Abstract
The transmigration of polymorphonuclear leukocytes (PMNs; neutrophils) into the intestinal lumen is a classical phenomenon associated with a wide variety of disease states, including those of both pathogenic and autoimmune/idiopathic origin. While PMNs are highly effective at killing invading pathogens by releasing microbiocidal products, excessive or unnecessary release of these substances can cause substantial damage to the intestinal epithelium. Therefore, it is necessary to understand the underlying mechanisms that lure neutrophils into the lumen allowing them to perform their desired functions, so that researchers may begin to identify which processes may be potential targets for inhibiting the transmigration of PMNs during non-infectious states.
Keywords: Inflammation, neutrophils, eicosanoids, Salmonella enterica serotype Typhimurium, Shigella flexneri, hepoxilin A3
Introduction
Polymorphonuclear leukocytes (PMNs; neutrophils) are the most abundant class of white blood cells, comprising approximately 60% of all white blood cells during non-infectious states [1]. It is well understood that neutrophils play an important role in the battle against invading pathogens by the delivery of anti-microbial molecules and reactive oxygen intermediates. In addition, neutrophils help to regulate other aspects of the immune response by generating signals that recruit monocytes and dendritic cells, and help determine macrophage differentiation (reviewed in [2]). While these activities are necessary during states of active infection, aberrant activation of these processes leads to rapid tissue destruction and a cascade of events that further augments pathology. It is important to understand not only the desired and beneficial actions of neutrophils, but also the events that lead to their involvement so that they may be limited during non-infectious states, such as ulcerative colitis and Crohn's disease.
Neutrophils during infectious vs non-infectious states: The right place at the right time
Given the daily exposure of the intestinal tract to commensal microflora, pathogens and non-pathogenic microorganisms, the state of the intestine spans a fine line between health and disease. One key player in the immune response, the neutrophil, holds the title as both the most abundant form of white blood cell present in the human body and the first line of defense against uninvited guests. Its well described killing abilities stem from the efficiency of the phagolysome, where the invading microbe is trapped and exposed to an assortment of enzymes, anti-microbial peptides and reactive oxygen intermediates.
Following onset of infection, neutrophils first mount an anti-microbial attack via phagocytosis. However, an additional weapon of the neutrophil is a recently described mechanism that involves the production of neutrophil extracellular traps (NETs), complex extracellular structures comprised of chromatin with proteins from neutrophilic granules [3**]. Such NETs, formed by activated neutrophils, serve to concentrate the neutrophil's antimicrobial agents and trap and kill bacteria [4]. While these activities are desirable during active infections, unrestricted neutrophil activation and movement is capable of causing significant tissue damage.
Signaling molecules and events involved in neutrophil transepithelial migration
With the goal of maintaining homeostasis within the intestine, there are a variety of activies and signaling events that are required to appropriately draw neutrophils into the lumen. In order to reach their destination, neutrophils are required to travel from the circulation to and through a number of barriers including the endothelium, basement membrane and, finally, the epithelium. The various signals that direct such migration include the coordinated efforts of cytokines, adhesion molecules and highly specific chemokines directed at neutrophils. The precise mechanisms by which these signaling molecules are produced/secreted are of increasing interest, particularly given their potential overlap between bacterial-induced inflammation and inflammatory bowel diseases (IBD).
