Abstract
Apicomplexan parasites generally invade their host cells by anchoring the parasite to the host membrane through a structure called the moving junction (MJ). This moving junction is also believed to sieve host proteins from the nascent parasitophorous vacuole membrane, which likely protects the pathogen from lysosomal destruction. Previously identified constituents of the Toxoplasma MJ have orthologues in Plasmodium, indicating a conserved structure throughout the Apicomplexa. We report here two novel MJ proteins, RON5 and RON8. While RON5 is conserved in Plasmodium, RON8 appears restricted to the coccidia. RON8, which is likely essential, coimmunoprecipitates RON5 and known MJ proteins from extracellular parasites, indicating a preformed complex exists within the parasites. Upon secretion, we show that RON8 within the MJ localizes to the cytoplasmic face of the host plasma membrane. To examine interactions between RON8 and the host cell, we expressed RON8 in mammalian cells and show that it targets to its site of action at the periphery in a manner dependent on the C-terminal portion of the protein. The discovery of RON5 and RON8 provides new insight into conserved and unique elements of the MJ, furthering our understanding of how the moving junction contributes to the intricate mechanism of Apicomplexan invasion.
Keywords: Toxoplasma gondii, rhoptry neck protein, moving junction, invasion, tachyzoites
Introduction
Apicomplexan parasites represent an important group of eukaryotic pathogens responsible for a variety of illnesses impacting nearly every form of vertebrate life (Hill and Dubey, 2002). This phylum of obligate intracellular parasites includes Plasmodium, the causative agent of malaria, responsible for 1–3 million deaths per year (Breman, 2001). Infection with another prominent Apicomplexan, Toxoplasma gondii, is a common occurrence around the world, with seroprevalence of up to 1/3 of the human population (Jackson and Hutchison, 1989). Given the ease with which Toxoplasma can be isolated, cultured, and genetically modified, considerable insight into the Apicomplexan lifestyle has been derived from this organism, especially concerning the conserved mechanism of host cell invasion (Weiss and Kim, 2004).
Most Apicomplexans actively invade their target cells using a modified form of gliding motility that utilizes a parasite actin-myosin motor to force the host membrane to invaginate and envelop the parasite within the resulting parasitophorous vacuole (PV), the sole environment in which the parasite can grow and replicate (Keeley and Soldati, 2004; Sibley, 2004). Initial attachment of the parasite to the host cell via GPI-anchored surface antigens (Grimwood and Smith, 1996; Mineo and Kasper, 1994) prefaces a coordinated series of secretion events from the Apicomplexa’s defining secretory organelles: the micronemes, rhoptries, and dense granules (Carruthers and Boothroyd, 2007; Carruthers and Sibley, 1997). While micronemal proteins are implicated in attachment to the host cell and contact with the actin-myosin motor, rhoptry secretion is concomitant with the appearance of a unique structure in pathogen entry, the moving junction (MJ). This tight contact, first witnessed in Plasmodium 30 years ago (Aikawa et al., 1978), is the interface between parasite and host membranes that is believed to provide a stable anchor against which productive forward motion can be generated, and in electron microscopy appears as a constricted region through which the parasite squeezes to enter the nascent PV (Michel et al., 1980). The stable contact represented by the MJ is required for successful invasion, although how this apparatus connects to the parasite’s actin-myosin motor and whether or not host components link to the MJ remains unknown.
In addition to providing an anchor for entry, the MJ is believed to function as a molecular sieve that selectively filters host transmembrane proteins from the nascent vacuole (Charron and Sibley, 2004; Mordue et al., 1999b). In general, type I transmembrane proteins and multimeric protein complexes are barred entry at the MJ, while GPI-anchored, acylated, and lipid raft associated proteins flow freely into the nascent parasitophorous vacuole membrane (PVM). Filtering of the type I transmembrane protein ICAM1 is dependent on the cytoplasmic tail of the protein, as removal of the tail or replacement of the transmembrane domain with a GPI anchor allows the protein to bypass filtration by the MJ. The cytoplasmic tails of type I transmembrane proteins are known to link these proteins to the host cell’s cortical cytoskeleton (Carpen et al., 1992), suggesting that filtration occurs on the cytoplasmic face of the host cell membrane at the cortical cytoskeleton. This selective removal of host proteins is likely critical for parasite survival, as it is believed to result in the non-fusogenic nature of the PV and the prevention of targeting to host lysosomes (Morisaki et al., 1995).
A major breakthrough in our knowledge of the protein constituents of the moving junction came about with the identification of a complex of rhoptry neck proteins (RONs) that coprecipitate with the microneme protein AMA1 and are associated with the Toxoplasma MJ (Alexander et al., 2005; Lebrun et al., 2005). Only RON4 and AMA1 have been localized to the MJ, but two other proteins identified in the rhoptry proteome, RON2 and an unidentified protein (incorrectly annotated as three gene models, 583.m00636, 583.m09192 and 583.m09191, which we refer to as 583.m00636 for ease of reference), co-precipitate as members of the complex. Precisely how these proteins enable entry into the host cell and/or filter membrane proteins from the vacuole remains an enigma, but considerable interest has surrounded these proteins in part because they all have significant homology to proteins in Plasmodium (Alexander et al., 2005; Lebrun et al., 2005) and thus support conservation of invasion machinery across the phylum.
In this work, we expand our understanding of the moving junction complex in Apicomplexan parasites. We show that 583.m00636 is a MJ/RON protein that we call RON5, which is processed into two major fragments upon arrival in the rhoptries. We further identify RON8, a novel MJ/RON protein in Neospora caninum and Toxoplasma gondii that has significant homology to a predicted protein in Eimeria tenella, but surprisingly does not appear to be present in Apicomplexans outside the coccidia (e.g. Plasmodium spp., Theileria spp., or Cryptosporidia spp.). RON8 forms a macromolecular protein complex with known components of the MJ and is likely essential as it is refractory to genetic deletion. Intriguingly, RON8 is not exposed to the extracellular milieu during invasion, but is secreted to the inner face of the host cytoplasmic membrane, indicating this component of the moving junction directly contacts the host cell cytoplasm. To investigate potential RON8 interactions with its host cell in the absence of other moving junction components, we expressed RON8 in mammalian cells and demonstrate that it traffics to the plasma membrane or cortical cytoskeleton at the cell periphery, consistent with its site of function as assessed by our topology experiments. By deletion analysis, we further demonstrate that the C-terminal region of RON8 is both necessary and sufficient for this distinctive localization. This localization suggests that RON8 may function in the sieving activity of the MJ or in anchoring the invading parasite to host proteins linked at the plasma membrane/cortical cytoskeleton. The discovery of RON5 and RON8 illustrates alternatives for moving junction formation between members of the coccidia and other Apicomplexa and may help to explain differences in host range or host cell specificity between Apicomplexan parasites.
Results
RON5, a conserved rhoptry neck protein, is processed and secreted to the moving junction
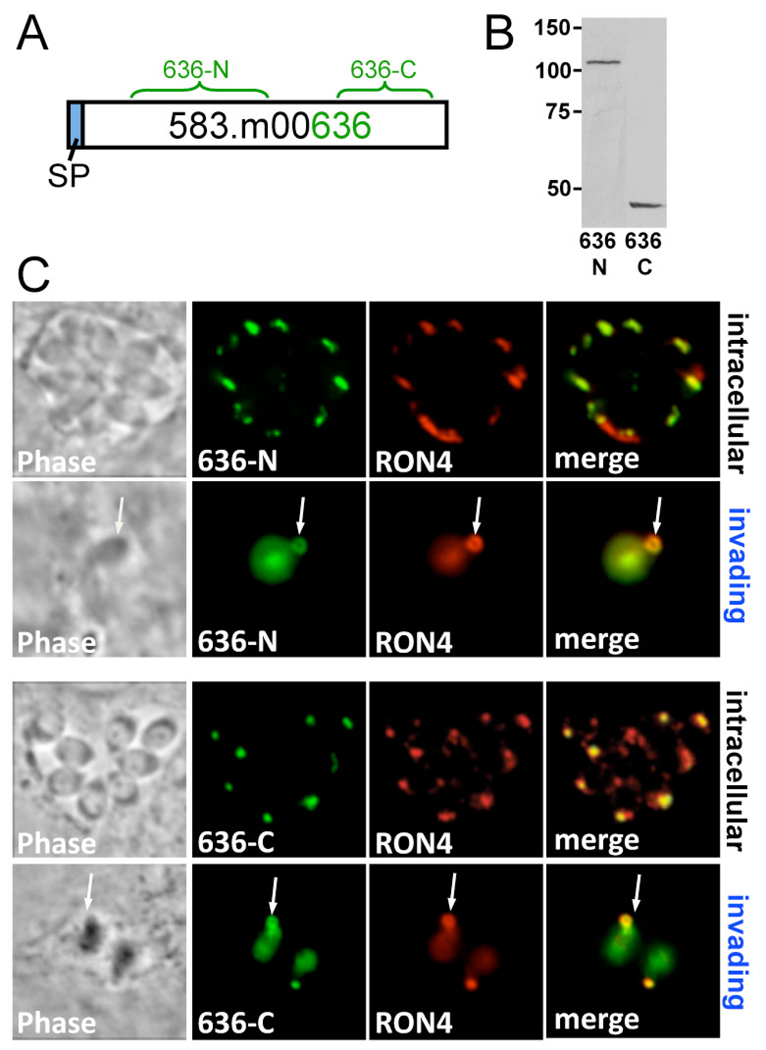
Our efforts to understand the Toxoplasma moving junction have focused on identifying rhoptry neck proteins that are released into this structure during invasion. RON2 and RON4 are known rhoptry neck proteins that co-precipitate with AMA1 as members of the moving junction complex. An additional protein, gene model 583.m00636 (Fig. 1A), has been suggested to be a MJ/RON protein because it is present in the rhoptry proteome and immunoprecipitates with the moving junction complex as 110 and 45 kDa fragments that are revealed by mass spectrometry (Alexander et al., 2005; Bradley et al., 2005). To determine if this protein is indeed a MJ/RON protein, we raised antibodies against the N-terminal and C-terminal portions of the protein and demonstrate that the 110 kDa and 45 kDa forms are present in lysates of extracellular parasites (Fig. 1A and 1B). We then used the antibodies in immunofluorescence assays and were able to show that both fragments are present in the rhoptry necks and are released into the moving junction in invading parasites (Fig. 1C). These results demonstrate that 583.m00636 is a processed MJ/RON protein that we have named RON5, and its cleavage products RON5N and RON5C, respectively.
