Abstract
Imaging in medicine has been classically based on the anatomical description of organs. In the past 15 years, new imaging techniques based on gene expression that characterize a pathological process have been developed. Molecular imaging is the use of such molecules to image cell-specific characteristics. Here, we review recent advances in molecular imaging, taking as our prime example lymph node (LN) metastasis in prostate cancer. We describe the new techniques and compare their accuracy in detecting LN metastasis in prostate cancer. We also present new molecular strategies for improving tumor detection using adenoviruses, molecular promoters and amplification systems. Finally, we present the concept of ‘in vivo pathology’, which envisages using molecular imaging to accurately localize metastatic lesions based on the molecular signature of the disease.
The importance of staging cancers
Cancer is the second most common cause of death in North America. Oncologists aim to provide the best outcomes for patients while minimizing the morbidity associated with the treatments. Treatment decisions are made after the oncological team has staged the cancer to be treated. Staging is based on physical examination, pathology at biopsy and imaging techniques. It determines whether a cancer is localized, locally advanced with a high risk of future metastasis or already metastatic. Based on the pre-treatment evaluation and the natural history of each cancer, there follows a local treatment (typically radiotherapy or surgery), a systemic treatment (often chemotherapy or hormonal deprivation therapy), or a combination of these. For most cancers, the status of the lymph nodes (LNs) is of primary importance in the treatment decision-making process because lymphatic involvement is considered to be an early step of metastatic spread and an independent prognostic factor [1]. Unfortunately, the classical pre-operative methods for assessing LNs are mainly based on LN structural abnormalities such as abnormal size and shape. Therefore, our ability to distinguish between absence of nodal involvement and metastatic disease when a LN is structurally normal is poor. There is a great need to develop an imaging modality for lymphatic metastasis, based on the molecular signature of the cancer. Here, we review the classical clinical imaging technologies for detection of LN metastasis in prostate cancer and the new technologies that have been clinically tested in trials and we consider future technologies that could improve the accuracy of current detection methods.
The importance of identifying LNs in prostate cancer
Choice of treatment modality
Pre-operative localized prostate cancer staging has been simplified by A. V. D’Amico and colleagues into three subcategories based on the probability of prostate-cancer-specific mortality [2]. The three categories have been named low-, intermediate- and high-risk prostate cancers (Box 1). It is based on these categories that treatment recommendations by the American Urological Association are made. Pre-operative prostate cancer staging helps the clinician to decide between surveillance, minimally invasive local therapies (cryotherapy, brachytherapy and experimental therapies) or a radical local treatment such as surgery or external beam radiotherapy. The main goal of staging is to limit the morbidity of the treatment while giving excellent oncologic results. If it is judged based on pre-clinical parameters that a cancer has a low-risk of LN involvement, a localized treatment without LN dissection could be proposed (i.e. surveillance, brachytherapy or radical prostatectomy without LN dissection). However, this classification is not precise enough to completely exclude LN metastasis. Indeed, more precise pre-operative predictive models for LN metastasis have been developed but, still, their accuracy is ~80%, which could be improved by combining these models with molecular imaging [3]. Finally, the precise location of nodes cannot be determined through use of predictive clinical tools, hence molecular imaging techniques that identify LN metastasis would be strongly advantageous.
Box 1. Risk categories in prostate cancer.
Clinical staging of a patient with prostate cancer relies on three parameters: (i) biopsy results; (ii) digital rectal examination; and (iii) level of serum PSA at diagnosis. Biopsy results reveal the histological differentiation of a prostate cancer using the classification of Gleason. The Gleason grade indicates the glandular differentiation patterns of the prostate carcinoma and a grade from 1 to 5 is given, 5 being a poorly differentiated cancer. By reporting the two most prevalent Gleason patterns in a biopsy and by adding them, a sum is generated. For example, the addition a Gleason pattern 4 (main pattern) and a Gleason pattern 3 (secondary pattern) in a biopsy core will give a Gleason sum of 4 + 3 = 7 for that core. Prostate cancers with higher Gleason score will have a more aggressive behaviour.
Digital rectal exam (DRE) enables the clinician to estimate the volume of the tumor and to ascertain if it is confined to the prostate. A clinical stage (cTNM) is then given based on this evaluation using the clinical tumor-node-metastasis system for prostate cancer. For example, if the tumor is not palpable, it is a T1 clinical stage; if the tumor is palpable but confined to the prostate, it is a T2 clinical stage; if the tumor is felt to be outside of the prostate capsule or to invade the seminal vesicles, it is staged as a T3, and if it invades anatomic structures surrounding the prostate, it is staged as a T4. DRE is not a precise evaluation tool because it is very subjective.