Chemokines and cytokines
Many chemokine and cytokine neutrophil chemoattractants are known to be produced at onset of infection and have also been found to be highly expressed during active states of IBD. Such molecules that aid in the transepithelial migration of neutrophils include GRO α/ β /γ, CXCL5/ENA-78 and CXCL8/IL-8 [5-7]. Among these, substantial weight has been placed on CXCL8/IL-8, the potent chemokine secreted from macrophages, endothelial cells, and intestinal epithelial cells [8-10]. While IL-8 undeniably plays a role in directing the traffic of neutrophils, its responsibilities occur prior to the final step of the neutrophils crossing the epithelium into the lumen. Although IL-8 is secreted from intestinal epithelial cells, it is secreted solely from the basolateral surface and associates with the extracellular matrix in a long-lived haptotactic gradient. This gradient serves to guide neutrophils from the lamina propria to the subepithelium, but does not induce movement across the epithelium, as observed in both model epithelia and a double transgenic mouse model with the ability to induce the expression human IL-8 [11,12]. In the latter in vivo study, doxycycline induced human IL-8 was expressed solely by intestinal epithelial cells and resulted in a 50-fold increase in caecal IL-8 concentration following three days of induction [12]. This coincided with an influx of neutrophils into the lamina propria with no signs of degranulation [12]. Furthermore, utilizing an in vitro model of neutrophil transepithelial migration, we have shown that an NF-kB inhibitor dramatically reduced the amount of IL-8 secreted from a polarized monolayer of colonic epithelial cells (T-84) infected with the enteric pathogen Shigella flexneri, without having an adverse effect on neutrophil migration (basolateral to apical movement) across the infected monolayer (unpublished observations, Mumy and McCormick). While significant emphasis is placed on IL-8, these data taken together would support the need for the production of other inflammatory mediators responsible for inducing the final step of neutrophil migration into the lumen (i.e., across the epithelium).
Hepoxilin A3
Separate from chemokines and cytokines, intestinal epithelial cells produce additional molecules that play a role in host defense. Among these are eicosanoids, a group of lipid molecules with pro- and anti-inflammatory properties, which include prostaglandins, leukotrienes, lipoxins and hepoxilins. The latter of these are known to be capable of a number of different biological activities. Specifically, hepoxilin A3 (HXA3) has a wide range of activities that include the ability to potentiate glucose dependent insulin secretion [13], and increase vascular permeability in rat skin [14]. Of greatest relevance for this topic, HXA3 also acts as a highly potent neutrophil chemoattractant at concentrations as low as 30-40 nm [15]. More recently we have determined that HXA3 is secreted from pathogen-infected intestinal epithelial cells exclusively from the apical surface [16-18*]. Once secreted from the epithelial cells, HXA3 behaves as a “pure” neutrophil chemoattractant and, although it does activate Ca2+ signaling, it does not activate neutrophils during the process as evidenced by the lack of release of reactive oxygen intermediates or secondary granule contents [15,16,19]. The direct interaction between HXA3 and neutrophils is not well understood and needs to be further elucidated.
Other chemoattractants have shown the ability to change the expression of specific adhesion molecules on the surface of neutrophils, with one example being that of f-Met-Leu-Phe exposure resulting in increased expression of CD18/CD11b [20]. However, the reliance on a specific adhesion molecule(s) varies depending on the type of chemoattractant [21]. Our recent studies with neutrophils and HXA3 have indicated that neutrophil transepithelial migration CD47, CD44 and CD55 [22*].
Generation and apical secretion of HXA3
HXA3 is a hydroxy epoxide derivative formed from 12S-hydroperoxyeicosa-5Z,8Z,10E,14Z- tetraenoic acid (12-HpETE), the primary product of arachidonic acid (AA) formed by 12/15-lipoxygenase (12/15-LOX). We have demonstrated the production and release of HXA3 in response to insult by various pathogens and in different tissues. With respect to the intestine are Salmonella enterica serovar Typhimurium and Shigella flexneri. Given that HXA3 is a product of 12/15-LOX activity, we determined that disruption of the 12/15-LOX pathway dramatically reduced S. typhimurium and S. flexneri-induced neutrophil transepithelial migration, supporting the notion that this molecule is a critical regulator of mucosal inflammation [16,17]. The pathway initiating 12/15-LOX activity leading to the generation of HXA3 has only recently begun to be elucidated.
