Abstract
We tested the possibility that it is possible to express unique peptide probes on cell surfaces and detect site-specific glycosylation on these peptides using flow cytometry. Such development can enhance the application of flow cytometry to detect and quantify post-translational modifications in proteins. To this end, the N-terminal section of the human leukocyte glycoprotein PSGL-1 (P-selectin glycoprotein ligand-1) was modified to contain a poly-histidine tag followed by a proteolytic cleavage site. Amino acids preceding the cleavage site have a single O-linked glycosylation site. The recombinant protein called PSGL-1 (HT) was expressed on the surface of two mammalian cell lines, CHO and HL-60, using a lentiviral delivery approach. Results demonstrate that the N-terminal portion of PSGL-1 (HT) can be released from these cells by protease, and the resulting peptide can be readily captured and detected using cytometry-bead assays. Using this strategy, the peptide was immunoprecipitated onto beads bearing mAbs against either the poly-histidine sequence or the human PSGL-1. The carbohydrate epitope associated with the released peptide was detected using HECA-452 and CSLEX-1, monoclonal antibodies that recognize the sialyl Lewis-X epitope. Finally, the peptide released from cells could be separated and enriched using nickel chelate beads. Overall, such an approach that combines recombinant protein expression with flow cytometry, may be useful to quantify changes in site-specific glycosylation for basic science and clinical applications.
Keywords: Glycoprotein, carbohydrate, glycosylation, inflammation, flow cytometry, leukocyte-endothelial cell adhesion, PSGL-1, selectin
Introduction
Glycosylation is an important post-translational modification (PTM) that is observed in 50% of all proteins (1). The site-specific nature of this modification plays a critical role in numerous cell recognition and signaling processes that regulate human development, immunity and disease. For example, during inflammation, the binding of adhesion molecules belonging to the selectin family on the activated vascular endothelial cells to the leukocyte cell-surface glycoprotein P-selectin glycoprotein ligand-1 (PSGL-1) plays a crucial role in controlling the rate of leukocyte-endothelial cell adhesion (2,3). The specific glycan on PSGL-1 that binds selectins is located near the N-terminus of this protein (4). In another example, cancer is thought to be associated with aberrant glycosylation (5,6). Many of the early tumor specific antibodies were developed against carbohydrate epitopes presented by tumor glycoproteins and glycolipids. More recent studies show that site-specific N-linked glycosylation of serum haptoglobin is a biomarker of pancreatic cancer (7). Further, Fuster and Esko (8) provide a comprehensive review of the roles of glycans in regulating various steps of tumor progression including proliferation, invasion and metastasis. Thus, cytometry based methodologies to detect site-specific glycosylation can aid a wide range of applications.
While the above examples illustrate the importance of identifying and quantifying the nature of site-specific modification on glycopeptides, tools to achieve these ends are still in development. Mass spectrometry and flow cytometry have emerged as two key technologies that can enable such research (9,10). While mass spectrometry tends to be a discovery-based approach in terms of identifying unknown modifications, flow cytometry is a more quantitative method. The later is more amenable to application in a routine laboratory and clinical setting.
In the current manuscript, proof-of-concept experiments are presented to test the hypothesis that it is possible to detect site-specific glycosylation in proteins using flow cytometry. This has not been attempted previously. To test this possibility, a poly-histidine sequence followed by a thrombin cleavage site was introduced after the first 22 amino acids at the N-terminus of PSGL-1. This modified PSGL-1 was termed PSGL-1 (HT). The N-terminus of this protein, released upon digestion at cleavage site, contains a unique glycosylation site at Thr-57 (amino acid numbering begins with signal peptide) that binds all three members of the selectin family with high affinity and under fluid shear conditions (2,11). The carbohydrate structure at this N-terminal site is thought to be an O-linked glycan with a core-2 structure bearing the sialyl Lewis-X epitope (NeuAcα2,3Galβ1,4[Fucα1,3]GlcNAc) (4,12,13). PSGL-1 (HT) was expressed stably in both easy-to-transfect CHO cells and hard-to-transfect human promyelocytic leukemia HL-60 cells using a lentiviral strategy. Results demonstrate that it is possible to specifically detect the isolated PSGL-1 (HT) peptide using flow cytometry by separating it from PSGL-1 (HT) bearing cells, using thrombin to release the peptide and a variety of cytometry-beads to immunoprecipitate the fragment. Glycan structures on the immunoprecipitated peptide probe can also be assayed using flow cytometry by employing fluorescent carbohydrate recognizing antibodies for detection. Such a strategy to minimally modifying the natural glycoprotein for the purpose of quantifying site-specific glycosylation likely preserves the transport of the protein in the cellular endoplasmic reticulum and Golgi. The residence/passage time of the proteins in these compartments likely plays an important role in regulating the micro/macro-heterogeneous pattern of glycosylation on these molecules. While mucinous proteins like PSGL-1 have 71 Ser/Thr sites for O-linked glycosylation and 3 sites for N-glycans, our strategy of expressing a cleavable fragment allows us to focus on a single site. It also obviates the need to lyse cells and to separate proteins of interest from a complex mixture. Studies are performed in the current manuscript using a single recombinant peptide with focus on a single site of glycosylation. This concept may be generalized in the future to examine multiple recombinant peptide probes and glycan structures.