PSA is a protease that is secreted by prostate epithelial cells but it is not specific to prostate cancer. However, the serum levels of PSA increase with the risk to discover prostate cancer at biopsy. Moreover, once prostate cancer is diagnosed, PSA level is an indicator of the tumor volume and pathological stage at prostatectomy.
D’Amico and colleagues have used clinical parameters to define risk categories of dying of prostate cancer. These categories help the clinician to decide which treatment to recommend to patients [62]. Low-risk category prostate cancers are those with all of the following criteria: (i) a Gleason sum <7; (ii) a clinical stage of T1 or T2a (only half of one lobe invaded by tumor at DRE); and (iii) a serum PSA level ≤10 ng/mL. Intermediary risk categories must have one of these criteria: (i) Gleason sum of 7; (ii) clinical stage T2b (more than a half of one lobe invaded at DRE but not two lobes); and (iii) a PSA level between 10 and 20 ng/mL (excluded). Prostate cancers in the high-risk category include cancers that have any of these features: (i) a Gleason sum >7; (ii) a clinical stage of cT2c (two lobes invaded at DRE), T3 or T4; or (iii) a PSA level ≥20 ng/mL.
Guiding urologic surgeons to LN metastasis
The role of lymphadenectomy (i.e. LN dissection) in the treatment of prostate cancer is currently a debate in urologic oncology and its curative role goes beyond the scope of this review. However, there is evidence in the literature suggesting that long-term survival can be achieved even in the presence of LN metastatic disease. Bader and colleagues have demonstrated that prostate-specific antigen (PSA)-free survival (i.e. no biochemical recurrence) after radical prostatectomy (RP) and lymphadenectomy was possible even in the presence of LN metastasis [4]. In their series, 39% of their patients with a single positive node were free of PSA recurrence without adjuvant treatment, showing that prostatectomy and lymphadenectomy might have a curative role. In another study, Daneshmand and colleagues have shown that long-term recurrence-free survival was possible ten years after RP and LN dissection in patients with positive LN pathology [5]. 65% of patients were free of clinical recurrence at 10 years despite the fact that only 31% of patients received adjuvant hormonal therapy, again suggesting that surgery was curative. Interestingly, the authors have shown that recurrence-free survival was strongly associated with density of positive LNs resected (number of positive LN/ total LN resected). Others studies have examined cancer-specific survival after RP and LN dissection in patients with positive nodes and have shown that long-term cancer-specific survival ranged between 62% and 84%. The patients with fewer than two involved LNs by pathological assessment had the best chances of survival [1]. Taken together, these results show that long-term recurrence-free and disease-specific survival can be achieved after surgery when LN metastasis is present, but the best chances of survival for the patients are when LNs are resected early in the disease. Moreover, the difference in survival between single and multiple node metastasis prostate cancer could potentially be explained by the presence of LN disease which has escaped the lymphadenectomy surgery. Therefore, we believe that these data justify the need to develop more sensitive and specific molecular imaging technologies to improve detection of LN metastasis and to guide surgeons during lymphadenectomy.
Classical imaging techniques to assess LN status
Cross-sectional imaging of the LNs has been widely used in clinical practice. Two technologies commonly used are computerized tomodensitometry (CT-scan) and magnetic resonance imaging (MRI). However, the ability of these techniques to distinguish between benign and malignant LNs is mainly based on the size of the node. The 1 cm size has been classically reported as the lower size limit to distinguish a benign from a malignant LN [6]. Other secondary anatomical signs of benign nodes include a fatty LN hilum, regular contours and homogenous signals [7,8]. In prostate cancer, the sensitivity (see Glossary) of CT-scan and MRI to detect LN metastasis is ~40% and the specificity is 80% [6]. Moreover, other inflammatory processes can mimick metastasis even in the presence of a clearly diagnosed primary tumor. Indeed, despite helping the clinician to detect gross LN metastasis, the actual sensitivity and specificity of cross-sectional imaging is low, justifying the development of new imaging tools.