Generation of HXA3
The observation that inhibitors of 12/15-LOX blocked neutrophil migration in response to infection in model intestinal underscores the importance of this enzyme in inflammation. Previous studies have suggested that the 5-lipoxygenase (5-LOX) pathway is also a prominent pro-inflammatory cascade [23], however, we determined that an inhibitor of the 5-LOX pathway failed to inhibit pathogen-induced neutrophil migration [16,17]. These observations also suggest that the rate-limiting step in HXA3 production, and therefore neutrophil movement, may be at the level of availability of arachidonic acid, the substrate for the 12/15-LOX pathway. In humans there are four enzymes with 12/15-LOX activity: platelet-type, 12(R), epidermal-type 12-LOX, and 12/15-LOX [24-26]. Of these, platelet-type 12-LOX is thought to be expressed largely in human platelets, and 12(R)-LOX, distinctively displaying R-steriospecificity of oxygen insertion, is expressed almost solely in skin [27]. While initial observations have suggested tissue-specific expression of these enzymes, the possibility exists for expression in additional tissues. Investigations into relevant expression profiles have proven to be difficult, with expression considered to occur within narrow time frames and the potential for false positive data as a result of induction from biological sources (e.g. IL-4), however this topic remains an active and important line of study [27]. Given that 12/15-LOX is highly expressed in intestinal epithelial cells, this pathway may play a major role in the synthesis of 12-HpETE. 12/15-LOX is capable of synthesizing both 15-HpETE and 12-HpETE at a 4:1 ratio, where 12-HpETE is the minor product [27,28]. At present it is unclear which 12-LOX genes specifically contribute to HXA3 production, so the potential contribution of the other enzymes with 12/15-LOX activity cannot be excluded. Further experiments will help determine the involvement of the respective 12-LOX genes involved in HXA3 production.
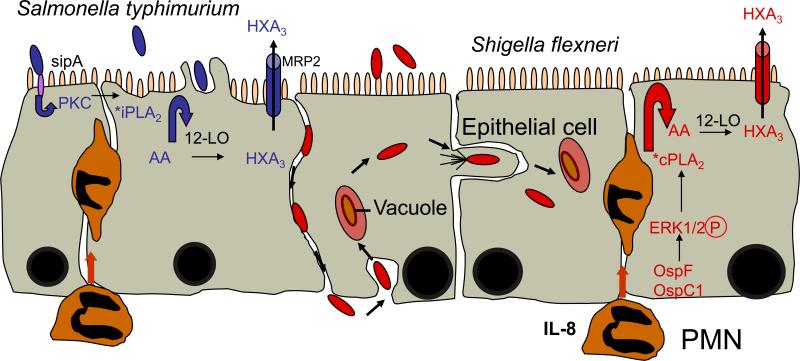
Due to the potent pro- and anti-inflammatory effects of eicosanoids, the enzymatic release of arachidonic acid from the membrane phospholipids must be highly regulated and the concentrations of free arachidonic acid inside the cell must be kept at low levels in resting cells. One major pathway involved in the release of arachidonic acid from the membrane is the hydrolysis of membrane glycerophospholipids by the action of phospholipase A2 (PLA2) [29]. There are three broad classes of phospholipases involved in eicosanoid generation by releasing arachidonic acid from the membrane: sPLA2, iPLA2, and cPLA2 [30]. Interestingly, we found that S. typhimurium and S. flexneri induce neutrophil transepithelial migration through the utilization of distinct PLA2 enzymes; S. typhimurium is dependent on iPLA2, whereas S. flexneri is dependent on cPLA2 activity [17]. The activation of these PLA2 enzymes is triggered by different cellular processes that are initiated by the infringing virulence proteins of the two pathogens; SipA of S. typhimurium leads to the activation of PKC, which in turn induces the activation iPLA2, whereas the Osp effector proteins of S. flexneri are involved in regulating the activation of the mitogen activated protein kinase/ERK pathway [31-33] which culminates in cPLA2 activation [17]. One major finding of this work is the understanding that neutrophil transepithelial migration induced by these pathogens converge at the point of arachidonic acid release, suggesting that the distal portion of the signal transduction pathway ultimately leading to the generation of HXA3 is conserved (Figure 1).