Materials and Methods
Materials
Cell culture products were purchased from Invitrogen (Carlsbad, CA), chemicals from Sigma (St. Louis, MO) and plasmids from Addgene (Cambridge, MA). Human promyelocytic leukemia HL-60 cells and Chinese Hamster Ovary cells (CHO) were cultured as recommended by ATCC (Manassas, VA). Purified and fluorescently-labeled monoclonal antibodies (mAbs) against Lewis-X/CD15 (clone HI98), sialyl Lewis-X (sLeX)/CD15s (clone CSLEX-1), sLeX-like antigen called cutaneous lymphocyte associated antigen (anti-CLA mAb HECA-452), human PSGL-1/CD162 (clones KPL-1 and PL2), and isotype-matched controls were from BD-Pharmingen (San Diego, CA). Anti-PSGL-1 mAb TB5 was from BioVendor (Candler, NC), and anti-His antibodies (tetra-His and penta-His) were from Qiagen (Valencia, CA). P-selectin fusion protein (mouse IgG2a) was produced in our laboratory using baculovirus expression system (Invitrogen). Where applicable, Alexa 488 fluorochrome conjugation was performed using an Alexa Fluor 488 monoclonal antibody labeling kit (Invitrogen).
Lentiviral preparation and cell transduction
Standard molecular biology methods were used to develop a lentivirus containing modified PSGL-1 cDNA. PCR was first used to insert a 6X-histidine tag followed by a thrombin cleavage site (LVPRGS) after the first 22 amino acids of the mature human PSGL-1 (Figure 1). To achieve this, a pBluescript vector containing the entire human PSGL-1 sequence was used as a template. Poly-His and thrombin cleavage sites were inserted into this template using primers 5′-[Phos] ATGGTGATGGTGATGATGCAGCATTTCTGGAGGCTCCG-3′ and 5′-CTGGTTCCGCGTGGATCCCCTCTGACTGGGCCTGGAACCCCTG-3′, followed by self-ligation of PCR product. Next, the lentiviral vector, pCSCG-GFP (14) was digested with BamHI and EcoRI, and its ends were blunted with DNA polymerase I (Klenow) to remove these restriction enzyme sites. The resulting product was religated. Subsequently, the GFP gene in pCSCG-GFP was excised using NheI and XhoI. PSGL-1 containing the 6X-histidine- and thrombin- sites was introduced in place of GFP in pCSCG-GFP by PCR amplifying PSGL-1 with primers that had sites for these restriction enzymes (underlined), 5′-CTAGCTAGCTAGGGGACTGCCGCAGGGGGT-3′ and 5′-CCGCTCGAGCGGAGATGGCAGAGTGAGCTAAG-3′, and ligating this product into the digested vector. This final product was termed pCSCG-PSGL-1 (HT).
Figure 1.
PSGL-1(HT) sequence compared to wild-type PSGL-1. Binding site for mAbs KPL-1 and PL-1 are indicated by straight lines. 6X Histidine-Thrombin cleavage site shown in shaded box substitute for selected amino acids in wild type molecule as indicated. Arrow denotes the thrombin cleavage site. *denotes site for tyrosine sulfation. Bold, italicized T denotes unique site for O-linked glycosylation.
VSV-G-pseudotyped recombinant HIV lentiviral virions were produced by co-transfection of HEK 293T cells with either 20μg pCSCG-PSGL-1 (HT) or pCSCG-GFP, along with VSV-G-expressing construct pMD2.G (10μg), and the packaging constructs pSRV-Rev (10μg) and pMDLg/pRRE (10μg) in a tissue culture dish using calcium phosphate method (14). Cells were induced with 25mM chloroquine in DMEM medium with 10% FBS for 8-10 hours after transfection. Chloroquine was removed thereafter and cell culture in fresh media containing 10mM sodium butyrate continued for 48 hours. Supernatant harvested after 2 days contained lentivirus. This was concentrated 100-fold using ultra centrifugation. The final lentivirus, resuspended in DMEM medium without serum, was aliquoted and either used immediately or it was frozen at -80°C.
CHO/HL-60 cells were seeded in 96-well plates at 5000 cells/well the day before transduction. These cells were incubated with 50μl of lentivirus encoding for either PSGL-1 (HT) or GFP in the presence of 6μg/ml polybrene in 200μl DMEM medium with FBS for two days. Transduction media was changed to normal growth media free of virus after 2 days. Vehicle control contained polybrene without lentivirus. Following transduction, HL-60 cells expressing PSGL-1 (HT) were sorted using tetra-His antibody along with goat anti-mouse Alexa488 F (ab′) 2 secondary antibody using a BD FACS Aria flow cytometer (San Jose, CA). Similarly, GFP-positive HL-60 cells were also sorted based on fluorescence.