Glossary
- Sensitivity
a sensitivity of 100% means that a test can diagnose disease in all patients with disease. If a test has a high sensitivity, a negative result means that the probability of having the disease is low. However, obtaining a positive test does not necessarily confirm the presence of the disease.
- Specificity
a specificity of 100% means that a test can identify a particular disease. A diagnostic test having high specificity is useful for confirming the presence of the disease.
- Positive predictive value (PPV)
the PPV is the proportion of patients with positive test results that are correctly diagnosed (low number of false positive results). It is the most important measure of a diagnostic method because it reflects the probability that a positive test means that a patient has the disease.
- Negative predictive value (NPV)
the NPV is the proportion of patients with negative test results who do not have the disease. This means that a test with a high NPV has a low number of false-negative results.
Advances in molecular imaging techniques
Molecular imaging involves the use of molecules to image tumor-cell-specific characteristics. The molecules can be an antibody bearing a radioactive label that recognizes a tumor-specific antigen, a virus that expresses a reporter gene in a tumor-specific manner, a molecule that is metabolized in proliferating cells or a molecule that indirectly shows microanatomic disruption of a normal LN by a metastasis. These approaches all aim to image LN metastasis before it has enlarged a node and to distinguish the presence of tumor cells from inflammation. Multiple approaches have been studied to image pathophysiological processes in vivo. In the next sections, for clarity purposes, we describe only those that have been studied for imaging LN metastasis in prostate cancers. This will enable the reader to compare the accuracy of different imaging modalities.
Clinically tested technologies
MRI lymphoangiography
Nanoparticle-enhanced MRI lymphoangiography (MRL) was recently developed and assessed for sensitivity and specificity in the detection of nodal metastases. Iron-oxide contrast agents (Combidex or Sinerem) are injected intravenously and are selectively taken up by macrophages that ultimately traffic through the lymphatics to LNs. On MRI T2 weighted images*, macrophages containing iron particles are hypointense and, therefore, the node seems black (Figure 1). Tumor infiltration of a LN prevents macrophages from spreading uniformly throughout the LN, and LN metastasis seems to be a hyperintense signal in the LN (Figure 1). Because this approach is based on anatomical disruption of the LN by the metastasis, it increases the sensitivity and specificity of MRI for the diagnosis of LN metastasis by defining the microanatomy of the LN. In a prospective study, Harisinghani and colleagues evaluated the sensitivity and specificity of MRL in detecting prostate cancer LN metastasis. All patients underwent histological confirmation of LN status when this was positive for metastasis by MRL [9]. The sensitivity and specificity for LN metastasis on a per-patient basis were 90.5% and 97.8%, respectively. However, detection of smaller LN lesions was difficult because only 40% of LN metastasis <5 mm could be detected by MRL. In another prospective multicentric study, Heesakkers and colleagues evaluated the precision of MRL in intermediate to high risk clinically localized prostate cancers [6]. All patients had a radical prostatectomy with limited LN dissection within eight weeks of MRL. LN metastasis was found in 16% of patients at pathology. MRL had a positive predictive value (PPV) of 69% and an excellent negative predictive value (NPV) of 96%, showing a good ability to exclude metastatic node disease in this population. MRL could also detect LN metastasis outside of the pelvic LN dissection field in 24–30% of patients and, therefore, could guide surgeons to unexpected positive LNs [6,10]. Thus, these two studies have demonstrated that MRL is an accurate imaging technique that can detect LN disease. However, this technique is new and not available widely for clinical use. Moreover, it relies only on the disturbance of LN anatomy and not on cancer-specific gene expression, which limits its use for LN metastasis.
Figure 1.
MRL. MRL uses the ability of macrophages to phagocytose iron-oxide particles. (a) Before iron oxide particle infusion, the LN has high signal intensity and is regular in shape. (b) When macrophages that contain iron particles migrate to LNs, the LN has low signal intensity, as indicated by the asterisk. However, when there is a micrometastasis, the macrophages cannot distribute evenly through the LN and some areas of the LN have a high signal intensity. Compare areas of metastasis (arrows) with black portion of the node (asterisk) in (b). Figure adapted, with permission, from Ref. [6].