Figure 1. The distal portion of the signal transduction pathway leading to S. typhimurium and S. flexneri-induced HXA3 release is conserved.
S. typhimurium interacts with intestinal epithelial cells from the apical surface and the effector protein, SipA, activates a novel signal transduction cascade that leads to the activation of PKC. We postulate that this PKC-dependent signal transduction cascade leads to the activation of iPLA2, which liberates archidonic acid (AA) from membranes for subsequent metabolism by the 12/15-LOX pathway culminating in the synthesis of HXA3 (pathway shown in blue). S. flexneri gains access to and interacts with the intestinal epithelium from the basolateral surface. Once inside the host cell, S. flexneri secretes the effector proteins OspF and OspC1 that leads to the phosphorylation of ERK1/2. ERK1/2 activation is a prominent trigger, which leads to the activation of cPLA2. This enzyme releases arachidonic acid (AA) from membranes where ii becomes an available substrate for the 12/15-LOX pathway and leads to the eventual production of HXA3 (pathway shown in red).
Apical secretion of HXA3
Given the role of HXA3, it is imperative that it be exclusively secreted from the apical surface of the intestinal epithelium in order to establish the concentration gradient across the tight junctions to lure neutrophils from the sub-epithelium, through the paracellular space and into the lumen. To this end, we evaluated the capacity of cellular transporters to efflux HXA3 from the apical surface. We considered the involvement of the ATP-binding cassette (ABC) transporters P-gp and MRP2 due to their localization on the apical surface and the structural characteristics of HXA3. We found HXA3 can serve as a substrate for MRP2 and that blocking the transport function and/or expression of MRP2 inhibited neutrophil transmigration in S. typhimurium-infected model intestinal epithelia, while similar attempts to attenuate the function of P-gp did not elicit the same effect [18]. Moreover, we determined that MRP2 is profoundly up-regulated in states of intestinal inflammation. Examination of inflamed intestinal epithelia from human biopsies also showed up-regulation of MRP2 [18], providing convincing support for its involvement in the inflammatory process.
Potential therapeutic targets to reduce neutrophil transepithelial migration
Given that HXA3 is implicated in PMN transepithelial migration during both infectious and non-infectious intestinal inflammation, disrupting the generation or release of this eicosanoid, or the recognition of it by neutrophils, are attractive candidates for limiting neutrophil involvement during aberrant states of inflammation. Therefore, obvious therapeutic targets include key enzymes involved in the production of HXA3. One such enzyme that has shown potential for success is that of 12/15 LOX. Inhibitors targeting this enzyme activity (i.e., baicalein) have shown the ability to significantly reduce neutrophil transepithelial migration in response to Salmonella and Shigella in model systems [16,17]. In addition, as mentioned previously, we found that HXA3 can serve as a substrate for MRP2 and that blocking the transport function and/or expression of MRP2 prevented neutrophil transmigration [18].
Alternatively, blocking the ability of the neutrophils to respond/recognize HXA3 would also function to inhibit neutrophil movement. This approach is based on the fact that the adhesion interaction profile of neutrophil transepithelial migration in response to HXA3 differs from the profile shown by similar eicosanoids, in that neutrophil - HXA3 interactions are critically dependent on four major surface adhesion molecules [22]. The blockage of one of these adhesion molecules might reduce inflammation. Thus, HXA3 as a key mediator of mucosal inflammation represents the discovery of a new class of chemoattracting agents that act across intact epithelial cell barriers. It is likely that this finding will have far-reaching clinical implications as it opens an entirely new era of pharmaceutical targets for the treatment of chronic inflammation in the gut (and perhaps other mucosal surfaces, i.e., lung).