Fig. 1. Toxoplasma 583.m00636 is RON5, which is processed into N and C-terminal fragments that are secreted into the moving junction.
A. Schematic of 583.m00636. The predicted signal peptide is shown in blue, and green brackets highlight residues 503–983 used to generate N-terminal antisera, and residues 1333–1675 to generate C-terminal antisera. B. Extracellular Toxoplasma lysate probed with 583.m00636 N and C-terminal antisera reveals two separate fragments migrating at ~110 and ~45 kDa. C. 583.m00636 N and C-terminal antisera used in immunofluorescence of intracellular Toxoplasma tachyzoites (1st, 3rd panels) or partially invaded Toxoplasma (2nd, 4th panels) show colocalization with Toxoplasma RON4 in both the rhoptry neck and the moving junction (arrows).
Monoclonal antibody 8E3 recognizes a novel member of the moving junction complex in Neospora caninum
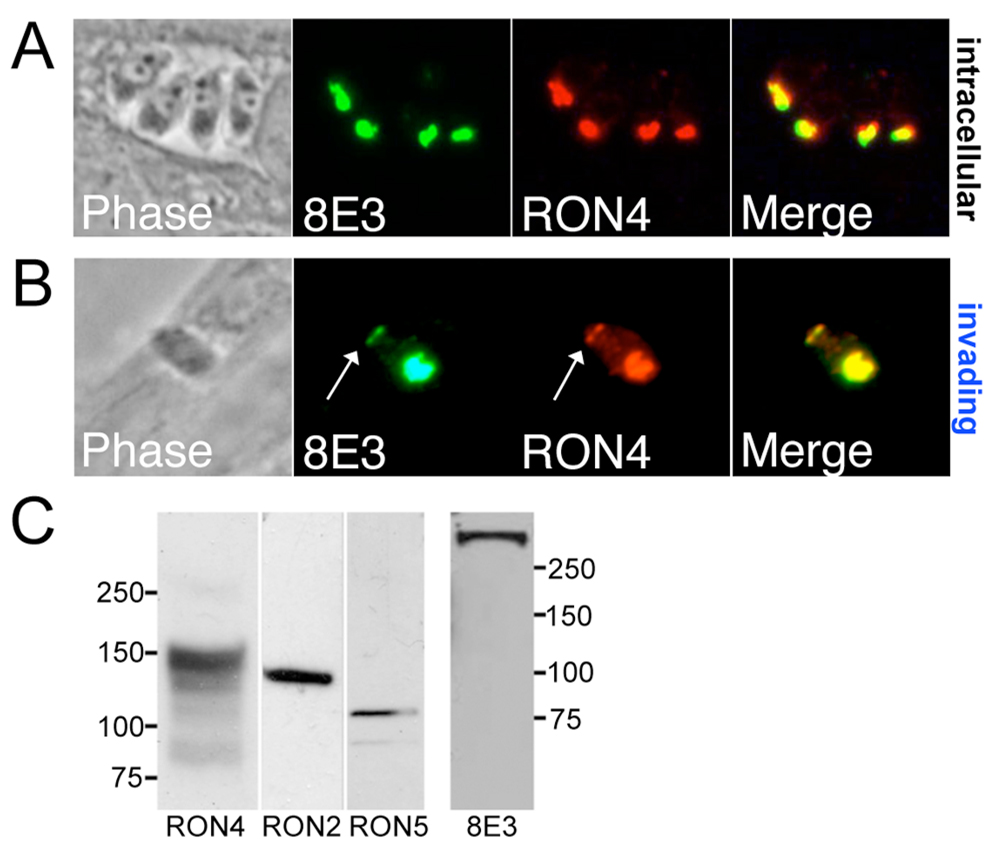
To identify novel components of the invasion machinery in Apicomplexans, we raised monoclonal antibodies against a purified fraction of organelles from a parasite closely related to Toxoplasma, Neospora caninum. One monoclonal antibody, 8E3, detects a Neospora protein in the rhoptry necks of intracellular Neospora tachyzoites as assessed by colocalization with cross-reactive Toxoplasma anti-RON4 polyclonal antisera (Fig. 2A). The protein detected by 8E3 is also secreted into the moving junction of invading parasites (Fig. 2B, arrow). Western blot analysis of Neospora lysate using 8E3 detected a protein >250 kDa, considerably larger than any previously identified MJ component as seen using cross-reactive Toxoplasma antibodies against RON2, RON4, and RON5 homologs in Neospora (Fig. 2C). While 8E3 did not cross-react with Toxoplasma, this data demonstrates 8E3 recognizes a novel player in the Neospora moving junction.
Fig. 2. Monoclonal antibody 8E3 detects a novel Neospora moving junction RON protein.
A. IFA of intracellular Neospora parasites shows colocalization of 8E3 with Neospora RON4 (using Toxoplasma cross-reactive polyclonal RON4 antisera). B. 8E3 antibody staining colocalizes with RON4 in partially invaded parasites in both the rhoptry neck and the Neospora MJ (arrows). C. Western blot analysis of Neospora lysate reveals the protein detected by 8E3 is >250 kDa, considerably larger than the MJ/RONs 2, 4, and 5.
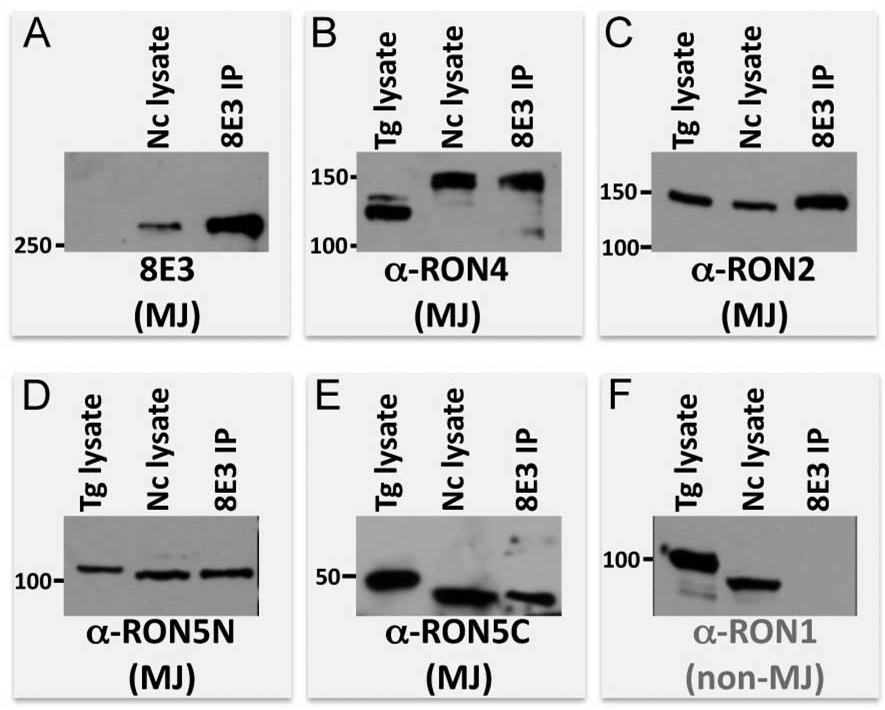
Since RONs 2, 4, and 5 collaborate in the moving junction, we investigated whether the protein recognized by 8E3 associates with these known components of the MJ complex. We immunoprecipitated the 8E3 target protein from extracellular Neospora parasites and probed for Neospora RON2, RON4, RON5N, and RON5C again using cross-reactive antisera (Fig. 3A–E). While all of these MJ/RONs coprecipitated along with the 8E3 protein, the non-MJ rhoptry neck protein RON1 was not coprecipitated (Fig. 3F), establishing the specificity of the novel component’s association with the other members of the complex. While we do not have an antibody probe for Neospora AMA1, an ~70 kDa protein was observed on Coomassie-stained gels of the complex immunoprecipitated by 8E3 that likely corresponds to Neospora AMA1 (data not shown). Together, this data demonstrates that the protein detected by 8E3 is a member of the MJ complex. As the complex was purified from extracellular parasites, this further indicates that the MJ complex is likely preformed within the rhoptries. We also observed subtle differences in migration between Toxoplasma and Neospora components, particularly RON4 (which migrates substantially slower in Neospora). This may be due to simply a difference in migration, or could result from variations in protein processing as is known to occur in many rhoptry proteins (Bradley and Sibley, 2007; Bradley et al., 2002; Sadak et al., 1988).