Flow cytometry studies with whole cells
Either CHO or HL-60 cells were washed and resuspended in 30mM HEPES buffer (30 mM HEPES, 110 mM NaCl, 10 mM KCl, 1 mM MgCl2, pH=7.2) containing 1.5mM CaCl2 and 0.1% human serum albumin (HSA) at 0.5-1×106 cells/ml prior to cytometry analysis. In runs that examined cell surface antigen expression or lectin binding, fluorescent mAbs or isotype-matched reagents were added for 15min at RT at 10-30μg/ml. For P-selectin binding, cells were incubated with a 1:3 dilution of P-selectin-IgG (supernatant of baculovirus expression system) along with a 1:40 dilution of FITC-conjugated F (ab′) 2 goat-anti-mouse secondary antibody (Jackson Immuno, West Grove, PA) for 25min at RT (27). Following incubation, cells were washed quickly just prior to flow cytometry (FACSCalibur, BD-Biosciences) measurements. Fluorescence signal from secondary Ab alone, in the absence of selectin fusion protein was at least one order of magnitude lower than in the presence of selectin. Signal from secondary Ab alone was thus subtracted from the positive signal. To confirm the specificity of the interaction, appropriate isotype-controls with fluorescent IgG and IgM antibodies were performed. Specificity of P-selectin binding function was confirmed by blocking antibody (clone G1) against the P-selectin fusion protein.
Cytometry-bead assays with N-terminal PSGL-1 fragment
For the cytometer-bead assays, carbodiimide chemistry was used to covalently link various mAbs to 6μm carboxyl polystyrene beads as described earlier (15). Monoclonal antibodies immobilized on these beads include anti-PSGL-1 mAb TB5 (mouse IgG1), anti-tetra-His mAb (mouse IgG1) and isotype-control anti-CD18 mAb IB4 (mouse IgG1). These three types of beads are termed “TB5-beads”, “anti tetra-His-beads” and “IB4-beads”. The density of mAbs on these beads was ∼16,000, 3,000 and 2,000/μm2 respectively. “Blank-beads” used in some studies were prepared identically to above, only mAbs were not incubated with beads during the covalent conjugation step.
HL-60-PSGL-1 (HT) and CHO-PSGL-1 (HT) were treated with thrombin to release the PSGL-1 (HT) peptide fragment into solution. Wild-type CHO/HL-60 cells lacking the PSGL-1 (HT) construct were treated identically in control runs. For this, typically, 3 million cells were washed and resuspended in PBS buffer (pH=7.2). Thrombin was added at a concentration of 50 U/ml for 2h at 37°C in 200μl volume. Supernatant was harvested by pelleting the cells.
In each experiment, a fraction of supernatant obtained following thrombin treatment of 3×106 cells (both WT and PSGL-1 (HT) transfected cells), was incubated with 30,000-50,000 beads (TB5/anti-tetra-His/IB4) in 100μl volume for 1h at RT with gentle agitation. Following three washes with PBS containing 0.025% Tween-20, aliquots of beads (∼5,000-10,000) were incubated with antibodies: 10-20μg/ml of phycoerythrin conjugated anti-PSGL-1 mAb (KPL-1), Alexa488 conjugated anti-His mAb (tetra-His), or unconjugated anti-carbohydrate mAbs (HECA-452 and CSLEX1) for 20min at RT in the same buffer. Following a wash step, Alexa488-conjugated goat-anti-mouse IgM (μ chain specific) was additionally added for 20min in the last cases which detected the sLeX epitope. Beads were then washed prior to flow cytometry analysis.
In competitive inhibition/blocking assays that confirmed the specificity of PSGL-1 (HT) capture onto cytometry-beads, the cell supernatant obtained following thrombin mediated cleavage was incubated with excess TB5, tetra-His or isotype control Ab (DREG-56, IgG1) for 15min at RT before addition of beads for antigen capture.
Purification of recombinant PSGL-1 using magnetic beads
In some cases, in order to verify our ability to purify the PSGL-1 peptide, supernatant from thrombin treated cells was incubated with MagneHis beads (Promega, USA) for 30min at RT. After 3-times wash with wash buffer (10mM HEPES, 500mM NaCl, 10mM imidazole), the recombinant PSGL-1 was eluted in elution buffer (10mM HEPES, 500mM NaCl, 500mM imidazole). A fraction of the eluted solution was incubated with TB5- or anti-tetra-His beads for PSGL-1 capture, and subsequent flow cytometry analysis was performed as described in the previous section.
Statistics
Error bars represent standard error mean (SEM). Student's t-test was performed for dual comparison.* p< 0.05 was considered significant.
Results
Design of recombinant PSGL-1 with His-tag and thrombin cleavage site
The anionic N-terminal peptide sequence of PSGL-1 has three sites of tyrosine-sulfation and a threonine residue that bears an O-linked glycan (Figure 1). This O-linked glycan participates in selectin mediated leukocyte adhesion (2,11). We tested if the glycan structure at this site could be detected/studied using flow cytometry. To this end, human PSGL-1 was modified by inserting a His-tag and thrombin cleavage site following the first 22 amino acids of the mature protein. The remaining portions of the protein, including the transmembrane and cytosolic sections, were identical to the wild-type protein. Here, we deliberately chose to preserve the first 22 amino acids of PSGL-1 because it has a unique site of glycosylation at Thr-57 (2,11). This peptide motif is also recognized by two anti-PSGL-1 mAbs, KPL-1 which binds a sulfation dependent segment between amino acids 46-52 (16) and PL-1 which binds between residues 49-62 (17). The availability of multiple mAbs that recognize both the PSGL-1 peptide and the his-tag segment simplified peptide detection during cytometry assays.
