig 2B), striatal extracellular DA, DOPAC and HVA levels remained high throughout the post-stimulation period in stimulated DA PL rats, strongly suggesting an effect of STN-HFS (Fig. 2B). Indeed, a comparison of DA levels in stimulated DA PL rats during the post-stimulation period with those obtained in similar non-stimulated animals at the same time points after L-DOPA treatment shown that DA levels tended to return to basal values in the absence of STN-HFS (Fig. 2A). In contrast to what was observed in intact rats, DA turnover index during the stimulation and post-stimulation periods was not significantly affected in DA PL animals (Table 2).
Discussion
The mechanisms by which STN-HFS improves motor symptoms in PD patients, particularly in those with motor fluctuations such as the wearing-off phenomenon, remain unclear. One interesting question concerns possible modification of the response to L-DOPA by STN-HFS. We report here the first evidence that STN-HFS interacts in a synergic manner with L-DOPA-induced changes in striatal extracellular dopamine concentration. In DA PL rats, STN-HFS stabilised the L-DOPA-induced increase in striatal dopamine levels during the stimulation period (1 h). These levels remained roughly constant throughout the post-stimulation period (2.5 h), rather than falling back to basal levels, as observed in stimulated intact and non-stimulated rats. Our data, and particularly those for DA PL animals, shed light on the mechanisms underlying the beneficial clinical effects observed in PD patients treated by STN-HFS, including the decrease in L-DOPA requirements and, therefore, in L-DOPA-induced dyskinesia.
Effects of acute L-DOPA injection on striatal extracellular DA, DOPAC and HVA contents
A single acute administration of L-DOPA significantly increased extracellular DA, DOPAC and HVA levels in the striatum of intact and DA PL rats.
Baseline extracellular DA levels in DA PL rats were similar to those in intact animals (Table 1, Fig. 2), suggesting that partial destruction of the SNc probably induces changes in striatal DA content through modifications to DA turnover and reuptake rates. This observation is consistent with published data for rats with similar DA lesions, showing a massive decrease in presynaptic dopaminergic vesicles and an increase in DA turnover (Hefti et al., 1980; Robinson and Wishaw, 1988; Zigmond et al., 1984; 1989). Moreover, the massive dopaminergic terminal loss in the dorsolateral part of the ipsilateral striatum may also be responsible for a major decrease in presynaptic DA uptake and/or an increase in the ability of dopaminergic neurons to release DA in response to impulse flow (Chritin et al., 1996; Zigmond et al., 1984; 1989, Dentresangle et al., 2001). The mechanisms involved in this compensation of DA levels in DA PL rats are unlikely to involve a tonic increase in the electrical activity of the dopaminergic neurons. Indeed, more than 80% denervation is required to modify the firing activity of dopaminergic neurons after nigrostriatal lesion (Hollerman and Grace, 1990), suggesting that DA level compensation in DA PL rats is correlated with changes in DA synthesis. Several authors have already shown that DA release is more strongly affected by the inhibition of DA synthesis in 6-OHDA-lesioned rats than in controls (Heffner et al., 1977; Marshall and Teitelbaum, 1973; Snyder et al., 1990). This is consistent with lower levels of amine storage in the dopaminergic terminals and an increase in TH activity (Hefti et al., 1985; Stachowiak et al., 1987; Snyder et al., 1990). Partial lesions of nigrostriatal dopaminergic neurons result in a condition mimicking the early or preclinical phase of PD (Carman et al., 1991; Zigmond et al., 1984; 1990). The lack of symptoms in patients with this disease before the dopaminergic terminal deficit becomes severe may be a consequence of the passive compensation we observed in DA PL rats. Both dopamine uptake sites (Laihinen et al., 1995) and the messenger RNA for the DA transporter (Uhl et al., 1994) have been shown to decrease in abundance in clinically diagnosed PD patients. Thus, the consequences of the simultaneous loss of DA release and uptake observed in this animal model may provide direct clues to how normal DA tone is maintained in preclinical subjects.
The L-DOPA-induced increase in striatal extracellular DA levels was more pronounced in DA PL animals (ipsilateral to the lesion) than in intact control rats (Fig 2A; Table 1). In intact striatum, increases in extracellular DA in response to exogenous L-DOPA are more limited because of the lower diffusion coefficient for DA in the extracellular space of the striatum due to the effects of high-affinity DA uptake (Kuhr et al., 1986; Kelly et al., 1987; Nicholson and Rice, 1991; Garris and Wightman, 1994). It is also possible that DA effectively clears DA synthesised from exogenous L-DOPA from the extracellular fluid of the intact striatum via the DA transporter,{NdT: OK}thereby restricting the diffusion of DA to the microdialysis probes or to the appropriate postsynaptic receptors. This suggests that compensatory mechanisms handle extracellular DA very efficiently in normal conditions, thereby preventing excessive increases in DA concentration in non-denervated areas, even when L-DOPA concentration is high.