Positron emission tomography
Positron emission tomography (PET) is a nuclear imaging technology based on the ability of a PET scanner consisting of a ring of high-energy photon detectors to detect gamma photons emitted by an isotope (11C, 18F, 15O or 13N), which is chemically integrated in a molecule metabolized predominantly in pathlogical tissues [11]. In oncology, the increased metabolism of glucose in tumor cells has enabled PET scans to identify tumor tissues by using the radioactive glucose metabolism marker 18F-fluorine-deoxyglucose (18F-FDG). When PET is combined with a CT scan (PET–CT), the fused image can enable the physician to associate increased metabolism with its anatomical location (Figure 2).
Figure 2.
PET-CT. 11C-choline positron emission tomography of a patient with prostate cancer LN metastasis. (a) A positive signal is localized in the pelvis (white arrow). (b) A positive signal is located within a LN, increasing the specificity of the signal. Note the non-specific signals from the gut above the LN (red arrow). Therefore, CT enables the correlation of an anatomic structure like a LN or the gut to an 11C-choline positive signal. Image reproduced, with permission, from Ref. [6].
The application of PET or PET–CT to image LNs has been studied thoroughly in many cancers, including prostate cancers [12-22]. The advantage of PET over CT or MRI is its ability to detect increased metabolism of tumors cells in a structurally normal node before tumor cells enlarge this node. In a prospective study, Antoch and colleagues studied the ability of PET–CT to accurately correlate with tumor-node-metastasis (TNM) staging at pathology on 98 patients with different cancer histological types (i.e. mainly non-prostate) and showed that PET–CT was an accurate imaging tool to assess LN invasion status [23].
The ability of 18F-FDG–PET–CT to detect prostate cancers is hampered by the tracer being excreted mostly in the urine, which renders genitourinary organs difficult to image owing to the presence of a high non-specific background from urine. Moreover, prostate cancers have a low metabolic activity and poor glucose uptake relative to other cancers [24]. Indeed, in primary tumors, the sensitivity of 18F-FDG–PET for localized prostate cancer was only 4% in one study [25]. However, 18F-FDG was shown to better image high-grade prostate cancers (Box 1), which are prone to metastasize to LNs [26], and three studies have shown that 18F-FDG–PET had a sensitivity of only 0–50% for LN metastasis [27-29].
Other molecules specifically metabolized in tumor cells have been tested to evaluate LN status by PET–CT. Choline is a molecule that is metabolized into phosphorylcholine and is incorporated into cellular membranes [24]. It can be labeled with two positron-emitting atoms: 18F and 11C. 11C-choline sensitivity for LN metastasis in prostate cancer has been studied in the context of PSA recurrence after a prostatectomy (Figure 2). When PSA increases in the serum after a prostatectomy, the increase might be due to tumor cells in the surgical site, LNs or distant metastases. Scattoni and colleagues have studied the sensitivity, specificity, PPV and NPV of 11C-choline to identify LN metastasis in patients with PSA recurrence (median PSA = 1.98 ng/ml) and negative biopsy in the surgical site to exclude local recurrence [30]. All patients had a 11C-choline PET–CT followed by pelvic lymphadenectomy and a pathological analysis of LNs. 30% of these patients had positive LN at final pathology and 42% of LN metastases were <1 cm. 11C-choline PET–CT had a sensitivity, specificity, PPV and NPV of 64, 90, 86 and 72% for node metastasis when analysed node by node after resection. However, when the data were analysed patient by patient, the PET–CT could detect metastasis with a sensitivity of 90% (19/21 N+ status) and a negative predictive value of 100%. These excellent results for 11C-choline to detect LN metastasis were supported by another study in which sensitivity and specificity of 11C-choline was 80% and 96% [31]. However, one limitation with the use of 11C-choline as a tracer is its short half-life, which requires a cyclotron to be close to the hospital. To avoid this limitation, 18F-choline PET–CT has been used to image positive prostate cancer LNs but because no systematic histological confirmation of postulated metastases was performed in these studies, the accuracy of 18F-choline detection is unknown [32,33].
Other tracers such as 11C-acetate or 18F-acetate reflect another aspect of lipid metabolism, which is increased in tumor cells. 11C-acetate has been shown to be better than 18F-FDG to visualize possible LN metastasis and has a putative sensitivity of 75% [34]. Other studies using 11C-acetate have also reported putative positive LNs for prostate cancer [35-38]. However, no details regarding node size or histological proof of metastasis were systematically provided in these studies, rendering the interpretation of the data difficult [39]. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (anti-18F-FACBC) is a synthetic L-leucine analog that is taken up via the sodium-independent large neutral amino acid transport system (LAT). In a heterogeneous population of patients with prostate cancers, anti-18F-FACBC was shown to be a good tracer to detect prostate cancer LN metastasis. Pelvic nodal status correlated with anti-18F-FACBC findings in seven of nine patients and was indeterminate in two of nine [40]. Other positron-emitting molecules such as 16β-(18)F-fluoro-5α-dihydrotestosterone (18F-FDHT) and 11C-methionine are in development and could potentially be used to detect LN metastasis [41,42].