Recombinant PSGL-1 (HT) expressed on CHO and HL-60 cells using lentivirus
HL-60 is a hard-to-transfect cell line which is resistant to conventional methods of gene transfer including lipofection, electroporation and nucleofection. Lipofection using lipofectamine™ 2000 (Invitrogen) and Fugene 6 (Roche Applied Science, Indianapolis, IN) resulted in no detectable expression of PSGL-1 (HT) or green fluorescence protein (GFP) in these cells. Electroporation using a variety of approaches resulted in excessive cell death and low transient transfection. Nucleofection resulted in efficient transfection in the first 24-48 hours, though the transformed cells lost their capacity to proliferate. Similar negative effects of nucleofection are reported by others (18).
In order to overcome the challenges of the above mentioned conventional approaches that prevent the stable transfection of HL-60 cells, we tested the possibility that VSV-G-pseutotyped lentivirus can be used to transduce HL-60. Transduction of CHO cells with virus that encode for either GFP or PSGL-1 (HT) typically leads to 85-95% of the cells expressing these genes (Fig. 2). HL-60 cell transduction lead to low, but non-zero, stable expression of the gene of interest. Typically, at the highest titer, 2-5 % of the HL-60 cells expressed PSGL-1 (HT) after 1 week of transduction. Thus, HL-60 cells containing PSGL-1 (HT) were sorted using flow cytometry to select for cells that had the highest binding to anti-tetra-His mAb.
Figure 2.
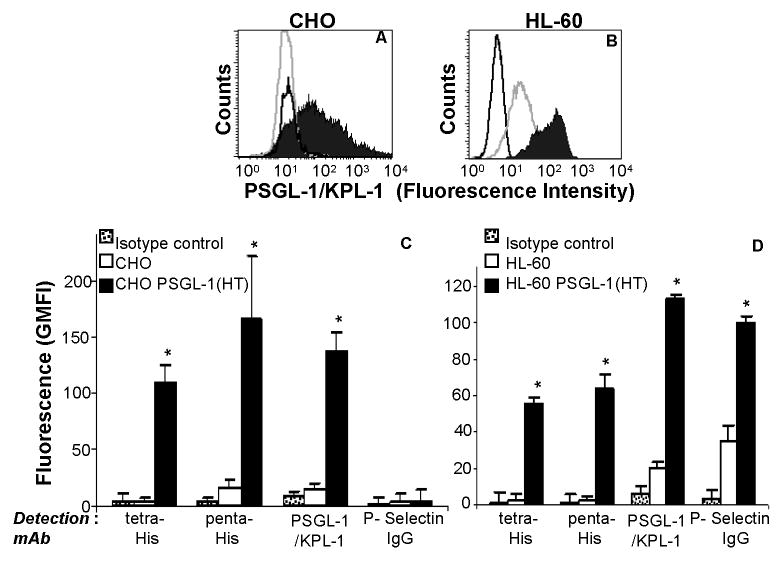
Detection of PSGL-1(HT) on CHO and HL-60 cell surface. Wild type CHO and HL-60 cells, and these same cell lines expressing PSGL-1(HT) were probed with monoclonal antibodies against PSGL-1 (clone KPL-1). Panels A corresponds to representative histogram for CHO and CHO PSGL-1(HT), while panel B are for HL-60 and HL-60 PSGL-1(HT). Black-filled peak corresponds to cells expressing PSGL-1(HT), grey-empty peaks correspond to wild type CHO/HL-60 that did not express PSGL-1(HT). Black-empty peaks correspond to isotype control antibody. Panel C and D presents geometric mean fluorescence intensity (GMFI) + SEM data for CHO and HL-60 cells respectively (N=3). As seen, PSGL-1 expression on HL-60 PSGL-1(HT) was 5.7 times higher and P-selectin binding was elevated 2.9-fold in comparison to wild-type HL-60. *p<0.05 with respect to wild-type CHO/HL-60 cells.
Studies were conducted with CHO and HL-60 cells that stably express recombinant PSGL-1 (HT). Both cell types bound monoclonal antibodies against the tetra-His (recognizes HHHH), penta-His (recognizes HHHHH) and N-terminal PSGL-1 epitopes efficiently (Fig. 2, Supplemental Fig. 1). PSGL-1 expression on these HL-60 PSGL-1 (HT) cells was 5-fold higher than wild-type HL-60 cells (Fig. 2D). P-selectin IgG chimeric fusion protein binding to CHO cells was absent since these cells lack key glycosyltransferases, core-2 β1,6-N-acetylglucosaminyltransferase and α(1,3) fucosyltransferases, that constitute selectin-ligand function (19) (Fig. 2C). HL-60 transduced with PSGL-1 (HT), however, bound P-selectin IgG at 3-fold higher levels compared to normal HL-60 (Fig. 2D). Overall, these data confirm the presence of PSGL-1 (HT) on both transduced CHO and HL-60 cells.