The unrestricted diffusion of DA in the extracellular fluid of the striatum in DA PL animals allows both the detection of extracellular DA at the microdialysis probe and, probably, the activation of dopaminergic receptors (Miller and Abercrombie, 1999). This might account for the marked increase in DA availability after the creation of a partial dopaminergic lesion in the striatum, indicating a disruption of the normal regulation of extrasynaptic DA. These observations confirm previous studies reporting greater selectivity of L-DOPA in DA-lesioned brain areas (therapeutic response) than in normally innervated regions (Abercrombie et al., 1990; Maeda et al., 1999; Miller and Abercrombie, 1999). Extracellular DOPAC and HVA levels showed a similar marked, longer-lasting response to L-DOPA than to DA (Table 1). However, it is possible that when the rate of DA synthesis exceeds that of DA usage, DA is immediately converted to DOPAC (Arbuthnott et al., 1990; Butcher et al., 1988; Fairbrother et al., 1990a; 1990b; Westerink and Van Putten, 1987). In this case, a portion of the administered L-DOPA is directly metabolised and therefore has no effect on dopaminergic neurotransmission.
Effect of STN-HFS on L-DOPA-induced increases in striatal extracellular DA, DOPAC and HVA levels
Several clinical studies have reported an excellent clinical outcome of STN stimulation in L-DOPA-responsive forms of PD — patients with selective brain dopaminergic lesions — and a moderate clinical outcome in patients with axial motor symptoms and cognitive impairment known to be less responsive or unresponsive to L-DOPA treatment — development of brain non-dopaminergic lesions in addition to degeneration of the nigrostriatal dopaminergic system (Welter et al., 2002; Kleiner-Fisman et al., 2003; Pahwa et al., 2005). Chronic bilateral STN stimulation has also been shown to allow the discontinuation of L-DOPA or equivalent treatment or large reductions in daily dose (Moro et al., 1999; Fraix et al., 2000; Molinuevo et al., 2000; Lopiano et al., 2001), resulting in a decrease in L-DOPA-induced dyskinesia (Krack et al., 1997; 1999; 2003; Bejjani et al., 2000). These clinical observations suggest that the beneficial effects of STN-HFS on PD symptoms are “L-DOPA-like”, but it remains unclear whether the mechanisms of action of STN-HFS and L-DOPA are similar, or even synergic. However, interactions with the dopaminergic system seem to be one key factor in the efficacy of both treatments. It has been suggested that STN-HFS acts directly on dopaminergic neurons in rodents by increasing their firing rate (Benazzouz et al., 2000), TH gene expression in the remaining dopaminergic neurons of the SNc, or by eliciting striatal DA release and the activation of striatal DA, together with striatal TH activity metabolites of PD (Paul et al., 2000; Bruet et al., 2001; Meissner et al., 2001; 2002; 2003; Henning et al., 2007). However, PET studies in humans have given conflicting results, suggesting that an increase in DA transmission cannot be the mechanism underlying the beneficial effects of STN-HFS in advanced PD (Hilker et al., 2003; Abosch et al., 2003; Strafella et al., 2003b). However, all these studies in humans were carried out in parkinsonian patients on long-term L-DOPA treatment. The cellular and molecular consequences of such treatment in PD patients, in terms of plasticty within the functional basal ganglia circuitry, remain unknown and it remains unclear how this plasticity interferes with STN-HFS mechanisms or facilitates the alleviation of PD motor symptoms by this treatment. We cannot totally exclude the possibility that STN-HFS mechanisms shunt the dopaminergic system and related circuitries, but this appears unlikely, as the outcome of STN stimulation seems to be correlated with L-DOPA-responsive forms of PD.