Fig. 3. The MJ protein immunoprecipitated by 8E3 specifically associates with known moving junction components.
Western blots of 8E3 immunoprecipitate compared to Toxoplasma and Neospora lysate were stained with A. 8E3 monoclonal antibody, B–E. cross-reactive polyclonal antisera against moving junction components RON2, RON4, RON5N, RON5C or F. the non-MJ protein RON1.
8E3 recognizes RON8, a novel MJ/RON protein restricted to the coccidia
To identify the protein detected by 8E3, the antibody was cross-linked to Protein G-Sepharose and used to purify its target protein by immunoaffinity chromatography from Neospora lysates. The eluted material was separated by SDS-PAGE, and the dominant band at >250 kDa was identified by tandem mass spectrometry. Significant peptide hits were then compared with the translated first draft of the Neospora genome (Table S1). Ten tryptic peptides were identified that corresponded to a region of the Neospora genome with highly significant homology to a predicted ~330 kDa protein in Toxoplasma gondii, 541.m00141 (Table S1). 541.m00141 had been previously identified in the rhoptry proteome (Bradley et al., 2005); however, anti-peptide antisera raised against 541.m00141 detected the apicoplast. This localization was noted to be suspect, since no other apicoplast proteins were identified in the rhoptry proteomic analysis (Bradley et al., 2005). Thus, we re-examined this predicted protein’s localization in T. gondii.
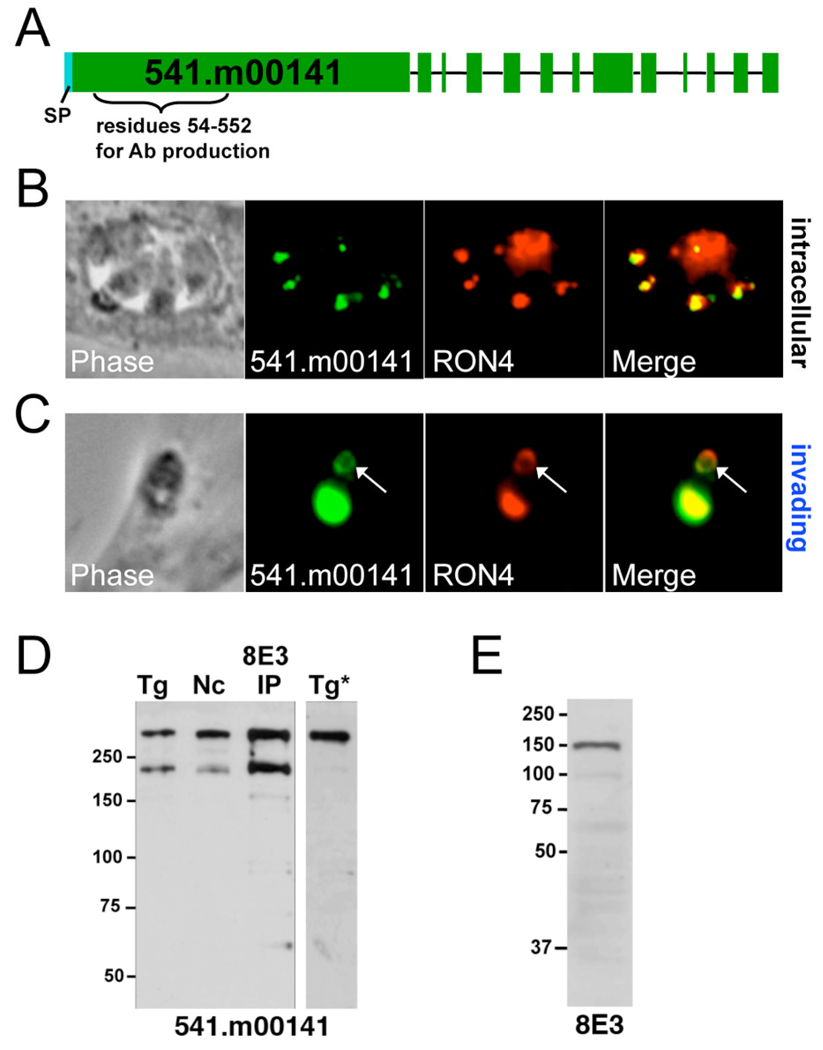
To resolve 541.m00141’s localization, we generated polyclonal antisera against a recombinant hexahistidine-tagged portion of the protein (corresponding to residues 54–552, Fig. 4A) and used the antisera in immunofluorescence assays of intracellular and partially invaded T. gondii. As seen in Fig. 4B and 4C, anti-541.m00141 stains both the rhoptry necks and the moving junction of invading parasites as assessed by RON4 colocalization. The antisera detects a high molecular weight band at the predicted size for 541.m00141 in Western blot analysis of both Toxoplasma and Neospora lysates and also recognizes the immunoprecipitated 8E3 protein (Fig. 4D). An additional smaller band is also detected in lysates that were incubated on ice during the 8E3 immunoprecipitation and in the immunoprecipitated protein, but this is presumably a degradation product, as it is not seen in freshly prepared lysates (Tg*, Fig. 4D). Finally, we ensured that the 8E3 antibody detects the Neospora ortholog of 541.m00141 by showing that it reacts against a recombinant portion of the protein expressed in E. coli (Fig. 4E). Together, these data unequivocally demonstrate 541.m00141 is the Toxoplasma ortholog of the Neospora 8E3 protein, which we now call rhoptry neck protein 8 (RON8).
Fig. 4. Toxoplasma 541.m00141 is RON8, the ortholog of the MJ protein detected by Neospora 8E3.
A. Gene model of 541.m00141 predicted by ToxoDB 4.2. Exons are highlighted in green, and the predicted signal peptide (a.a. 1–26) shown in blue. Amino acids 54–522 were used for generation of mouse polyclonal antisera. B, C. Immunofluorescence on intracellular (B.) or invading (C.) Toxoplasma tachyzoites utilizing antisera against 541.m00141 and α-RON4 show colocalization in both the rhoptry necks and the MJ (arrows). D. Toxoplasma lysate, Neospora lysate, and 8E3 immunoprecipitate were stained with α-541.m00141, which detects a >250 kDa band by Western blot. An ~200 kDa band also appears as a result of degradation that is not detected in Western analysis of fresh Toxoplasma lysates (Tg*). E. Lysate of E. coli BL21 cells expressing recombinant Neospora RON8 residues 1–1921 was probed with 8E3 monoclonal antibody, producing a ~200 kDa band revealed by Western blot.
BLAST analysis of Toxoplasma and Neospora RON8 reveals an orthologous protein in Eimeria tenella, but not in the more distantly related Apicomplexans Babesia bovis, Theileria annulata, Cryptosporidium parvum or Plasmodium spp. or in any species outside of the Apicomplexa. PFAM and PROSITE searches of the RON8 sequence failed to identify any conserved motifs or domains. Aside from its predicted signal peptide and an unusually long first exon (responsible for encoding ~2/3 of the protein), RON8 lacks any other distinctive features that might provide clues to its function, further highlighting the uniqueness of this protein. To directly address RON8’s function, we attempted to disrupt residues 1–1716 with a knockout cassette containing 5’ and 3’ flanking regions (~3.3 kb each) surrounding HXGPRT, which would be expected to disable RON8 expression in the haploid organism. In spite of screening knockout populations and clones from multiple independent transfections by immunofluorescence and PCR, no successful knockout was ever obtained. Non-essential genes [e.g. PP2C-hn, (Gilbert et al., 2007)] were readily disrupted in parallel as controls, suggesting RON8 is an essential gene.
RON8 is secreted to the cytoplasmic face of the host membrane during invasion
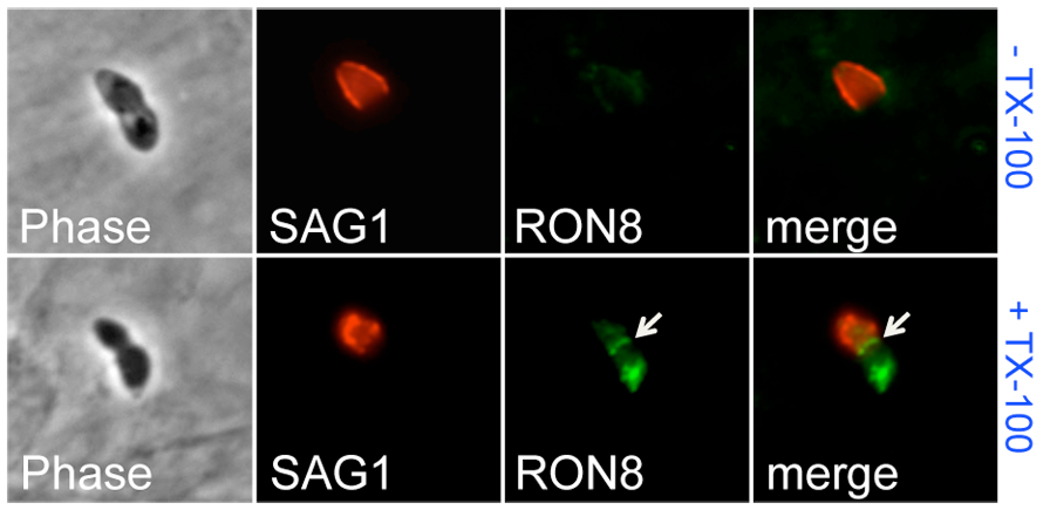
Selective filtration of host proteins attached to the cortical cytoskeleton by the moving junction suggests that this activity takes place on the inner face of the host membrane (Charron and Sibley, 2004; Mordue et al., 1999b). Soluble components of the junction (RON4, RON5, RON8) could be delivered to the host cell cytoplasm during the burst of rhoptry secretion that occurs concomitant with a transient breach in the host cell membrane (Boothroyd and Dubremetz, 2008; Suss-Toby et al., 1996). To determine RON8 topology during invasion, we conducted immunofluorescence assays on partially-invaded Toxoplasma parasites under permeabilizing or non-permeabilizing conditions (Fig. 5). To define the portion of the parasite outside of the host cell, we first stained with antibodies against the surface antigen SAG1 without permeabilizing the cells. We then used our RON8 antibody to detect the moving junction in the presence or absence of permeabilization. RON8 is clearly detected within the moving junction of parasites invading host cells upon permeabilization (+ TX-100, Fig. 5), but is not detected when detergent is omitted (− TX-100, Fig. 5). This data indicates that RON8 injected into the MJ is delivered to the inner face of the host plasma membrane.