Detection of N-terminal PSGL-1 peptide and associated carbohydrate using cytometry-beads
We determined if the carbohydrate signature of the cleavable PSGL-1 peptide can be detected using flow cytometry. Experiments were thus undertaken with N-terminal PSGL-1 peptide released from both CHO (Fig. 3A-3C, Supplemental Fig. 2) and HL-60 cells (Fig. 3D, Supplemental Fig. 3) expressing PSGL-1 (HT) following thrombin treatment. The phosphate-saline based solution containing the cleaved peptide was incubated with 6μm beads bearing anti-PSGL-1 (clone TB5), anti-tetra-His or isotype control (clone IB4) mAb. Fluorescent tagged antibodies recognizing PSGL-1 (clone KPL-1), His-tag and carbohydrate structures (CLA and sLeX) were then incubated with these beads. Fluorescent signal due to antibody binding was quantified. Data in literature suggest that both the anti-sLeX mAbs chosen, HECA-452 (20) and CSLEX-1 (21), inhibit the binding of selectins with leukocyte PSGL-1.
Figure 3.
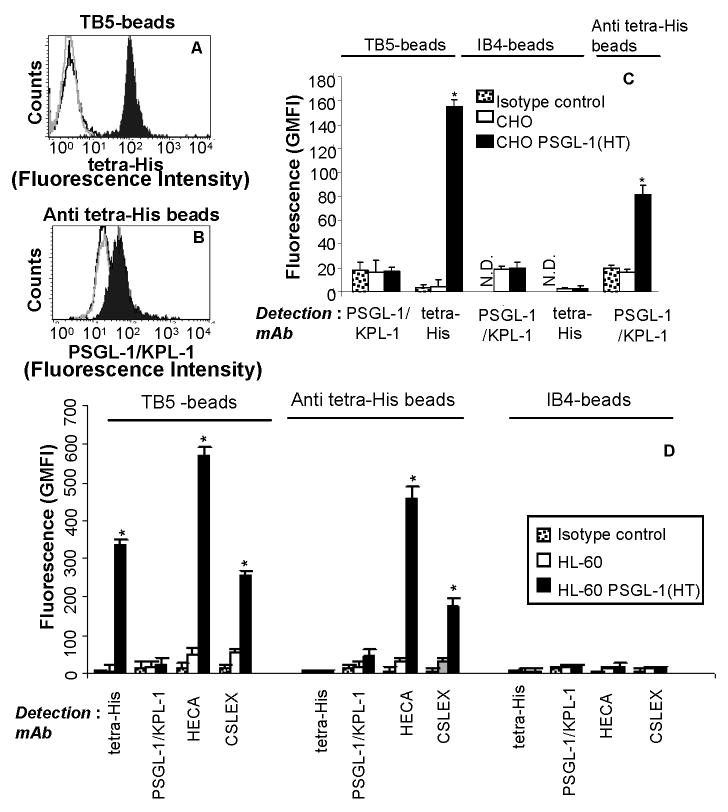
Cytometry-bead experiments used to detect N-terminal PSGL-1(HT) peptide expressed in CHO and HL-60 cells. CHO/HL-60 PSGL-1 (HT) or wild-type CHO/HL-60 cells were treated with 50U/mL bovine thrombin in 200μl volume for 2h at 37°C. Supernatant containing cleaved peptide was collected and this was allowed to bind either TB5-beads that bear anti-PSGL-1 mAb (panels A) or anti tetra-His beads (panel B). PSGL-1 binding to these beads was detected using either anti-tetra-His mAb for panel A or anti-PSGL-1 mAb KPL-1 for B. Representative cytometry histogram are presented for CHO cells where black-filled peaks correspond to supernatant from cells expressing PSGL-1 (HT), grey-empty peaks correspond to wild type cells, and black-empty peaks represent isotype control sample. Panels C and D present a summary of the cytometry data for CHO and HL-60 cells respectively as mean + SEM (N=3). In both panels, immunoprecipitated PSGL-1 fragment is detected using both TB5- and anti tetra-His beads, but not isotype IB4-beads. Also, the binding sites for clones TB5 and KPL-1 overlap. Glycans on the PSGL-1(HT) peptide from HL-60 is detected using both anti-CLA mAb HECA-452 and anti-CD15s mAb CSLEX-1, on both TB5- and Anti tetra-His beads. N.D.: Not done since these are isotype controls themselves. *p<0.05 with respect to wild-type CHO/HL-60 cells.