We applied STN-HFS to a dopaminergic system activated by L-DOPA in both intact and DA PL rats. The lack of enhancement of the L-DOPA-elicited increase in extracellular DA levels by STN-HFS during the stimulation period suggests that the capacity of the intact or remaining dopaminergic neurons to synthesise and/or release DA has reached its maximum (Table 1 and Fig 2). However, only in DA PL animals did STN-HFS stabilise high striatal DA levels, resulting in an increase in DOPAC and HVA levels during the stimulation and post-stimulation periods. This is consistent with previous push-pull studies showing an increase in DOPAC levels during STN-HFS (100 Hz) with no change in extracellular DA content (Mintz et al., 1986). The equilibrium between the maintenance of high levels of DA and DA degradation resulted in the relative stabilisation/normalisation of DA turnover index (Table 2). This STN-HFS-induced stabilisation of DA turnover index is probably related to the dopaminergic lesion and the capacity of the remaining L-DOPA-activated dopaminergic neurons to respond to STN stimulation directly or indirectly, as this stabilisation is not observed in stimulated intact or non-stimulated DA PL animals, probably as the increase in DA levels is not maintained in these animals (Table 2). These data indicate that STN-HFS interferes with DA turnover, probably by modulating DA uptake and synthesis in DA PL rats, suggesting that adaptive mechanisms are involved in the stabilisation of striatal DA concentrations potentially involved in alleviating L-DOPA-related motor fluctuations, such as the wearing-off phenomenon (Nimura et al., 2005). However, the mechanism underlying the stabilisation of striatal DA concentration remains unclear. It may involve the restoration of autoregulation for presynaptic DA release in the striatum. Torstenson et al. (1997) demonstrated that the effect of L-DOPA infusion on striatal presynaptic DA activity differs significantly between patients in early and advanced stages of PD. Moreover, Ekesbo et al. reported that in mild and stable PD, upregulation of the presynaptic inhibitory feedback system maintains congruity with the dopaminergic system after the administration of antiparkinsonian medication (Ekesbo et al., 1999). The functional tone of the nigrostriatal DA system seems to be regulated at two sites of action: inhibitory autoreceptors located on presynaptic dopaminergic terminals and controlling the synthesis or release of DA, and receptors on the soma or dendrites of these neurons, involved in regulating impulse flow (Bunney et al., 1973; Roth, 1979; 1984). This inhibitory feedback regulation decreases with the progression of nigrostriatal degeneration. It seems plausible that STN-HFS alters inputs from the STN to the somatodendritic receptors at the SNc. Autoreceptor function can therefore be restored in the striatum. Alternatively, a relaxation of outputs from the STN may activate the premotor and motor cortices, leading to the attenuation of DA release in the striatum. Repetitive transcranial magnetic stimulation of the human motor cortex has been reported to lead to focal DA release in the ipsilateral striatum, consistent with a corticostriatal mode of DA release (Strafella et al., 2003a). STN-HFS attenuates SNr outputs, overcoming the inhibition of premotor and primary cortices. The decrease in the binding of dopaminergic D2 receptors observed in the known projection area of the disinhibited cortical site indicates that DA release is mediated by a direct effect of the corticostriatal neurons on striatal dopaminergic nerve terminals. Pallidotomy or pallidal stimulation alters the binding of postsynaptic dopaminergic D2 receptors in the striatum, demonstrating an effect of the cortex on the dopaminergic system (Nakajima et al., 2003). STN-HFS also increases striatal glutamate levels in both normal and hemiparkinsonian rats, suggesting an increase in the activity of striatal excitatory glutamatergic afferents (Bruet et al., 2003). Indeed, the inhibition of basal ganglial output activity by STN-HFS (Benazzouz et al., 1995; Windels et al., 2000; 2003; 2005; Salin et al., 2002; Boulet et al., 2006) may overcome inhibition of the thalamo-cortical pathway (Anderson et al., 2003), activating bilateral corticostriatal projections via cortical collaterals and, probably, also the thalamostriatal pathway. This disinhibitory effect is also supported by our recent data showing that STN-HFS increases extracellular GABA levels in the SNr, strongly suggesting that it is involved in inhibiting the BG output structures projecting onto the thalamus (Windels et al., 2000; 2003; 2005; Boulet et al., 2006). A direct corticostriatal influence on striatal dopaminergic terminals could probably account for the spatial selectivity of STN-HFS effects in our study, but we cannot exclude the involvement of other anatomical pathways. Frontal cortical neurons also project onto the SNc (Naito and Kita, 1994), where they can modulate the firing of dopaminergic neurons projecting onto the striatum. Consistent with a previous report, STN-HFS modulated DA concentration in the caudate nucleus after L-DOPA administration, although the degree of change was smaller than that in the putamen (Torstenson et al., 1997; Ekesbo et al., 1999). The difference between the caudate nucleus and the putamen may reflect the smaller L-DOPA response in the putamen. This may be due to the more severe effects on the dopaminergic terminals of the putamen than on those of the caudate nucleus in PD (Pearson et al., 1979; Graybiel et al., 1987; Miller et al., 1997; 1999).
In conclusion, these new neurochemical data suggest that STN-HFS interferes with L-DOPA effects, probably via synergic action, stabilising dopamine levels in the striatum. However, the mechanism underlying this stabilisation remains unclear. Our data show that STN-HFS interferes with DA turnover, probably by modulating DA uptake and synthesis in DA PL rats, suggesting a prolonged smooth DA action and adaptive mechanisms for alleviating L-DOPA-related motor fluctuations, such as the wearing-off phenomenon, shedding light on the mechanisms of STN-HFS in PD.
Acknowledgments
This work was supported by the Institut National de la Santé et de la Recherche Médicale, Ministère de la Recherche et des Nouvelles Technologies and Région Rhône-Alpes (Cluster n°11). We thank Drs M. Albrieux, V. Sgambato-Faure and P. Krack for critical reading of this manuscript.