Fig. 5. RON8 is secreted to the cytoplasmic face of the host cell membrane within the moving junction.
Partially-invaded Toxoplasma tachyzoites were stained with anti-SAG1 without permeabilization, then washed and incubated with anti-RON8 under either non-permeabilizing (− TX-100) or permeabilizing (+ TX-100) conditions. RON8 within the moving junction (arrow) and the rhoptry necks of the invading parasite is only detected under permeabilizing conditions. Equivalent exposure times were used for fluorescent imaging of the signal from RON8 in both permeabilizing and non-permeabilizing conditions.
RON8 expressed in mammalian cells traffics to the cell periphery
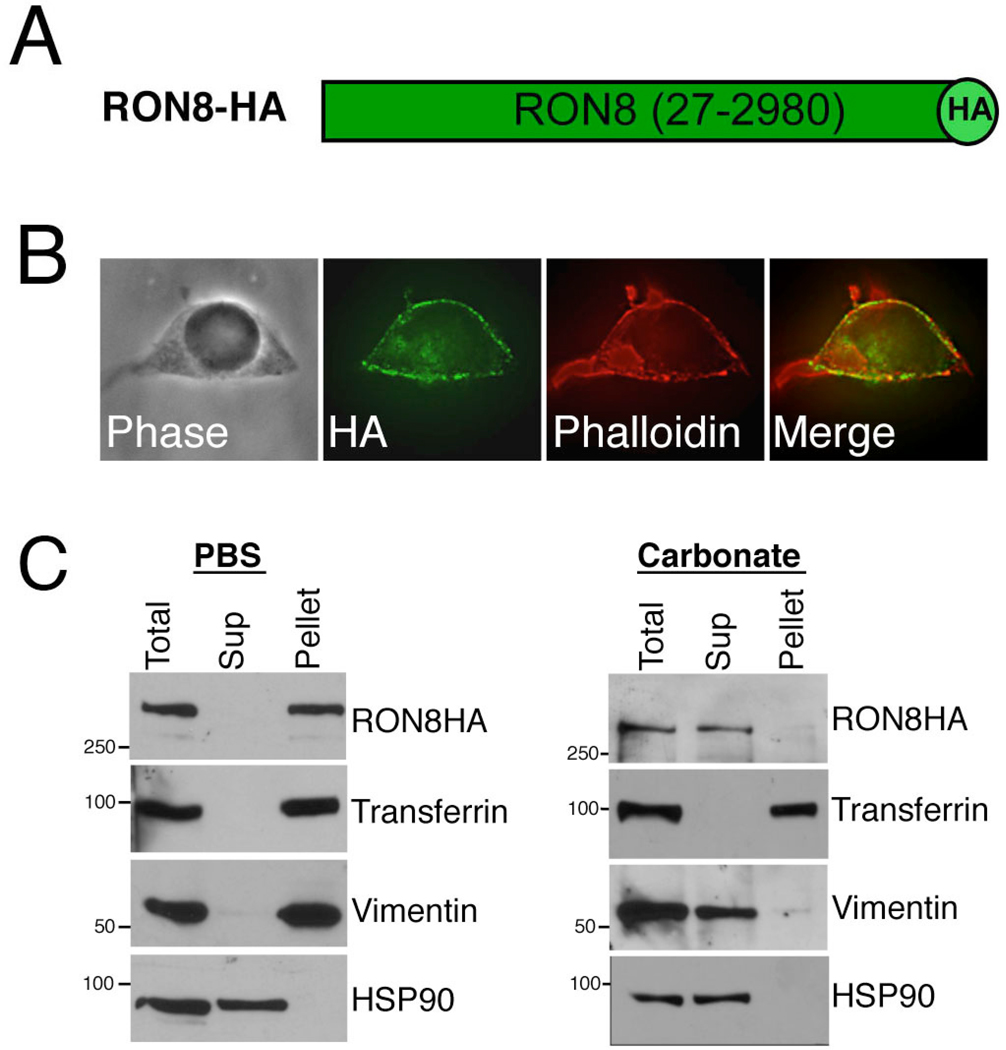
Given RON8’s topology during invasion, we investigated the possibility that it traffics to its intracellular location within the host cell in the absence of other RONs by direct expression in mammalian cells. We therefore engineered the RON8 coding sequence lacking its predicted signal peptide with a C-terminal HA tag and subcloned it into the mammalian expression vector pMSCV-puromycin for transfection into HEK293 cells (Fig. 6A). Intriguingly, RON8-HA protein expressed in the resulting cell line was largely trafficked to the periphery of the host cell, corresponding to the plasma membrane or cortical cytoskeleton (Fig. 6B). Given that selective filtration of host cell proteins by the MJ excludes proteins linked to the cortical cytoskeleton (Mordue et al., 1999b), we addressed whether RON8-HA at the periphery localized with this structure by phalloidin staining. A near-perfect overlap of signal was observed, demonstrating that RON8 traffics to the sub-membrane area of the mammalian cell where it could interact with constituents of the plasma membrane or cortical cytoskeleton. We further investigated the trafficking of RON8-HA by subcellular fractionation. In a gentle fractionation of sonicated cell lysates in PBS, RON8 partitioned to the membrane/cytoskeleton pellet as assessed by probing for the plasma membrane marker transferrin and the cytoskeletal marker vimentin, whereas the cytosolic protein HSP90 was completely released into the supernatant (Fig. 6C). RON8 is not firmly attached to the membrane, as it is completely solubilized along with vimentin by sodium carbonate extraction (Fig. 6C). These results demonstrate that RON8 traffics to and loosely associates with components of the periphery of the cell corresponding to the membrane or cortical cytoskeleton. Taken together, RON8 topology and exogenous expression implicate the cytoplasmic face of the host plasma membrane as RON8’s site of action during Toxoplasma invasion.
Fig. 6. Toxoplasma RON8 traffics to the periphery upon expression in mammalian cells.
A. Diagram of mammalian expression construct encoding RON8 with a C-terminal HA tag (bright green). Note that no signal peptide is present. B. HEK293 cells transfected with pMSCV-puro-RON8-HA were stained with α-HA (which detects the C-terminal HA tag encoded by the construct) and a phalloidin costain to label the cortical cytoskeleton by immunofluorescence assay. RON8 expression roughly follows the perimeter of the cell corresponding to the cell periphery and colocalizes with the cortical cytoskeleton. C. Subcellular fractionation of RON8-HA cells. Cells were sonicated in PBS or sodium carbonate and fractionated into soluble supernatant (Sup) and membrane pellet (Pellet), then stained with antibodies against HA, transferrin, vimentin, or HSP90.
The RON8 C-terminus is necessary and sufficient for targeting
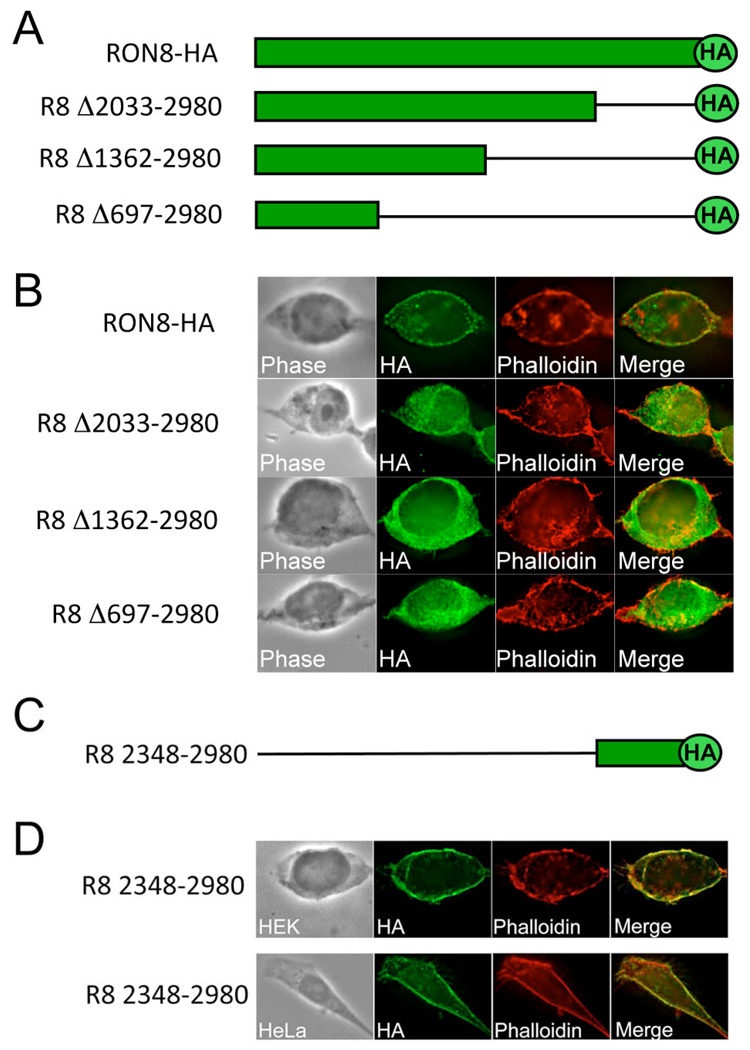
The sequence of RON8 lacks identifiable regions such as transmembrane domains or cytoskeletal binding domains that could account for its targeting within the host cell. Therefore, we designed deletions from the RON8 C-terminus, each of which retained the C-terminal HA tag, to determine regions of RON8 that are necessary for intracellular targeting (Fig. 7A). The deletion constructs and RON8-HA control were then separately transfected into HEK293 cells for transient expression and localization was assessed by IFA (Fig. 7B). IFA analysis of the transfected cells showed that each of the deletions resulted in cytoplasmic localization (Fig. 7B), with the truncated proteins failing to either traffic to the membrane or colocalize with phalloidin. These experiments establish that the C-terminal portion of RON8 is necessary for its localization to the periphery.