In studies performed with thrombin cleaved product from CHO cells, the PSGL-1 peptide immunoprecipitated onto TB5-beads could be detected using mAb against the His-epitope (Fig. 3A) but not anti-PSGL-1 mAb KPL-1 (Supplemental Fig. 2A). PSGL-1 bound to anti-tetra-His beads could also be detected using phycoerythrin conjugated KPL-1 (Fig. 3B). Isotype control beads incubated with thrombin cleaved product bound neither fluorescently labeled anti-PSGL-1 nor directly conjugated anti-His mAb (Fig. 3C). Binding of anti-CD15/Lex (clone HI98), anti-CLA (HECA-452) and anti-CD15s/sLex (CSLEX-1) was absent in all three bead preparations (data not shown). Thus, the N-terminal PSGL-1 fragment from CHO PSGL-1 (HT) cells do not express these carbohydrate antigens. Overall, the results demonstrate that N-terminal PSGL-1 peptide cleaved from CHO PSGL-1 (HT) cells can be immunoprecipitated and detected on anti-His and anti-PSGL-1 bearing beads. Also, TB5 and KPL-1 compete for the same or overlapping sites at the N-terminus of PSGL-1. This last observation is consistent with platelet-neutrophil cell adhesion studies where both KPL-1 and TB5 efficiently and completely inhibit heterotypic cell adhesion by blocking the interaction of platelet P-selectin with PSGL-1 expressed on neutrophils (22). Finally, the detection of PSGL-1 on anti-tetra-His beads was lower than that on TB5-beads due to the lower density of immobilized mAb on the former beads (3,000 on anti-tetra His vs. 16,000 sites/μm2 on TB5-beads).
When media containing thrombin cleaved product from HL-60 PSGL-1 (HT) cells was incubated with the three types of beads (Fig. 3D), similar to experiments with CHO cells, we detected immobilized PSGL-1 peptide in runs where TB5-beads bearing the peptide were probed with fluorescent tetra-His mAb (Supplemental Fig. 3A), and also when anti-tetra-His beads with peptide were detected using fluorescent anti-PSGL-1 mAb KPL-1 (Supplemental Fig. 3F). The detection of the sLex epitope on these beads was also evident using both mAbs HECA-452 and CSLEX-1 (Fig. 3D). Accessibility of the carbohydrate epitope on these beads was high as evidenced by the high fluorescence signal in runs performed with TB5- and anti-tetra-His beads, compared to isotype control IB4-beads which displayed low binding to both the PSGL-1 (HT) peptide and anti-carbohydrate antibodies (last column of Supplemental Fig. 3).
Competition studies with excess soluble mAbs confirm the specificity of the measured interaction
Additional studies were undertaken to confirm the specificity of the measured fluorescence signal. Here, the binding of the PSGL-1 peptide to anti-TB5 beads was competed away using excess soluble TB5 mAb to both thrombin cleaved product from CHO cells (Supplemental Fig. 4A-C) and that from HL-60 cells (Supplemental Fig. 4D-F) in the cytometry-bead assay. In studies with CHO cells, ∼50-60% reduction in PSGL-1 (HT) signal was observed when soluble TB5 concentration was 70 μg/ml, and this increased to 80% when soluble TB5 dose was increased to 140 μg/ml. Similar observations were made with HL-60 cells (Supplemental Fig. 4D-F) where the binding of PSGL-1 (HT) to beads was reduced by ∼80 and 90% when soluble TB5 was added at 70 and 140 μg/ml. In additional studies, we show that HECA-452 binding to anti-tetra-His beads bearing immobilized PSGL-1 (HT) from HL-60 was also reduced by ∼75 % in the presence of 10 and 20 μg/ml anti-His mAb (Supplemental Fig. 4G-I). Isotype control antibody DREG-56 (mouse IgG1) did not affect PSGL-1 (HT) peptide binding in any of the control runs performed (Supplemental Fig. 4B, E, H). Overall, these competition binding assays further confirm that the fluorescence signal measured in this work is specific to the PSGL-1(HT) peptide.
Purification and enrichment of PSGL-1 (HT) using nickel beads
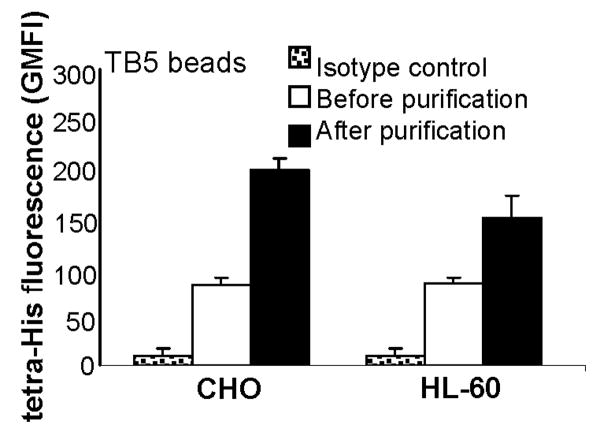
Purification and enrichment of PSGL-1 (HT) may be helpful for biochemical studies that aim to further characterize the N-terminal peptide of PSGL-1. With this goal, we demonstrate the utility of magnetic nickel beads for convenient purification of the PSGL-1 (HT) peptide from cell culture supernatants of thrombin treated CHO PSGL-1 (HT) and HL-60 PSGL-1 (HT) cells (Fig. 4). As seen, fluorescence signal emitted by the eluted PSGL-1 (HT) peptide was ∼2-2.5 fold higher than that observed for sample before purification. This increased signal is due to 5-fold sample concentration during the purification step.
