Abstract
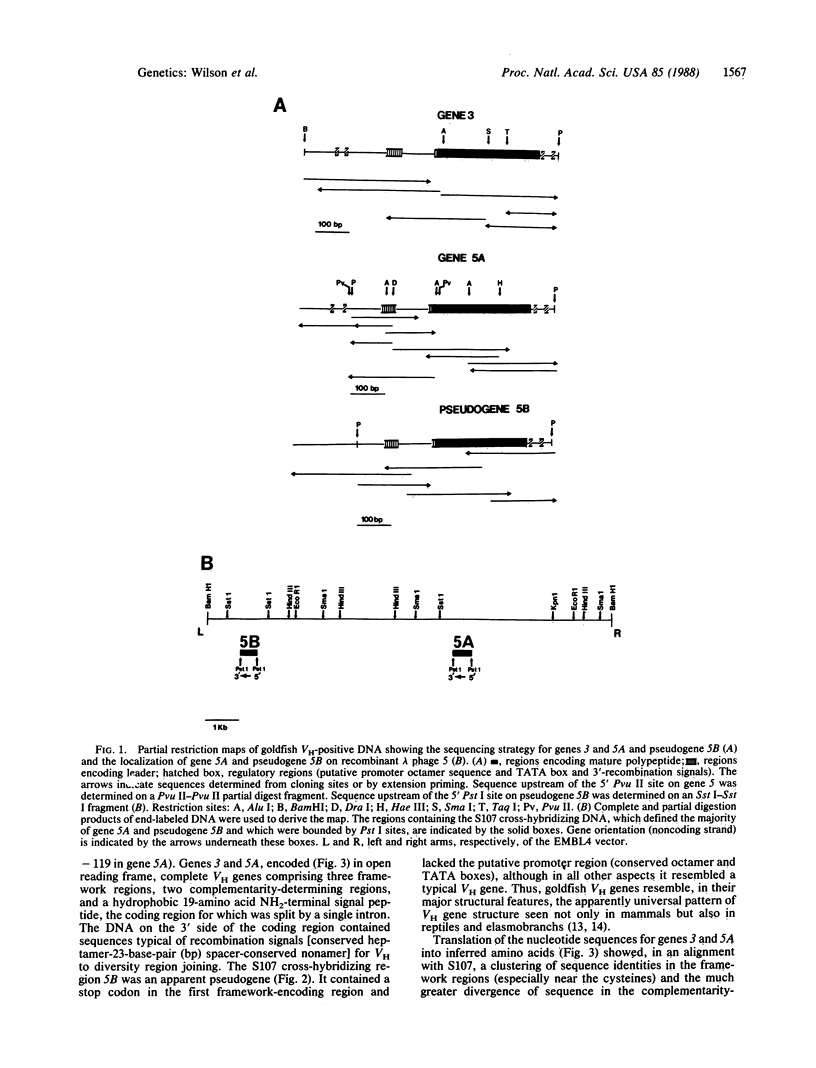
Nucleotide sequences for two immunoglobulin heavy chain variable region (VH) genes and one pseudogene in the goldfish (Carassius auratus) and the family relationships and distribution of these genes in individual fish are presented. Comparison of the nucleotide and inferred amino acid sequences of goldfish and other vertebrate VH genes indicates that goldfish VH genes show the major VH gene regulatory and structural features (5'-putative promoter region, split hydrophobic leader, three framework and two complementarity-determining regions, and 3'-recombination signals for VH to diversity region joining) and that goldfish VH genes are not more closely related to one another than they are to VH genes of evolutionarily distant vertebrates such as the mammals. Goldfish VH genes appear to exist in distinct families, and individual goldfish can carry from none to apparently greater than 15 genes of a given family. These results suggest that whereas the basic structure of VH genes has been conserved in evolution, there may be substantial variation in the nature and population distribution of VH gene families in the vertebrates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Dover G. A., Flavell R. B. Molecular coevolution: DNA divergence and the maintenance of function. Cell. 1984 Oct;38(3):622–623. doi: 10.1016/0092-8674(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Hinds K. R., Litman G. W. Major reorganization of immunoglobulin VH segmental elements during vertebrate evolution. Nature. 1986 Apr 10;320(6062):546–549. doi: 10.1038/320546a0. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Preuss D., Grisafi P., Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987 Jan 16;235(4786):312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- Litman G. W., Berger L., Murphy K., Litman R., Hinds K., Erickson B. W. Immunoglobulin VH gene structure and diversity in Heterodontus, a phylogenetically primitive shark. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2082–2086. doi: 10.1073/pnas.82.7.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman G. W., Murphy K., Berger L., Litman R., Hinds K., Erickson B. W. Complete nucleotide sequences of three VH genes in Caiman, a phylogenetically ancient reptile: evolutionary diversification in coding segments and variation in the structure and organization of recombination elements. Proc Natl Acad Sci U S A. 1985 Feb;82(3):844–848. doi: 10.1073/pnas.82.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livant D., Blatt C., Hood L. One heavy chain variable region gene segment subfamily in the BALB/c mouse contains 500-1000 or more members. Cell. 1986 Nov 7;47(3):461–470. doi: 10.1016/0092-8674(86)90603-3. [DOI] [PubMed] [Google Scholar]
- Mäkelä O., Litman G. W. Lack of heterogeneity in antihapten antibodies of a phylogenetically primitive shark. Nature. 1980 Oct 16;287(5783):639–640. doi: 10.1038/287639a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Mori N., Matsunaga T. Antigen-binding specificities of antibodies are primarily determined by seven residues of VH. Proc Natl Acad Sci U S A. 1985 May;82(9):2945–2949. doi: 10.1073/pnas.82.9.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parslow T. G., Blair D. L., Murphy W. J., Granner D. K. Structure of the 5' ends of immunoglobulin genes: a novel conserved sequence. Proc Natl Acad Sci U S A. 1984 May;81(9):2650–2654. doi: 10.1073/pnas.81.9.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Bienz B., Ram D., Ben-Neriah Y., Cohen J. B., Zakut R., Givol D. Organization and evolution of immunoglobulin VH gene subgroups. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4405–4409. doi: 10.1073/pnas.79.14.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. E., Jung H. D., Palm W., Hilschmann N. Beitrag zur dreidimensionalen Strukturaufklärung der Antikörper. Die Primärstruktur des kristallisierbaren monoklonalen Immunoglobulins IgG1 KOL, I. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):713–747. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Toraño A., Putnam F. W. Complete amino acid sequence of the alpha 2 heavy chain of a human IgA2 immunoglobulin of the A2m (2) allotype. Proc Natl Acad Sci U S A. 1978 Feb;75(2):966–969. doi: 10.1073/pnas.75.2.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. R., Middleton D., Alford C., Sullivan J. T., Litman G. W., Warr G. W. Putative immunoglobulin VH genes of the goldfish, Carassius auratus, detected by heterologous cross-hybridization with a murine VH probe. Vet Immunol Immunopathol. 1986 Jun;12(1-4):21–28. doi: 10.1016/0165-2427(86)90106-6. [DOI] [PubMed] [Google Scholar]
- Winter E., Radbruch A., Krawinkel U. Members of novel VH gene families are found in VDJ regions of polyclonally activated B-lymphocytes. EMBO J. 1985 Nov;4(11):2861–2867. doi: 10.1002/j.1460-2075.1985.tb04015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]