Fig. 7. The RON8 C-terminus is necessary and sufficient for trafficking and cytoskeletal colocalization.
A. Schematic of RON8 deletion series involving successive removal of 947, 1618, and 2283 residues from the C-terminus of RON8-HA, while remaining in frame with the HA epitope tag. B. HEK293 cells transiently transfected with each deletion construct were stained with α-HA and phalloidin; all three constructs lack membrane/cytoskeleton targeting seen with full length RON8-HA expressing HEK293 cells. C. Diagram depicting residues 2348–2980 of RON8 fused to an HA tag, used for assessing targeting sufficiency. D. Transfection of this C-terminal region into either HEK293 or HeLa cells produces colocalization of RON8 (2348–2980) with the cortical cytoskeleton by IFA with α-HA sera and phalloidin costain.
To determine whether the C-terminal region of RON8 is sufficient for this localization, we expressed residues 2348–2980 of RON8 as a HA-tag fusion and again assessed localization by IFA (Fig. 7C and 7D). Expression of this C-terminal domain resulted in targeting to the membrane/cytoskeleton and costained with phalloidin in a pattern similar to that seen for full-length RON8 (Fig. 7D). A similar localization was also seen upon expression in HeLa cells, indicating that the targeting is not cell-type specific. This demonstrates residues 2348–2980 of RON8 contain the membrane/cytoskeletal targeting information and therefore encompasses the first functional domain identified in any rhoptry neck protein.
Discussion
We have identified RON5 and RON8, two novel rhoptry neck proteins that contribute to the moving junction, a crucial structure for entry of most Apicomplexans into their host cells. These new components reinforce the predominance of proteins from the rhoptry necks in assembling this remarkable structure. RON5 homology with Plasmodium supports the notion that the rhoptry neck proteins and the moving junction are generally conserved in the Apicomplexa; RON5 and previously known RONs all have orthologs in Plasmodium, in contrast to only four of 24 rhoptry bulb proteins (Boothroyd and Dubremetz, 2008). Using antisera against both N-terminal and C-terminal portions of the protein, we show that RON5 is processed into its N and C terminal fragments either en route to or within the rhoptries, and that both pieces are secreted into the MJ. The RON5 sequence contains two perfect ROP1-like processing sites (SFVE^, at residues 311–314 and 1259–1262) that could generate fragments approximately the size observed here and which agree with mass spectrometry of the immunoprecipitated proteins and those identified in the rhoptry proteome (Alexander et al., 2005; Bradley et al., 2005), although processing could also occur at nearby sites. A third smaller RON5 fragment (consisting of the region downstream of the predicted signal peptide to the N-terminal fragment shown here) could also be present, or may be a propeptide that is degraded as is common for several other rhoptry proteins (Bradley and Sibley, 2007; Bradley and Boothroyd, 1999). Whether the N-terminal and C-terminal fragments of RON5 directly associate with each other in the moving junction complex has yet to be determined.
The discovery of RON8, the first moving junction component without homology to Plasmodium proteins, adds a compelling twist to Apicomplexan host cell invasion that may suggest specialized evolution amongst coccidian members of the phylum in building the junction complex. It is conceivable that we did not find a Plasmodium RON8 homolog detectable by BLAST analysis due to extensive sequence drift. Indeed, Toxoplasma RON4 and RON5 appear to have significantly diverged from their Eimeria and Plasmodium counterparts. However, Toxoplasma RON2 and RON8 both have high homology to their Eimeria orthologs (e-values ~ 10−170), yet only a RON2 Plasmodium ortholog can be found (e-value ~ 10−63). It seems more likely, therefore, that Plasmodium has evolved other proteins to carry out the same function of RON8 or that its function is not required for Plasmodium invasion.
RON8’s exclusivity within the coccidia does not appear to make it dispensable, as we could not ablate even a portion of RON8 using relatively long flanking regions of homologous sequence and extensive screens of populations and clones from multiple KO attempts by immunofluorescence and PCR. This suggests RON8 is essential for parasite survival, similar to that seen for junction components AMA1 and RON4 (Alexander et al., 2005; Mital et al., 2005). Conditional expression should circumvent this obstacle, as it has done for AMA1 and other essential invasion-related genes (Kessler et al., 2008; Huynh and Carruthers, 2006; Mital et al., 2005; Meissner et al., 2001). Our initial attempts at conditional knockouts of rhoptry proteins have been complicated by improper targeting from the SAG1 or SAG4 promoters and problems with regulation from rhoptry promoter derived constructs (data not shown). One can envision two potential outcomes for a parasite with RON8 conditionally repressed. In the first scenario, parasites would fail to invade, indicating a role for RON8 in serving as part of the anchor for parasite entry. In the second, parasites would invade, but the failure to sieve out host membrane proteins would result in targeting of the PV to the host lysosomes and parasite destruction.
By coimmunoprecipitation of RON8 from extracellular parasites, we show that RON8 specifically associates with the other moving junction components, in what appears to be a preformed complex established prior to invasion (Fig. 3). The necessity of micronemal AMA1 for RON4 secretion into a ring-shaped junction (Alexander et al., 2005) indicates that AMA1 likely makes contact with the RON complex shortly after secretion of the RON complex at the initial stages of invasion. It has been alternatively speculated that micronemes discharge their contents through the rhoptry necks during invasion (Bannister et al., 2003), but no studies have ever confirmed the presence of AMA1 in parasites anywhere besides the micronemes. It is also conceivable that the RON complex seen in Fig. 3 only forms upon contacting the AMA1 freed in the extracellular lysate, but a preassembly of RON proteins en route to the rhoptries or within the rhoptries seems more likely. The recent finding that RON4 does not even secrete to the host/parasite interface in MIC8-depleted parasites (Kessler et al., 2008) indicates interaction between the micronemes and rhoptry necks occurs in rapid consecutive steps at the beginnings of invasion.
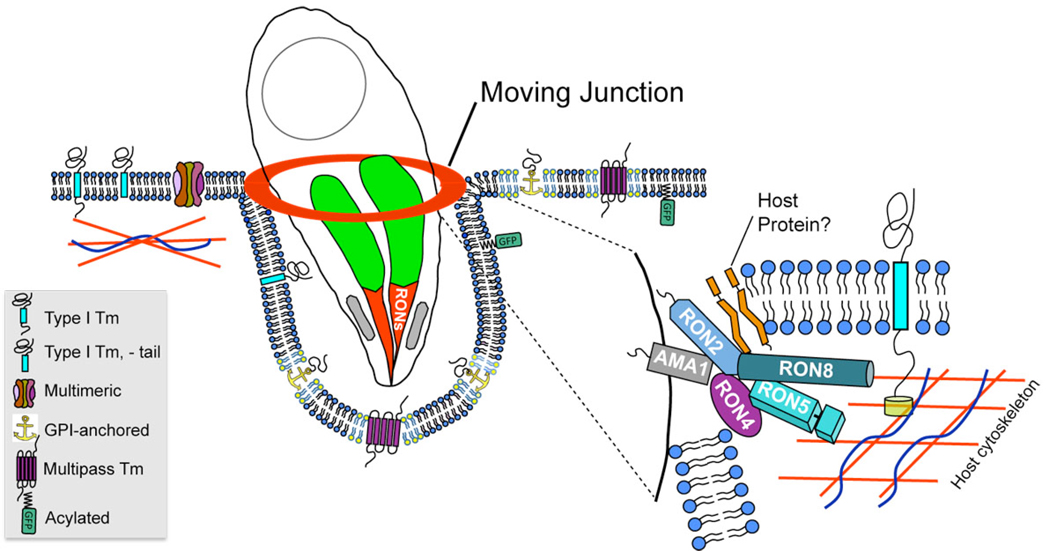
By assessing partially-invaded parasites in the presence or absence of permeabilization, we have addressed the topology of Toxoplasma RON8 secreted during invasion and determined that RON8 is largely submerged beneath the host cytoplasmic membrane where it can contact the host cytoplasm (Fig. 5). Previously, RON4 has been detected at the exterior surface of the MJ in non-permeabilized cells (Alexander et al., 2005). In our hands, however, both RON4 and RON8 in the moving junction are only seen under non-permeabilizing conditions when the integrity of the host plasma membrane at the point of invasion is compromised. Such events of localized permeabilization at the MJ may occur during sample processing of partially invaded parasites and are revealed by costaining with an antibody against host vimentin (data not shown). These experiments also argue against the need for detergent to detect RON8 secreted into the moving junction, as RON8 can be detected in cells whose plasma membrane is compromised without detergent. We have been unable to address RON5’s topology during invasion, as all of our anti-RON5 antibodies only detect their targets under methanol fixation, conditions that permeabilize the cell. As RON5 is a soluble junction component, however, we expect that it will be buried underneath the host cytoplasmic membrane, as are RON8 and RON4. Positioning of soluble MJ/RON proteins on the inner face of the cytoplasmic membrane could occur during a spike in conductivity observed across the host cell membrane immediately preceding the onset of invasion (Suss-Toby et al., 1996). This spike is consistent with a localized breach in the host plasma membrane and has also been proposed as the means by which rhoptry bulb effector proteins enter the host cytosol (Boothroyd and Dubremetz, 2008; Bradley and Sibley, 2007). Based on previous results and the data presented here, we therefore propose a model with RON2 attaching the moving junction to the host cell membrane via its predicted transmembrane domains while AMA1 anchors the junction complex to the parasite membrane (Fig. 8). Soluble MJ partners (RON8, RON5, RON4) could be tethered by RON2 to the inner face of the host membrane and either accomplish further linkage to the host or engage in molecular sieving.