Acknowledgements
We thank members of the McCormick lab past and present that have contributed to the studies highlighted in this review.
Financial Support: National Institutes of Health grants DK 56754 and DK3306, and a Senior Investigator Award from the Crohn's and Colitis Foundation of America (all to B.A.M). These funding sources were not involved in the writing of this report or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References and recommended reading
Paper of particular interest, published within the period of review having been highlighted as:
* of special interest
** of outstanding interest
- 1.Bainton DF, Ullyot JL, Farquhar MG. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971;134:907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- **3.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [** of outstanding interest** Important review that provides outstanding review on neutrophil production of neutrophil extracellular traps (NETs) that capture and kill pathogens] [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 5.MacDermott RP. Chemokines in the inflammatory bowel diseases. J Clin Immunol. 1999;19:266–272. doi: 10.1023/a:1020583306627. [DOI] [PubMed] [Google Scholar]
- 6.Eckmann L, Kagnoff MF. Cytokines in host defense against Salmonella. Microbes Infect. 2001;3:1191–1200. doi: 10.1016/s1286-4579(01)01479-4. [DOI] [PubMed] [Google Scholar]
- 7.Egesten A, Eliasson M, Olin AI, Erjefalt JS, Bjartell A, Sangfelt P, Carlson M. The proinflammatory CXC-chemokines GRO-alpha/CXCL1 and MIG/CXCL9 are concomitantly expressed in ulcerative colitis and decrease during treatment with topical corticosteroids. Int J Colorectal Dis. 2007;22:1421–1427. doi: 10.1007/s00384-007-0370-3. [DOI] [PubMed] [Google Scholar]
- 8.Phalipon A, Sansonetti PJ. Shigella's ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol Cell Biol. 2007;85:119–129. doi: 10.1038/sj.icb7100025. [DOI] [PubMed] [Google Scholar]
- 9.Figueiredo JF, Lawhon SD, Gokulan K, Khare S, Raffatellu M, Tsolis RM, Baumler AJ, McCormick BA, Adams LG. Salmonella enterica Typhimurium SipA induces CXC-chemokine expression through p38MAPK and JUN pathways. Microbes Infect. 2009;11:302–310. doi: 10.1016/j.micinf.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Varon C, Duriez A, Lehours P, Menard A, Laye S, Zerbib F, Megraud F, Laharie D. Study of Helicobacter pullorum proinflammatory properties on human epithelial cells in vitro. Gut. 2009;58:629–635. doi: 10.1136/gut.2007.144501. [DOI] [PubMed] [Google Scholar]
- 11.McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucharzik T, Hudson JT, 3rd, Lugering A, Abbas JA, Bettini M, Lake JG, Evans ME, Ziegler TR, Merlin D, Madara JL, et al. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54:1565–1572. doi: 10.1136/gut.2004.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pace-Asciak CR, Martin JM. Hepoxilin, a new family of insulin secretagogues formed by intact rat pancreatic islets. Prostaglandins Leukot Med. 1984;16:173–180. doi: 10.1016/0262-1746(84)90069-6. [DOI] [PubMed] [Google Scholar]
- 14.Laneuville O, Corey EJ, Couture R, Pace-Asciak CR. Hepoxilin A3 increases vascular permeability in the rat skin. Eicosanoids. 1991;4:95–97. [PubMed] [Google Scholar]
- 15.Sutherland M, Schewe T, Nigam S. Biological actions of the free acid of hepoxilin A3 on human neutrophils. Biochem Pharmacol. 2000;59:435–440. doi: 10.1016/s0006-2952(99)00345-7. [DOI] [PubMed] [Google Scholar]