Footnotes
Receiving Editor: Jean-Marc Fritschy
References
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of l-Dopa on extracellular dopamine in striatum of normal and 6- hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Abosch A, Kapur S, Lang AE, Hussey D, Sime E, Miyasaki J, Houle S, Lozano AM. Stimulation of the subthalamic nucleus in Parkinson’s disease does not produce striatal dopamine release. Neurosurgery. 2003;53:1095–1102. doi: 10.1227/01.neu.0000088662.69419.1b. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol. 2003;89:1150–1160. doi: 10.1152/jn.00475.2002. [DOI] [PubMed] [Google Scholar]
- Arbuthnott GW, Fairbrother IS, Butcher SP. Brain microdialysis studies on the control of dopamine release and metabolism in vivo. J Neurosci Methods. 1990;34:73–81. doi: 10.1016/0165-0270(90)90044-g. [DOI] [PubMed] [Google Scholar]
- Bejjani BP, Gervais D, Arnulf I, Papadopoulos S, Demeret S, Bonnet AM, Cornu P, Damier P, Agid Y. Axial parkinsonian symptoms can be improved: the role of levodopa and bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2000;68:595–600. doi: 10.1136/jnnp.68.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Benazzouz A, Pollak P. Mechanisms of deep brain stimulation. Mov Disord. 2002;17(suppl 3):S73–74. doi: 10.1002/mds.10145. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Piallat B, Pollak P, Benabid AL. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett. 1995;189:77–80. doi: 10.1016/0304-3940(95)11455-6. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Gao D, Ni Z, Benabid AL. High frequency stimulation of the STN influences the activity of dopamine neurons in the rat. Neuroreport. 2000;11:1593–1596. [PubMed] [Google Scholar]
- Blanchard V, Chritin M, Vyas S, Savasta M, Feuerstein C, Agid Y, Javoy-Agid F, Raisman-Vozari R. Long-term induction of tyrosine hydroxylase expression: compensatory response to partial degeneration of the dopaminergic nigrostriatal system in the rat brain. J Neurochem. 1995;64:1669–1679. doi: 10.1046/j.1471-4159.1995.64041669.x. [DOI] [PubMed] [Google Scholar]
- Boulet S, Lacombe E, Carcenac C, Feuerstein C, Sgambato-Faure V, Poupard A, Savasta M. Subthalamic stimulation-induced forelimb dyskinesias are linked to an increase in glutamate levels in the substantia nigra pars reticulata. J Neurosci. 2006;26:10768–10776. doi: 10.1523/JNEUROSCI.3065-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruet N, Windels F, Bertrand A, Feuerstein C, Poupard A, Savasta M. High frequency stimulation of the subthalamic nucleus increases the extracellular contents of striatal dopamine in normal and partially dopaminergic denervated rats. J Neuropathol Exp Neurol. 2001;60:15–24. doi: 10.1093/jnen/60.1.15. [DOI] [PubMed] [Google Scholar]
- Bruet N, Windels F, Carcenac C, Feuerstein C, Bertrand A, Poupard A, Savasta M. Neurochemical mechanisms induced by high frequency stimulation of the subthalamic nucleus: increase of extracellular striatal glutamate and GABA in normal and hemiparkinsonian rats. J Neuropathol Exp Neurol. 2003;62:1228–1240. doi: 10.1093/jnen/62.12.1228. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Aghajanian GK, Roth RH. Comparison of effects of L-dopa, amphetamine and apomorphine on firing rate of rat dopaminergic neurones. Nat New Biol. 1973;245:123–125. doi: 10.1038/newbio245123a0. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. J Neurochem. 1988;50:346–355. doi: 10.1111/j.1471-4159.1988.tb02919.x. [DOI] [PubMed] [Google Scholar]
- Carman LS, Gage FH, Shults CW. Partial lesion of the substantia nigra: relation between extent of lesion and rotational behavior. Brain Res. 1991;553:275–283. doi: 10.1016/0006-8993(91)90835-j. [DOI] [PubMed] [Google Scholar]
- Chritin M, Blanchard V, Raisman-Vozari R, Feuerstein C, Agid Y, Javoy-Agid F, Savasta M. DA uptake sites, D1 and D2 receptors, D2 and preproenkephalin mRNAs and Fos immunoreactivity in rat striatal subregions after partial dopaminergic degeneration. Eur J Neurosci. 1996;8:2511–2520. doi: 10.1111/j.1460-9568.1996.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Contin M, Riva R, Martinelli P, Procaccianti G, Cortelli P, Avoni P, Baruzzi A. Response to a standard oral levodopa test in parkinsonian patients with and without motor fluctuations. Clin Neuropharmacol. 1990;13:19–28. doi: 10.1097/00002826-199002000-00002. [DOI] [PubMed] [Google Scholar]