Fig. 8. Model of the moving junction during Toxoplasma invasion.
The model shows the moving junction as the point of contact between the parasite and host membranes during invasion. The sieving of transmembrane proteins from the nascent vacuole is shown in which type I transmembrane proteins and multimeric protein complexes are filtered from the forming vacuole. Filtering of type I transmembrane proteins is dependent on cytoplasmic tails, which are anchored in the cortical cytoskeleton. In contrast, GPI-anchored, raft-associated (rafts shown in yellow), multipass transmembrane, and acylated proteins are not filtered and enter the vacuole. The inset depicts a speculative arrangement of moving junction components; AMA1 and RON2 are shown serving as anchors for the MJ in the parasite and host membranes, respectively. Soluble RONs (RON4, RON5, RON8) are tethered to the cytoplasmic face of the plasma membrane by RON2 where they are positioned to perform further anchoring to the host cytoskeleton or sieving of host transmembrane proteins.
Our experiments with exogenously expressed RON8 show that this protein traffics to the periphery of mammalian cells (Fig. 6B), in agreement with our findings on the topology of RON8 within the moving junction. This makes RON8 a tantalizing candidate for fulfilling key tasks carried out by the moving junction, as per our model. If RON8 anchors the moving junction to an ubiquitous host partner (e.g. a cytoskeletal component), such an interaction could contribute to the incredibly broad host range displayed by Toxoplasma (Boothroyd and Dubremetz, 2008). Alternatively, RON8 binding to the cytoskeleton or membrane would make it ideally positioned to prevent the entry of transmembrane proteins with cytoskeletal links (such as ICAM-1) into the nascent PV, supporting the mechanism of host protein sieving performed by the moving junction (Charron and Sibley, 2004; Sibley, 2004; Mordue et al., 1999b). Selective filtration by the junction is likely the means by which the PVM remains devoid of endocytic markers that would target the parasite to lysosomal destruction (Mordue et al., 1999a; Mordue and Sibley, 1997), making RON8 or any other MJ sieving partner crucial for the parasite’s survival.
Fractionation of RON8-expressing HEK293 cells under mild conditions (PBS) support RON8 association with the membrane/cytoskeleton, but harsher treatment via sodium carbonate extraction releases RON8 into the soluble fraction (Fig. 6D). Rather than an integral attachment of RON8 to the membrane, a loose association of RON8 with either the cortical cytoskeleton, a host transmembrane protein, or host lipids would produce the results in the fractionation data seen here. Our initial attempts at immunoprecipitation of either RON8-HA or RON8 (2348–2980) from stably transfected HEK293 cells have yet to recover associated partners, but this could be due to the stringency of the conditions tested. Despite consistent membrane targeting across multiple cell lines expressing RON8 (2348–2980), we cannot dismiss the possibility that this localization is a consequence of expression in the absence of other moving junction partners. The fact that RON8 topology during invasion positions the protein at the same site of action suggests that it is binding its target within the host cell, but whether this link proves physiologically relevant awaits identification of its binding partner. Regardless, the C-terminal region described here represents the first activity ascribed to any portion of a moving junction protein.
In summary, this work describes the discovery of new moving junction components that support a conserved assemblage of rhoptry neck proteins in the junction and highlight a distinctive element for coccidial members of the Apicomplexa. The function of individual RONs within the moving junction and the presence of additional parasite or host proteins remains unknown. The RON complex in conjunction with AMA1 may be sufficient for host cell entry by contacting ubiquitous host proteins, which would help to explain Toxoplasma’s fascinating ability to invade nearly any nucleated cell type. This has striking parallels with the Tir proteins in enteropathogenic E. coli, which are inserted into the host cell membrane and act as the pathogen’s own receptor for tight adhesion to mammalian host cells (Kenny et al., 1997). RON8 mammalian expression is an attractive system to dissect host interactions and further functional domains within moving junction proteins, and thereby address the enigma of the moving junction’s architecture. Resolving this architecture promises incredible insight into the molecular means by which this apparatus enables pathogen entry, a process with fundamental implications for the propagation of disease.
Experimental Procedures
Parasite and host cell culture
Toxoplasma [RHΔhpt strain, (Donald et al., 1996)] and Neospora tachyzoites (NCI strain) were passaged on confluent monolayers of human foreskin fibroblasts (HFFs) grown in Dulbecco’s modified eagle medium supplemented with 10% fetal bovine serum and 2 mM glutamine (Bradley et al., 2005). Human Embryonic Kidney-293 (HEK293) cells and HeLa cells were also cultured and maintained in this growth medium, unless otherwise specified.
Antibodies and Western blot analysis
The 8E3 monoclonal antibody (mAb) was generated by immunization with a mixed organellar fraction that was purified from Neospora tachyzoites using a protocol for Toxoplasma organelles as described (DeRocher et al., 2008). Purified organelles (~300 µg) were injected into a BALB/c mouse for production of a panel of monoclonal antibodies and the hybridoma fusion was carried out as previously described (DeRocher et al., 2008) (Sohn et al, in preparation). For immunofluorescence and Western blot analysis, additional antibodies include: rabbit polyclonal anti-RON4 (1:7000) (Alexander et al., 2005), rabbit polyclonal anti-SAG1 (1:100000) (Rome et al., 2008), mouse polyclonal anti-RON2 (1:800) (Bradley et al., 2005), mouse polyclonal anti-RON5N and RON5C (both 1:600, see below), mouse polyclonal anti-RON8 (1:400, see below), mouse polyclonal anti-RON1 (1:5000) (Bradley et al., 2005), rabbit polyclonal anti-HA (1:300) (Invitrogen), mouse monoclonal anti-HA (1:500) (Covance), mouse anti-transferrin (1:500) (Invitrogen), mouse anti-heat shock protein-90 (1:1200) (BD Biosciences), and mouse anti-vimentin (1:10000) (Sigma). For colocalization to the cortical cytoskeleton, Texas-Red conjugated phalloidin (Invitrogen) was used at a dilution of 1:200. Western blot analysis was carried out on proteins separated on 8% or 12% SDS-PAGE gels as previously described (Bradley et al., 2002). Secondary antibodies were horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit used at a dilution of 1:2000 (Sigma) and detected using the ECL Western Blot Detection Kit (Amersham Biosciences).
Expression of recombinant RON5 and RON8 for antibody production
For expression of recombinant N-terminal and C-terminal regions of RON5 (Fig. 1A), sequences encoding residues 503–983 (636N, which is shown to be RON5N) and 1333–1675 (636C, shown to be RON5C) were PCR amplified from T. gondii cDNA using the primers CACCCCTCAGATGGTAGCCAACGCG/GAACGGCTTCAGCTCCTGGTG for RON5N and GCGAGGATCCGGCCCAGACGTCGATGCTAGT/GCGCAAGCTTTCTAACTTCAACGCTATCGCC for RON5C. The resulting PCR products were cloned into pET161-gW-D-TOPO (RON5N) or pET28a (RON5C). For Toxoplasma RON8, the region of 541.m00141 corresponding to amino acids 54–552 was PCR amplified from RH genomic DNA using primers CACCCCGACACTCGTTCTGACGCTG and GTCATCAAAATGGAGGGTTACG prior to subcloning into pET161-gW-D-TOPO (Invitrogen). The junctions of the expression constructs were sequenced to ensure the hexahistidine purification tags were in frame, and then the constructs were transformed into E. coli BL21DE3 cells for protein expression. Transformed bacteria were grown to A600 of 0.6–0.8 and induced using 1 mM isopropyl 1-thio-β-D-galactopyranoside (Sigma) for 5 hours at 37°C. Recombinant RON5N, RON5C, and RON8 were purified via Qiagen Ni-NTA agarose under denaturing conditions and eluted with a low pH buffer as per the manufacturer’s instructions. Eluted proteins were dialyzed against PBS and ~75 µg injected per immunization into BALB/c mice (Charles River) on a 21-day immunization schedule. Polyclonal antiserum was collected from multiple mice after the second boost and screened by IFA and Western blot analysis. Similarly, residues 1–1921 of Neospora RON8 were expressed in E. coli following amplification from Neospora genomic DNA with primers CACCATGGTGGCCGCCACACTTCG and CGGAAAGCTCGGTTGTGACCG and cloning of the product into pET101/D-TOPO (Invitrogen). Bacterial cell lysates expressing Neospora RON8 were probed by Western blot with the 8E3 antibody to demonstrate that the antibody detects the recombinant protein. The first antisera generated against Toxoplasma RON8 residues 54–552 only detected RON8 under methanol fixation conditions for immunofluorescence and was used in Fig. 4. A second mouse produced antisera against the same recombinant protein that detected RON8 under formaldehyde fixation conditions and was therefore used in topology experiments described in Fig. 5.