Figure 4.
His-tag purification: N-terminal PSGL-1 (HT) peptide from CHO/HL-60 cells obtained following thrombin treatment was purified using MagneHis beads. 500mM imidazole was used to elute peptides from these nickel beads. The presence of peptide in this eluate was determined using TB5-beads to capture PSGL-1 (HT) and anti-tetra-His mAb for detection. Data are presented as mean + SEM (N=3). Fluorescence signal from eluted peptide is higher than original sample due to sample concentration that occurs during purification.
Discussion
This manuscript demonstrates that novel peptide probes can be applied in cytometry-based assays to quantify the site-specific nature of glycosylation. A strategy is also described for the presentation of these proteins on the surface of hard-to-transfect HL-60 cells. These peptides, which are released following proteolytic cleavage from CHO and HL-60 cell surfaces using thrombin, were immunoprecipitated onto polystyrene beads. Monoclonal antibodies against carbohydrate antigens were applied to detect glycans on these immobilized proteins. Studies performed in the presence of excess soluble antibody that competed away the binding of the peptide to beads confirm that the measured glycan structures can be specifically attributed to those expressed by the peptide probe. Proteolytic fragments from 106 cells were sufficient for a typical run and thus this technique can be performed without the need for large-scale cell culture. The time required for a single run is also short compared to conventional western blot analysis. Thus, this methodology can be applied to rapidly and quantitatively test the effect of a range of experimental times and other variables on a particular glycan structure. Western blot analysis is also limited in that it is difficult or impossible to detect hydrophilic peptides with molecular weight less than 10kDa using this technique.
Based on the current investigation, improvements to the design of the peptide probe may be suggested. In this regard, elaborate attempts were made to analyze the carbohydrate structures on the peptides using tandem mass spectrometry. These studies were however unsuccessful, possibly due to the 6-histidine segment on the peptide which complicated such analysis. This issue will be addressed in the future by changing the design of the peptide probe and by introducing alternate, more specific sites for proteolysis like either the enterokinase or TEV protease (Nuclear Inclusion-a protein encoded by the Tobacco Etch Virus) cleavage site.
While results are presented in this manuscript for a single protein that is expressed on human leukocytes, the scope of the study may be expanded in the future using alternate peptide probes. This can facilitate the application of cytometry-bead methods for both basic science and clinical applications. In one aspect, the current methodology can be extended to other modifications of the PSGL-1 peptide substrate in order to study the effect of peptide backbone on the initiation and extension of O-linked glycosylation. In this regard, while it has been shown that N-linked glycosylation commonly occurs at Asn-Xaa-Ser/Thr sites, with Xaa being any amino acid residue except Pro, no consensus sequence for the initiation of O-linked glycosylation is known (23). Further, besides O-linked chain initiation, it remains unknown to what degree the peptide substrate affects chain extension. In this regard, it is necessary to note that while N-linked glycosylation is initiated by the transfer of a 14-sugar precursor from dolicol to the polypeptide chain in the endoplasmic reticulum, O-linked glycosylation is more complex. It involves the stepwise growth of the oligosaccharide chain at Ser/Thr sites using a series of Golgi resident glycosyltransferases. In another aspect, if site specific glycosylation correlates with disease progression as is suggested by recent literature (6,7), the cytometry-bead assay bearing a peptide probe along with a glycan specific antibody may be used to identify such specific glycan modifications. With the inclusion of other protein-specific antibodies for the capture of antigens, this cytometry-based immunoassay can thus be further multiplexed to simultaneously screen glycosylation of multiple proteins from the same source in a single sample run.
Overall, the current manuscript advances the concept of developing recombinant peptide probes to measure/characterize glycosylation processes in cells. Such methods may complement parallel efforts in the field of mass spectrometry that attempt to advance our understanding of the glycoproteome (24,25).
Supplementary Material
Acknowledgments
We gratefully acknowledge help from Jun Tian and Prof. Stelios Andreadis for construction of the lentivirus.