- De la Fuente-Fernandez R, Lu JQ, Sossi V, Jivan S, Schulzer M, Holden JE, Lee CS, Ruth TJ, Calne DB, Stoessl AJ. Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neurol. 2001;49:298–303. doi: 10.1002/ana.65.abs. [DOI] [PubMed] [Google Scholar]
- Dentresangle C, Le Cavorsin M, Savasta M, Leviel V. Increased extracellular DA and normal evoked DA release in the rat striatum after a partial lesion of the substantia nigra. Brain Res. 2001;893:178–185. doi: 10.1016/s0006-8993(00)03311-4. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Lozano AM. Mechanisms of deep brain stimulation. Mov Disord. 2002;17(suppl 3):S63–68. doi: 10.1002/mds.10143. [DOI] [PubMed] [Google Scholar]
- Ekesbo A, Rydin E, Torstenson R, Sydow O, Laengstrom B, Tedroff J. Dopamine autoreceptor function is lost in advanced Parkinson’s disease. Neurology. 1999;52:120–125. doi: 10.1212/wnl.52.1.120. [DOI] [PubMed] [Google Scholar]
- Fahn S. “On-off” phenomenon with levodopa therapy in Parkinsonism. Clinical and pharmacologic correlations and the effect of intramuscular pyridoxine. Neurology. 1974;24:431–441. doi: 10.1212/wnl.24.5.431. [DOI] [PubMed] [Google Scholar]
- Fairbrother IS, Arbuthnott GW, Kelly JS, Butcher SP. In vivo mechanisms underlying dopamine release from rat nigrostriatal terminals: I. Studies using veratrine and ouabain. J Neurochem. 1990a;54:1834–1843. doi: 10.1111/j.1471-4159.1990.tb04880.x. [DOI] [PubMed] [Google Scholar]
- Fairbrother IS, Arbuthnott GW, Kelly JS, Butcher SP. In vivo mechanisms underlying dopamine release from rat nigrostriatal terminals: II. Studies using potassium and tyramine. J Neurochem. 1990b;54:1844–1851. doi: 10.1111/j.1471-4159.1990.tb04881.x. [DOI] [PubMed] [Google Scholar]
- Fraix V, Pollak P, Van Blercom N, Xie J, Krack P, Koudsie A, Benabid AL. Effect of subthalamic nucleus stimulation on levodopa-induced dyskinesia in Parkinson’s disease. Neurology. 2000;55:1921–1923. doi: 10.1212/wnl.55.12.1921. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Hirsch EC, Agid YA. Differences in tyrosine hydroxylase-like immunoreactivity characterize the mesostriatal innervation of striosomes and extrastriosomal matrix at maturity. Proc Natl Acad Sci U S A. 1987;84:303–307. doi: 10.1073/pnas.84.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guesdon JL, Ternynck T, Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979;27:1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Zigmond MJ, Stricker EM. Effects of dopaminergic agonists and antagonists of feeding in intact and 6-hydroxydopamine-treated rats. J Pharmacol Exp Ther. 1977;201:386–399. [PubMed] [Google Scholar]
- Hefti F, Melamed E, Wurtman RJ. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res. 1980;195:123–137. doi: 10.1016/0006-8993(80)90871-9. [DOI] [PubMed] [Google Scholar]
- Hefti F, Enz A, Melamed E. Partial lesions of the nigrostriatal pathway in the rat. Acceleration of transmitter synthesis and release of surviving dopaminergic neurones by drugs. Neuropharmacology. 1985;24:19–23. doi: 10.1016/0028-3908(85)90090-5. [DOI] [PubMed] [Google Scholar]
- Henning J, Koczan D, Glass Ä, Karopka T, Pahnke J, Rolfs A, Benecke R, Gimsa U. Deep brain stimulation in a rat model modulates TH, CaMKIIa and Homer1 gene expression. Eur J Neurosci. 2007;25:239–250. doi: 10.1111/j.1460-9568.2006.05264.x. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Ghaemi M, Lehrke R, Rudolf J, Koulousakis A, Herholz K, Wienhard K, Sturm V, Heiss WD. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord. 2003;18:41–48. doi: 10.1002/mds.10297. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Grace AA. The effects of dopamine-depleting brain lesions on the electrophysiological activity of rat substantia nigra dopamine neurons. Brain Res. 1990;533:203–212. doi: 10.1016/0006-8993(90)91341-d. [DOI] [PubMed] [Google Scholar]
- Juncos JL, Engber TM, Raisman R, Susel Z, Thibaut F, Ploska A, Agid Y, Chase TN. Continuous and intermittent levodopa differentially affect basal ganglia function. Ann Neurol. 1989;25:473–478. doi: 10.1002/ana.410250509. [DOI] [PubMed] [Google Scholar]
- Kelly RS, Wightman RM. Detection of dopamine overflow and diffusion with voltammetry in slices of rat brain. Brain Res. 1987;423:79–87. doi: 10.1016/0006-8993(87)90827-4. [DOI] [PubMed] [Google Scholar]
- Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003;99:489–495. doi: 10.3171/jns.2003.99.3.0489. [DOI] [PubMed] [Google Scholar]