8E3 immunoaffinity purification for protein identification and coimmunoprecipitation of the moving junction complex
Immunoaffinity chromatography with 8E3 monoclonal antibody was carried out by first cross-linking 8E3 to Protein G-Sepharose beads (Amersham) using dimethyl pimelimidate, as described (Bradley et al., 2005). 8E3-linked beads were then incubated with Neospora tachyzoite lysates in radioimmunoprecipitation (RIPA) buffer [50 mM Tris-Cl pH 7.4, 150 mM NaCl, 1% NP40, 0.5% DOC, 0.1% SDS (Gilbert et al., 2007)]. In brief, ~1.2 × 1010 extracellular parasites were centrifuged at 3000g for 20 min. The parasites were washed once in 1X PBS and then lysed in RIPA buffer + Complete Protease Inhibitor (Roche). Lysates were kept on ice for 20 min prior to removing insoluble material by centrifugation at 10000g for 20 min. Antibody-coupled beads were incubated with Neospora lysate at 25°C for 3 hours before four washes with RIPA detergent. Bound proteins were eluted using 100mM triethylamine pH 11.5, and lyophilized to remove the triethylamine and concentrate the eluate. For coprecipitation of the moving junction complex, the 8E3 immunoprecipitations were carried out in a modified RIPA buffer + Complete Protease Inhibitor described in (Alexander et al., 2005) (50 mM Tris-Cl pH 8.0, 5 mM EDTA, 150 mM NaCl, 1% NP40, 0.5% DOC, 0.01% SDS). The resulting products were analyzed by Western blot using 8E3 and cross-reactive mouse polyclonal antisera against Toxoplasma RON proteins.
MS-MS analysis of the 8E3 protein
Elution fractions from 8E3 immunoprecipitation were separated on 8% gels via SDS-PAGE, stained with Coomassie Blue R250 for 30 minutes, destained, and the prominent high molecular weight band was excised for trypsin digestion and LC-MS/MS analysis performed at the Stanford Mass Spectrometry Facility (http://mass-spec.stanford.edu/, see supplementary material).
RON5 and RON8 gene sequencing and analysis
The complete Toxoplasma RON5 and RON8 coding sequences were compiled from ESTs and sequencing of Toxoplasma cDNA (see supplementary material). The predicted sequence of the RON8 ortholog in Neospora was generated in silico using BLAST analysis against the first draft of the translated Neospora genome at (http://www.sanger.ac.uk/sequencing/Neospora/caninum/) and visual examination of predicted intron/exon boundaries, using the Toxoplasma RON8 sequence as a model. BLAST searches for RON5 and RON8 orthologs were performed against Eimeria tenella (http://www.sanger.ac.uk/Projects/E_tenella/), Babesia bigemina (http://www.sanger.ac.uk/Projects/B_bigemina/), Babesia bovis (http://www.tigr.org/tdb/e2k1/bba1/), Theileria annulata (http://www.sanger.ac.uk/Projects/T_annulata/), Plasmodium spp. (http://www.plasmodb.org), and Cryptosporidium spp. (http://cryptodb.org). The Signal P program (http://www.cbs.dtu.dk/services/SignalP) was used to search for signal peptides, and searches for transmembrane domains were carried out by TMHMM2 (http://www.cbs.dtu.dk/services/TMHMM/) and DAS (http://www.sbc.su.se/~miklos/DAS/). Potential conserved domains were examined using PFAM (http://pfam.sanger.ac.uk/search) and PROSITE (http://www.ebi.ac.uk/Tools/ppsearch/index.html).
Immunofluorescence and early invasion assays
For immunofluorescence assays on intracellular parasites, confluent HFFs grown on glass coverslips were infected with parasites and incubated for 24–30 hours at 37°C, washed with PBS, and then fixed with either ice-cold methanol for 3 minutes or 3.7% formaldehyde/PBS for 15 minutes prior to quenching with phosphate-buffered saline (PBS) plus 100 mM glycine for 5 min. Coverslips were then washed in PBS and blocked with PBS/3% bovine serum albumin (BSA) or a completely permeabilizing solution of PBS/3%BSA/0.1% Triton X-100 (PBT buffer) for 30 minutes. Primary antibodies were diluted in PBS/3%BSA or PBT buffer for 1 hour except for monoclonal 8E3, which was used as undiluted hybridoma supernatant. Coverslips were washed five times in PBS and incubated with secondary antibodies Alexa-488 goat anti-mouse and Alexa-594 goat anti-rabbit (Molecular Probes, OR) diluted 1:2000 in PBS/3%BSA for 1 hour. Following secondary washes in PBS, coverslips were then mounted onto slides using Vectashield mounting medium for fluorescence microscopy using a Zeiss upright light microscope (Zeiss Axio Imager Z1) using a 100x oil immersion objective.
For IFAs of mammalian cells, transfected HEK293 or HeLa cells were seeded onto glass coverslips 24 hours prior to fixation. Fixation with methanol or 3.7% formaldehyde then proceeded as described above, as was primary and secondary antibody staining. Mounting and fluorescence microscopy was also as above although fluorescence was examined using a 40X objective. Serial Z-stacks of fluorescence images of mammalian cells collected at 40X were deconvolved through an inverse-filter algorithm and calculated point spread function, and merged using Axiovision software. All images were rendered using Axiovision software in conjunction with a Zeiss digital CCD camera (AxioCam MRm).
For early invasion assays, confluent HFF monolayers on coverslips were incubated in cold 4°C DMEM media prior to infection with freshly lysed Neospora or Toxoplasma tachyzoites, and incubated on ice for 20 minutes. Invasion was initiated following transfer of the coverslip plate to a 37°C water bath, and coverslips were fixed with either ice-cold methanol or EM-grade 3.7% formaldehyde/PBS (Biosciences, Inc.) following 1, 2, or 4 minutes at 37°C. For topology experiments with anti-RON8 serum in Toxoplasma, infected coverslips were fixed with 3.7% formaldehyde/PBS, blocked in PBS/3%BSA for 25 minutes and incubated with rabbit anti-SAG1 diluted in PBS/3%BSA for 1 hour, then washed five times in 1X PBS and incubated with anti-RON8 diluted 1:75 in PBS/3%BSA or PBT buffer as a second primary step. Secondary staining and fluorescence microscopy (without deconvolution) then proceeded as above.
Generation of RON8-expressing mammalian cells
The 8.9 kb of RON8 coding sequence was first assembled into the pGRA-HA_HPT vector (Rome et al., 2008) as follows: primers GTTAGGTACCCAGTCTCCACACAGAAGCCAG and CAAGCTCGAGCCTGTGGGTGTCTCTTTCAGCAGATAC were used to amplify a region 1.8 kb upstream of the start codon to the end of RON8’s large N-terminal exon, using RHΔhpt genomic DNA as template. This fragment was inserted into pGRA-HA_HPT using KpnI and XhoI sites, introducing a NsiI site that is unique to the RON8 sequence. A second fragment of RON8 (encompassing a further 2.2 kb) was amplified from Toxoplasma cDNA using primers CTATATGCATTCGTGACACATCTCC and GCGGCCGCAGCAGCCTTCGCAG and inserted into [pGRA-HA_HPT + fragment 1] using the introduced NsiI site and the endogenous NotI site. The final ~0.9 kb of RON8 was amplified from cDNA using primers GCGGCCGCAGAAGGTACCGAAG and GCGGCCGCGTGCGAAGTTATTCGCTTCTG and inserted into [pGRA-HA_HPT + fragment 1 + fragment 2] using NotI. The junctions linking each inserted fragment in the final construct were sequenced to confirm the correct frame was maintained and that the RON8 coding sequence was in frame with a C-terminal hemagglutinin (HA) tag added by pGRA-HA_HPT.
For subcloning RON8-HA into the pMSCV-puromycin mammalian expression vector (Clontech), primers CACCACTAGTCACCACCATGGGCCTTCACGAAAGTCCCGATGGC and GGTGTTAATTAAGGTGCTAGTGCGAAGTTATTCGCTTC were used to amplify the coding sequence lacking its signal peptide, but including the HA tag. The PCR fragment was then digested with SpeI and PacI, blunted and cloned into pMSCV-puro that had been cut with HpaI. The resulting vector was sequenced to ensure the RON8 coding sequence was in its proper orientation and remained in frame with the HA tag. The pMSCV-puro-RON8-HA vector was linearized by PciI digestion and ~30 µg of DNA was transfected into wildtype HEK293 cells via lipofectamine (Invitrogen), as per manufacturer’s instructions. For stable transfections, cells were grown in media containing 5 µg/ml puromycin for seven days with drug replacement every two days. Stably drug-resistant mammalian cells were cloned by limiting dilution, and RON8-HA expression was gauged by immunofluorescence and Western blot using rabbit anti-HA antibody and mouse polyclonal anti-RON8 antibody.