Footnotes
This work was supported by National Institutes of Health grant HL63014
Conflict-of-interest disclosure: The authors declare no competing financial interests
Literature Cited
- 1.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473(1):4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 2.Ebnet K, Vestweber D. Molecular mechanisms that control leukocyte extravasation: the selectins and the chemokines. Histochem Cell Biol. 1999;112(1):1–23. doi: 10.1007/s004180050387. [DOI] [PubMed] [Google Scholar]
- 3.Neelamegham S. Transport features, reaction kinetics and receptor biomechanics controlling selectin and integrin mediated cell adhesion. Cell Commun Adhes. 2004;11(1):35–50. doi: 10.1080/15419060490471793. [DOI] [PubMed] [Google Scholar]
- 4.Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J Biol Chem. 1996;271(31):18732–42. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- 5.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekaran EV, Xue J, Neelamegham S, Matta KL. The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr Res. 2006;341(8):983–94. doi: 10.1016/j.carres.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Miyoshi E, Nakano M. Fucosylated haptoglobin is a novel marker for pancreatic cancer: detailed analyses of oligosaccharide structures. Proteomics. 2008;8(16):3257–62. doi: 10.1002/pmic.200800046. [DOI] [PubMed] [Google Scholar]
- 8.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5(7):526–42. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 9.Bonilla L, Means G, Lee K, Patterson S. The evolution of tools for protein phosphorylation site analysis: from discovery to clinical application. Biotechniques. 2008;44(5):671–9. doi: 10.2144/000112800. [DOI] [PubMed] [Google Scholar]
- 10.Sklar LA, Edwards BS, Graves SW, Nolan JP, Prossnitz ER. Flow cytometric analysis of ligand-receptor interactions and molecular assemblies. Annu Rev Biophys Biomol Struct. 2002;31:97–119. doi: 10.1146/annurev.biophys.31.082901.134406. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Ramachandran V, Kang J, Kishimoto TK, Cummings RD, McEver RP. Identification of N-terminal residues on P-selectin glycoprotein ligand-1 required for binding to P-selectin. J Biol Chem. 1998;273(12):7078–87. doi: 10.1074/jbc.273.12.7078. [DOI] [PubMed] [Google Scholar]
- 12.Aeed PA, Geng JG, Asa D, Raycroft L, Ma L, Elhammer AP. Characterization of the O-linked oligosaccharide structures on P-selectin glycoprotein ligand-1 (PSGL-1) Glycoconj J. 1998;15(10):975–85. doi: 10.1023/a:1006985825141. [DOI] [PubMed] [Google Scholar]
- 13.Beauharnois ME, Lindquist KC, Marathe D, Vanderslice P, Xia J, Matta KL, Neelamegham S. Affinity and kinetics of sialyl Lewis-X and core-2 based oligosaccharides binding to L- and P-selectin. Biochemistry. 2005;44(27):9507–19. doi: 10.1021/bi0507130. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72(10):8150–7. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marathe DD, Chandrasekaran EV, Lau JT, Matta KL, Neelamegham S. Systems-level studies of glycosyltransferase gene expression and enzyme activity that are associated with the selectin binding function of human leukocytes. Faseb J. 2008;22(12):4154–67. doi: 10.1096/fj.07-104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snapp KR, Ding H, Atkins K, Warnke R, Luscinskas FW, Kansas GS. A novel P-selectin glycoprotein ligand-1 monoclonal antibody recognizes an epitope within the tyrosine sulfate motif of human PSGL-1 and blocks recognition of both P- and L-selectin. Blood. 1998;91(1):154–64. [PubMed] [Google Scholar]
- 17.Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem. 1996;271(6):3255–64. [PubMed] [Google Scholar]
- 18.Schakowski F, Buttgereit P, Mazur M, Marten A, Schottker B, Gorschluter M, Schmidt-Wolf IG. Novel non-viral method for transfection of primary leukemia cells and cell lines. Genet Vaccines Ther. 2004;2(1):1. doi: 10.1186/1479-0556-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez M, Joffraud M, Giraud S, Baisse B, Bernimoulin MP, Schapira M, Spertini O. Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin: role of human fucosyltransferase-IV and -VII. J Biol Chem. 2005;280(7):5378–90. doi: 10.1074/jbc.M410899200. [DOI] [PubMed] [Google Scholar]
- 20.Tu L, Murphy PG, Li X, Tedder TF. L-selectin ligands expressed by human leukocytes are HECA-452 antibody-defined carbohydrate epitopes preferentially displayed by P-selectin glycoprotein ligand-1. J Immunol. 1999;163(9):5070–8. [PubMed] [Google Scholar]
- 21.Zhou Q, Moore KL, Smith DF, Varki A, McEver RP, Cummings RD. The selectin GMP-140 binds to sialylated, fucosylated lactosaminoglycans on both myeloid and nonmyeloid cells. J Cell Biol. 1991;115(2):557–64. doi: 10.1083/jcb.115.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Z, Goldsmith HL, McIntosh FA, Shankaran H, Neelamegham S. Biomechanics of P-selectin PSGL-1 bonds: shear threshold and integrin-independent cell adhesion. Biophys J. 2006;90(6):2221–34. doi: 10.1529/biophysj.105.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrine C, Ju T, Cummings RD, Gerken TA. Systematic determination of the peptide acceptor preferences for the human UDP-Gal:glycoprotein-alpha-GalNAc beta 3 galactosyltransferase (T-synthase) Glycobiology. 2009;19(3):321–8. doi: 10.1093/glycob/cwn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaia J. Mass spectrometry and the emerging field of glycomics. Chem Biol. 2008;15(9):881–92. doi: 10.1016/j.chembiol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wuhrer M, Catalina MI, Deelder AM, Hokke CH. Glycoproteomics based on tandem mass spectrometry of glycopeptides. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;849(1-2):115–28. doi: 10.1016/j.jchromb.2006.09.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.