- 16.Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, McCormick BA. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci U S A. 2004;101:7421–7426. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mumy KL, Bien JD, Pazos MA, Gronert K, Hurley BP, McCormick BA. Distinct isoforms of phospholipase A2 mediate the ability of Salmonella enterica serotype typhimurium and Shigella flexneri to induce the transepithelial migration of neutrophils. Infect Immun. 2008;76:3614–3627. doi: 10.1128/IAI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Pazos M, Siccardi D, Mumy KL, Bien JD, Louie S, Shi HN, Gronert K, Mrsny RJ, McCormick BA. Multidrug resistance-associated transporter 2 regulates mucosal inflammation by facilitating the synthesis of hepoxilin A3. J Immunol. 2008;181:8044–8052. doi: 10.4049/jimmunol.181.11.8044. [* of special interest* This study describes the role of the ABC transporter MRP2 in HXA3 secretion, and discusses the potential development of therapeutic strategies for the treatment of epithelial-associated inflammatory conditions by targeting MRP2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- 20.Vedder NB, Harlan JM. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988;81:676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blake KM, Carrigan SO, Issekutz AC, Stadnyk AW. Neutrophils migrate across intestinal epithelium using beta2 integrin (CD11b/CD18)-independent mechanisms. Clin Exp Immunol. 2004;136:262–268. doi: 10.1111/j.1365-2249.2004.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22.Hurley BP, Sin A, McCormick BA. Adhesion molecules involved in hepoxilin A3-mediated neutrophil transepithelial migration. Clin Exp Immunol. 2008151:297–305. doi: 10.1111/j.1365-2249.2007.03551.x. [** of outstanding interest** This study reveals that the adhesion interaction profile of neutrophils in response to HXA3 differs from that of a related eicosanoid, with a critical dependency on CD18, CD47, CD44 and CD55. This research lends important information with respect to the potential for adhesion molecules to be targets for drug development] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S, Kishimoto K, Arakawa T, Suzuki H, Nakamura M, Yoshimoto T, Takao T, Shimonishi Y, Tanabe T. Arachidonate 12-lipoxygenases. Catalytic properties and regulation of the enzyme gene. Adv Exp Med Biol. 1997;407:191–196. [PubMed] [Google Scholar]
- 25.Yamamoto S, Suzuki H, Nakamura M, Ishimura K. Arachidonate 12-lipoxygenase isozymes. Adv Exp Med Biol. 1999;447:37–44. doi: 10.1007/978-1-4615-4861-4_4. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimoto T, Yamamoto S. Arachidonate 12-lipoxygenase. J Lipid Mediat Cell Signal. 1995;12:195–212. doi: 10.1016/0929-7855(95)00019-m. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn H, O'Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68-69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 29.Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2. Annu Rev Pharmacol Toxicol. 1999;39:175–189. doi: 10.1146/annurev.pharmtox.39.1.175. [DOI] [PubMed] [Google Scholar]
- 30.Diaz BL, Arm JP. Phospholipase A(2). Prostaglandins Leukot Essent Fatty Acids. 2003;69:87–97. doi: 10.1016/s0952-3278(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 31.Zurawski DV, Mitsuhata C, Mumy KL, McCormick BA, Maurelli AT. OspF and OspC1 are Shigella flexneri type III secretion system effectors that are required for postinvasion aspects of virulence. Infect Immun. 2006;74:5964–5976. doi: 10.1128/IAI.00594-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zurawski DV, Mumy KL, Badea L, Prentice JA, Hartland EL, McCormick BA, Maurelli AT. The NleE/OspZ family of effector proteins is required for polymorphonuclear transepithelial migration, a characteristic shared by enteropathogenic Escherichia coli and Shigella flexneri infections. Infect Immun. 2008;76:369–379. doi: 10.1128/IAI.00684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zurawski DV, Mumy KL, Faherty CS, McCormick BA, Maurelli AT. Shigella flexneri type III secretion system effectors OspB and OspF target the nucleus to downregulate the host inflammatory response via interactions with retinoblastoma protein. Mol Microbiol. 2008 doi: 10.1111/j.1365-2958.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]