- Krack P, Limousin P, Benabid AL, Pollak P. Chronic stimulation of subthalamic nucleus improves levodopa-induced dyskinesias in Parkinson’s disease. Lancet. 1997;350:1676. doi: 10.1016/s0140-6736(05)64273-0. [DOI] [PubMed] [Google Scholar]
- Krack P, Hamel W, Mehdorn HM, Deuschl G. Surgical treatment of Parkinson’s disease. Curr Opin Neurol. 1999;12:417–425. doi: 10.1097/00019052-199908000-00008. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Kuhr WG, Bigelow JC, Wightman RM. In vivo comparison of the regulation of releasable dopamine in the caudate nucleus and the nucleus accumbens of the rat brain. J Neurosci. 1986;6:974–982. doi: 10.1523/JNEUROSCI.06-04-00974.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laihinen AO, Rinne JO, Nagren KA, Lehikoinen PK, Oikonen VJ, Ruotsalainen UH, Ruottinen HM, Rinne UK. PET studies on brain monoamine transporters with carbon-11-beta-CIT in Parkinson’s disease. J Nucl Med. 1995;36:1263–1267. [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Lopiano L, Rizzone M, Bergamasco B, Tavella A, Torre E, Perozzo P, Valentini MC, Lanotte M. Deep brain stimulation of the subthalamic nucleus: clinical effectiveness and safety. Neurology. 2001;56:552–554. doi: 10.1212/wnl.56.4.552. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kannari K, Suda T, Matsunaga M. Loss of regulation by presynaptic dopamine D2 receptors of exogenous L-DOPA-derived dopamine release in the dopaminergic denervated striatum. Brain Res. 1999;817:185–191. doi: 10.1016/s0006-8993(98)01248-7. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Parkes JD. “On-off” effects in patients with Parkinson’s disease on chronic levodopa therapy. Lancet. 1976;1:292–296. doi: 10.1016/s0140-6736(76)91416-1. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Basal ganglia disease. Lancet. 1982;2:1141–1147. doi: 10.1016/s0140-6736(82)92797-0. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Teitelbaum PA. Comparison of the eating in response to hypothermic and glucoprivic challenges after nigral 6-hydroxydopamine and lateral hypothalamic electrolytic lesions in rats. Brain Res. 1973;55:229–233. doi: 10.1016/0006-8993(73)90507-6. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Meissner W, Reum T, Paul G, Harnack D, Sohr R, Morgenstern R, Kupsch A. Striatal dopaminergic metabolism is increased by deep brain stimulation of the subthalamic nucleus in 6-hydroxydopamine lesioned rats. Neurosci Lett. 2001;303:165–168. doi: 10.1016/s0304-3940(01)01758-x. [DOI] [PubMed] [Google Scholar]
- Meissner W, Harnack D, Paul G, Reum T, Sohr R, Morgenstern R, Kupsch A. Deep brain stimulation of subthalamic neurons increases striatal dopamine metabolism and induces contralateral circling in freely moving 6-hydroxydopamine-lesioned rats. Neurosci Lett. 2002;328:105–108. doi: 10.1016/s0304-3940(02)00463-9. [DOI] [PubMed] [Google Scholar]
- Meissner W, Harnack D, Reese R, Paul G, Reum T, Ansorge M, Kusserow H, Winter C, Morgenstern R, Kupsch A. High-frequency stimulation of the subthalamic nucleus enhances striatal dopamine release and metabolism in rats. J Neurochem. 2003;85:601–609. doi: 10.1046/j.1471-4159.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- Miller DW, Abercrombie ED. Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous L-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J Neurochem. 1999;72:1516–1522. doi: 10.1046/j.1471-4159.1999.721516.x. [DOI] [PubMed] [Google Scholar]
- Miller GW, Staley JK, Heilman CJ, Perez JT, Mash DC, Rye DB, Levey AI. Immunochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann Neurol. 1997;41:530–539. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Miller GW, Erickson JD, Perez JT, Penland SN, Mash DC, Rye DB, Levey AI. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson’s disease. Exp Neurol. 1999;156:138–148. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- Mintz I, Hammond C, Guibert B, Leviel V. Stimulation of the subthalamic nucleus enhances the release of dopamine in the rat substantia nigra. Brain Res. 1986;376:406–408. doi: 10.1016/0006-8993(86)90209-x. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Valldeoriola F, Tolosa E, Rumia J, Valls-Sole J, Roldan H, Ferrer E. Levodopa withdrawal after bilateral subthalamic nucleus stimulation in advanced Parkinson disease. Arch Neurol. 2000;57:983–988. doi: 10.1001/archneur.57.7.983. [DOI] [PubMed] [Google Scholar]
- Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology. 1999;53:85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- Mouradian MM, Heuser IJ, Baronti F, Chase TN. Modification of central dopaminergic mechanisms by continuous levodopa therapy for advanced Parkinson’s disease. Ann Neurol. 1990;27:18–23. doi: 10.1002/ana.410270105. [DOI] [PubMed] [Google Scholar]
- Muenter MD, Tyce GM. L-dopa therapy of Parkinson’s disease: plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin Proc. 1971;46:231–239. [PubMed] [Google Scholar]
- Naito A, Kita H. The cortico-nigral projection in the rat: an anterograde tracing study with biotinylated dextran amine. Brain Res. 1994;637:317–322. doi: 10.1016/0006-8993(94)91252-1. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Nimura T, Yamaguchi K, Ando T, Itoh M, Yoshimoto T, Shirane R. The impact of stereotactic pallidal surgery on the dopamine D2 receptor in Parkinson disease: a positron emission tomography study. J Neurosurg. 2003;98:57–63. doi: 10.3171/jns.2003.98.1.0057. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Rice ME. Diffusion of ions and transmitters in the brain cell microenvironment. In: Fuxe K, Agnati LF, editors. Volume Transmission in the Brain. Raven Press; New York: 1991. pp. 279–294. [Google Scholar]
- Nimura T, Yamaguchi K, Ando T, Shibuya S, Oikawa T, Nakagawa A, Shirane R, Itoh M, Tominaga T. Attenuation of fluctuating striatal synaptic dopamine levels in patients with Parkinson disease in response to subthalamic nucleus stimulation: a positron emission tomography study. J Neurosurg. 2005;103:968–973. doi: 10.3171/jns.2005.103.6.0968. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Woodward WR, Carter JH, Gancher ST. Effect of long-term therapy on the pharmacodynamics of levodopa. Relation to on-off phenomenon. Arch Neurol. 1992;49:1123–1130. doi: 10.1001/archneur.1992.00530350037016. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Carter JH, Woodward WR. Long-duration response to levodopa. Neurology. 1995;45:1613–1616. doi: 10.1212/wnl.45.8.1613. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Holford NH. The response to levodopa in Parkinson’s disease: imposing pharmacological law and order. Ann Neurol. 1996;39:561–573. doi: 10.1002/ana.410390504. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Macias R, Alvarez L, Guridi J, Vitek J, DeLong MR. Pathophysiologic basis of surgery for Parkinson’s disease. Neurology. 2000;55:S7–S12. [PubMed] [Google Scholar]
- Olanow CW, Obeso JA. Preventing levodopa-induced dyskinesias. Ann Neurol. 2000;47(Suppl 1):S167–178. [PubMed] [Google Scholar]
- Olanow W, Schapira AH, Rascol O. Continuous dopamine-receptor stimulation in early Parkinson’s disease. Trends Neurosci. 2000;23:S117–126. doi: 10.1016/s1471-1931(00)00030-6. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Freeman T, Kordower J. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;345:146–147. [PubMed] [Google Scholar]
- Pahwa R, Wilkinson SB, Overman J, Lyons KE. Preoperative clinical predictors of response to bilateral subthalamic stimulation in patients with Parkinson’s disease. Stereotact Funct Neurosurg. 2005;83:80–83. doi: 10.1159/000086866. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Paul G, Reum T, Meissner W, Marburger A, Sohr R, Morgenstern R, Kupsch A. High frequency stimulation of the subthalamic nucleus influences striatal dopaminergic metabolism in the naive rat. Neuroreport. 2000;11:441–444. doi: 10.1097/00001756-200002280-00003. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; London: 1982. [DOI] [PubMed] [Google Scholar]
- Pearson J, Goldstein M, Brandeis L. Tyrosine hydroxylase immunohistochemistry in human brain. Brain Res. 1979;165:333–337. doi: 10.1016/0006-8993(79)90565-1. [DOI] [PubMed] [Google Scholar]
- Quattrone A, Zappia M. Oral pulse levodopa therapy in mild Parkinson’s disease. Neurology. 1993;43:1161–1166. doi: 10.1212/wnl.43.6.1161. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA dopamine lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- Roth RH. Dopamine autoreceptors: pharmacology, function and comparison with post-synaptic dopamine receptors. Commun Psychopharmacol. 1979;3:429–445. [PubMed] [Google Scholar]
- Roth RH. CNS dopamine autoreceptors: distribution, pharmacology, and function. Ann N Y Acad Sci. 1984;430:27–53. doi: 10.1111/j.1749-6632.1984.tb14497.x. [DOI] [PubMed] [Google Scholar]