Fractionation of RON8-HA mammalian cells
For fractionation, 6 × 106 HEK293 cells stably expressing RON8-HA were pelleted at 2500g, washed in PBS, and sonicated in either PBS or 100 mM Na2CO3 pH 11.5 + Complete Protease Inhibitor (Roche). After incubation on ice for 30 minutes, lysates were then spun at 2500g for 15 min at 4°C to remove unbroken cells and other insoluble material, and the supernatant of this low speed spin was then subjected to centrifugation at 100,000g for 90 minutes at 4°C. Equivalent loads of low speed supernatant (Total), high speed supernatant (Sup), and high speed pellet (Pellet) were examined by Western blot probing with mouse anti-HA, mouse anti-transferrin (membrane control), mouse anti-vimentin, and mouse anti-heat shock protein-90 (cytosolic control).
Construction of RON8-HA deletion series and sufficiency constructs
The pMSCV-puro-RON8-HA vector was used as template for amplification using the forward primer GGAACGGTTAACATCAAATCCC and either reverse primer CACCGCGGCCGCAGGTTTCGACTTCAGGAGTTC, CACAGCGGCCGCGATCTCCGCGAGACTGCCAAG, or CACCGCGGCCGCGATACCTCTATGCTGCCGAAG. These three fragments were then cloned into pMSCV-puro-RON8-HA using HpaI and NotI. The resulting deletion constructs, R8 Δ2033–2980, R8 Δ1362–2980, and R8 Δ697–2980, were sequenced to confirm the C-terminal HA tag was in frame with the portion of RON8 encoded by the vector. ~30 µg of DNA for each of the three deletion constructs was linearized by PciI digestion and transfected into wildtype HEK293 cells by lipofectamine and screened by IFA as above. Transfection of full-length RON8-HA into HEK293 cells was used as a control.
To engineer the construct R8 (2348–2980), the region encoding residues 2348–2980 plus the HA tag were amplified using primers CACCGTTAACCACCACCATGGGCCGAGACTTCGGGCAGCTGTTC and GGTCGTTAACGGTGTCATGCGTAGTCGGGGACGTC using pMSCV-puro-RON8-HA as a template. This PCR product was inserted into pMSCV-puro using HpaI. Following sequencing to confirm proper orientation, this vector was linearized and lipofectamine-transfected into HEK293 or HeLa cells prior to immunofluorescence as described above.
Supplementary Material
The following supplementary material is available for this article online: A description of the experimental procedures for mass spectrometry of the immunoprecipitated 8E3 protein and Table S1, a list of the tryptic peptides identified. Also described is the generation of the RON8 knockout construct, the RON5 and RON8 protein sequences, and an alignment of Toxoplasma and Neospora RON8.
Acknowledgments
We thank Dan Howe for helpful discussions of the coccidia and also thank Jean Francois Dubremetz, Maryse Lebrun, and Sebastian Besteiro for discussion of results prior to publication. We thank members of the Bradley Lab for scientific input into the project and critical reading of the manuscript. This work was supported by a Ruth L. Kirschstein Natural Research Service Award (GM07185) to K.W.S and a UCLA Frontiers of Science Seed Grant, an Ellison Medical Foundation New Scholar Award in Global Infectious Disease (ID-NS-0162-04) and an NIH Grant (1R01AI064616) to P.J.B.
References
- Aikawa M, Miller LH, Johnson J, Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978;77:72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 2005;1:e17. doi: 10.1371/journal.ppat.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrizabalaga G, Ruiz F, Moreno S, Boothroyd JC. Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation. J Cell Biol. 2004;165:653–662. doi: 10.1083/jcb.200309097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister LH, Hopkins JM, Dluzewski AR, Margos G, Williams IT, Blackman MJ, et al. Plasmodium falciparum apical membrane antigen 1 (PfAMA-1) is translocated within micronemes along subpellicular microtubules during merozoite development. J Cell Sci. 2003;116:3825–3834. doi: 10.1242/jcs.00665. [DOI] [PubMed] [Google Scholar]
- Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6:79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- Bradley PJ, Boothroyd JC. Identification of the pro-mature processing site of Toxoplasma ROP1 by mass spectrometry. Mol Biochem Parasitol. 1999;100:103–109. doi: 10.1016/s0166-6851(99)00035-3. [DOI] [PubMed] [Google Scholar]
- Bradley PJ, Sibley LD. Rhoptries: an arsenal of secreted virulence factors. Curr Opin Microbiol. 2007;10:582–587. doi: 10.1016/j.mib.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PJ, Hsieh CL, Boothroyd JC. Unprocessed Toxoplasma ROP1 is effectively targeted and secreted into the nascent parasitophorous vacuole. Mol Biochem Parasitol. 2002;125:189–193. doi: 10.1016/s0166-6851(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, et al. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J Biol Chem. 2005;280:34245–34258. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64:1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- Carpen O, Pallai P, Staunton DE, Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007;10:83–89. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- Charron AJ, Sibley LD. Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic. 2004;5:855–867. doi: 10.1111/j.1600-0854.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- DeRocher AE, Coppens I, Karnataki A, Gilbert LA, Rome ME, Feagin JE, et al. A thioredoxin family protein of the apicoplast periphery identifies abundant candidate transport vesicles in Toxoplasma gondii. Eukaryot Cell. 2008;7:1518–1529. doi: 10.1128/EC.00081-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RG, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- Gilbert LA, Ravindran S, Turetzky JM, Boothroyd JC, Bradley PJ. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot Cell. 2007;6:73–83. doi: 10.1128/EC.00309-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood J, Smith JE. Toxoplasma gondii: the role of parasite surface and secreted proteins in host cell invasion. Int J Parasitol. 1996;26:169–173. doi: 10.1016/0020-7519(95)00103-4. [DOI] [PubMed] [Google Scholar]
- Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- Huynh MH, Carruthers VB. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2006;2:e84. doi: 10.1371/journal.ppat.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MH, Hutchison WM. The prevalence and source of Toxoplasma infection in the environment. Adv Parasitol. 1989;28:55–105. doi: 10.1016/s0065-308x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. doi: 10.1016/j.tcb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- Kessler H, Herm-Gotz A, Hegge S, Rauch M, Soldati-Favre D, Frischknecht F, Meissner M. Microneme protein 8--a new essential invasion factor in Toxoplasma gondii. J Cell Sci. 2008;121:947–956. doi: 10.1242/jcs.022350. [DOI] [PubMed] [Google Scholar]
- Lebrun M, Michelin A, El Hajj H, Poncet J, Bradley PJ, Vial H, Dubremetz JF. The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell Microbiol. 2005;7:1823–1833. doi: 10.1111/j.1462-5822.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- Meissner M, Brecht S, Bujard H, Soldati D. Modulation of myosin A expression by a newly established tetracycline repressor-based inducible system in Toxoplasma gondii. Nucleic Acids Res. 2001;29:E115. doi: 10.1093/nar/29.22.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel R, Schupp K, Raether W, Bierther FW. Formation of a close junction during invasion of erythrocytes by Toxoplasma gondii in vitro. Int J Parasitol. 1980;10:309–313. doi: 10.1016/0020-7519(80)90012-0. [DOI] [PubMed] [Google Scholar]
- Mineo JR, Kasper LH. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30) Exp Parasitol. 1994;79:11–20. doi: 10.1006/expr.1994.1054. [DOI] [PubMed] [Google Scholar]
- Mital J, Meissner M, Soldati D, Ward GE. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol Biol Cell. 2005;16:4341–4349. doi: 10.1091/mbc.E05-04-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordue DG, Sibley LD. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- Mordue DG, Hakansson S, Niesman I, Sibley LD. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp Parasitol. 1999a;92:87–99. doi: 10.1006/expr.1999.4412. [DOI] [PubMed] [Google Scholar]
- Mordue DG, Desai N, Dustin M, Sibley LD. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med. 1999b;190:1783–1792. doi: 10.1084/jem.190.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki JH, Heuser JE, Sibley LD. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci. 1995;108(Pt 6):2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- Rome ME, Beck JR, Turetzky JM, Webster P, Bradley PJ. Intervacuolar transport and unique topology of GRA14, a novel dense granule protein in Toxoplasma gondii. Infect Immun. 2008 doi: 10.1128/IAI.00782-08. In Press doi:10.1128/IAI.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadak A, Taghy Z, Fortier B, Dubremetz JF. Characterization of a family of rhoptry proteins of Toxoplasma gondii. Mol Biochem Parasitol. 1988;29:203–211. doi: 10.1016/0166-6851(88)90075-8. [DOI] [PubMed] [Google Scholar]
- Sibley LD. Intracellular parasite invasion strategies. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- Suss-Toby E, Zimmerberg J, Ward GE. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc Natl Acad Sci U S A. 1996;93:8413–8418. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Kim K. The International Congress on Toxoplasmosis. Int J Parasitol. 2004;34:249–252. doi: 10.1016/j.ijpara.2003.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following supplementary material is available for this article online: A description of the experimental procedures for mass spectrometry of the immunoprecipitated 8E3 protein and Table S1, a list of the tryptic peptides identified. Also described is the generation of the RON8 knockout construct, the RON5 and RON8 protein sequences, and an alignment of Toxoplasma and Neospora RON8.