- Salin P, Manrique C, Forni C, Kerkerian-Le Goff L. High-frequency stimulation of the subthalamic nucleus selectively reverses dopamine denervation-induced cellular defects in the output structures of the basal ganglia in the rat. J Neurosci. 2002;15:5137–5148. doi: 10.1523/JNEUROSCI.22-12-05137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Keller RW, Jr, Zigmond MJ. Dopamine efflux from striatal slices after intracerebral 6- hydroxydopamine: evidence for compensatory hyperactivity of residual terminals. J Pharmacol Exp Ther. 1990;253:867–876. [PubMed] [Google Scholar]
- Stachowiak MK, Keller RW, Jr, Stricker EM, Zigmond MJ. Increased dopamine efflux from striatal slices during development and after nigrostriatal bundle damage. J Neurosci. 1987;7:1648–1654. doi: 10.1523/JNEUROSCI.07-06-01648.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human cortex. Brain. 2003a;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Sadikot AF, Dagher A. Subthalamic deep brain stimulation does not induce striatal dopamine release in Parkinson’s disease. Neuroreport. 2003b;14:1287–1289. doi: 10.1097/00001756-200307010-00020. [DOI] [PubMed] [Google Scholar]
- Torstenson R, Hartvig P, Langstrom B, Westerberg G, Tedroff J. Differential effects of levodopa on dopaminergic function in early and advanced Parkinson’s disease. Ann Neurol. 1997;41:334–340. doi: 10.1002/ana.410410308. [DOI] [PubMed] [Google Scholar]
- Tossman U, Ungerstedt U. Microdialysis in the study of extracellular levels of amino acids in the rat brain. Acta Physiol Scand. 1986;128:9–14. doi: 10.1111/j.1748-1716.1986.tb07943.x. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Johnson PS. Neurotransmitter transporters: three important gene families for neuronal function. J Exp Biol. 1994;196:229–236. doi: 10.1242/jeb.196.1.229. [DOI] [PubMed] [Google Scholar]
- Welter ML, Houeto JL, Tezenas du Montcel S, Mesnage V, Bonnet AM, Pillon B, Arnulf I, Pidoux B, Dormont D, Cornu P, Agid Y. Clinical predictive factors of subthalamic stimulation in Parkinson’s disease. Brain. 2002;125:575–583. doi: 10.1093/brain/awf050. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Van Putten FM. Simultaneous determination of the rates of synthesis and metabolism of dopamine in various areas of the rat brain: application to the effects of (+)amphetamine. Eur J Pharmacol. 1987;133:103–110. doi: 10.1016/0014-2999(87)90211-1. [DOI] [PubMed] [Google Scholar]
- Windels F, Bruet N, Poupard A, Urbain N, Chouvet G, Feuerstein C, Savasta M. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. Eur J Neurosci. 2000;12:4141–4146. doi: 10.1046/j.1460-9568.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- Windels F, Bruet N, Poupard A, Feuerstein C, Bertrand A, Savasta M. Influence of the frequency parameter on extracellular glutamate and gamma-aminobutyric acid in substantia nigra and globus pallidus during electrical stimulation of subthalamic nucleus in rats. J Neurosci Res. 2003;72:259–267. doi: 10.1002/jnr.10577. [DOI] [PubMed] [Google Scholar]
- Windels F, Carcenac C, Poupard A, Savasta M. Pallidal origin of GABA release within the substantia nigra pars reticulata during high frequency stimulation of the subthalamic nucleus. J Neurosci. 2005;25:5079–5086. doi: 10.1523/JNEUROSCI.0360-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia M, Colao R, Montesanti R, Rizzo M, Aguglia U, Gambardella A, Oliveri RL, Quattrone A. Long-duration response to levodopa influences the pharmacodynamics of short-duration response in Parkinson’s disease. Ann Neurol. 1997;42:245–248. doi: 10.1002/ana.410420217. [DOI] [PubMed] [Google Scholar]
- Zappia M, Oliveri RL, Montesanti R, Rizzo M, Bosco D, Plastino M, Crescibene L, Bastone L, Aguglia U, Gambardella A, Quattrone A. Loss of long-duration response to levodopa over time in PD: implications for wearing-off. Neurology. 1999;52:763–767. doi: 10.1212/wnl.52.4.763. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Acheson AL, Stachowiak MK, Stricker EM. Neurochemical compensation after nigrostriatal bundle injury in an animal model of preclinical parkinsonism. Arch Neurol. 1984;41:856–861. doi: 10.1001/archneur.1984.04050190062015. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Berger TW, Grace AA, Stricker EM. Compensatory responses to nigrostriatal bundle injury. Studies with 6-hydroxydopamine in an animal model of parkinsonism. Mol Chem Neuropathol. 1989;10:185–200. doi: 10.1007/BF03159728. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–295. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]