Taking these data together, PET–CT is certainly the most clinically advanced imaging technology enabling correlation of LN size and tracer-specific signaling, which reflects tumor-specific metabolism or gene expression. Moreover, its accuracy can be constantly improved with the design of new tracers. However, the ability of PET–CT to diagnose LN micrometastasis accurately is uncertain.
Antibody detection of LN metastasis
Besides having an increased metabolism, tumor cells also express tumor-specific antigens. By directing a radioactive-labeled antibody against one tumor-specific antigen, it can be possible to detect tumor cells by planar single-photon emission computed tomographic (SPECT) imaging. It is the principle behind Capromab Pendetide imaging of prostate cancer cells [43]. 111-Indium-Capromab Pendetide (7E11 or ProstaScint) is a radioactively-labeled monoclonal antibody that is directed against the intracellular portion of the prostate-specific membrane antigen (PSMA or glutamate carboxypeptidase 2) protein, a transmembrane peptidase. In a prospective, randomized, controlled trial, Polascik and colleagues analysed the precision of 111-Indium-Capromab Pendetide to detect LN metastasis [44]. Patients selected for this study had >20% chance of LN metastasis, as assessed by clinical predictive tables based on clinical tumor stage, pre-operative PSA and Gleason score at biopsy (Box 1). All patients underwent pelvic LN dissection after planar single photon emission computed tomographic (SPECT) imaging was performed. Capromab Pendetide detected pathological LNs in 62% of patients. Globally, the Capromab Pendetide imaging had a sensitivity of 67%, a specificity of 80%, a PPV of 75% and an NPV of 73%. When compared with the clinical algorithm, Capromab Pendetide was more precise in predicting LN metastasis with an Area Under the Curve (AUC) by Receiver Operator Curve (ROC) analysis of 0.71 compared with 0.52–0.61 for clinical algorithms. Moreover, when combined with clinical algorithms, Capromab Pendetide could predict LN metastasis with an AUC of 0.77. However, this study was limited by an average PSA of 57 ng/ml, which is too high to translate the precision of this molecular tool in clinical practice where most patients at prostate cancer diagnosis have PSA levels of <10 ng/ml. Moreover, the specificity of Prostascint to detect prostate cancer is not perfect (as for many molecules presented earlier) because PSMA has also been reported to be positive in melanoma, renal cell carcinoma, myelolipoma and lymphoma [45]. One problem with the Capromab antibody is that it targets the intracellular domain of PSMA and cell membrane disruption by necrosis is necessary for binding of the antibody. Recently, an antibody targeting the extracellular portion of PSMA has been developed: the J591 (MLN591) antibody [46]. In a single study, J591 antibody labeled with 111-Indium was administered to prostate cancer patients and imaged with a SPECT camera [47]. Five soft tissue lesions were detected by either CT-scan or SPECT. Of these, three lesions were suspected to be LN metastasis on SPECT; however, none was confirmed after six months of follow-up. Hence, the potential of the J591 antibody to image LN metastasis needs further investigation.
Clinical trials have shown that radioactively labelled antibodies can be used for molecular imaging. However, their use has been limited in LN imaging owing to problems with specificity and the inability to detect cancer cells at a threshold that would change clinical management. New generations of antibodies such as minibodies might solve this problem [48].
Pre-clinical small animal imaging technologies
Current clinical molecular tools to detect LN metastasis in prostate cancer are based on the ability of tumor cells to disrupt the normal anatomy of a node (e.g. using MRL or CT-scan), express a specific antigen at levels high enough to be detected by an antibody (e.g. using Prostascint) or by their ability to differentially metabolize a tracer when compared with a LN without cancerous cells (e.g. using PET scan). Therefore, the detection of LN metastasis by molecular imaging is dependent on a specific tumor burden or cancer cell gene expression high enough to discriminate from background levels.
To sharpen the molecular imaging tools to achieve specific detection of metastasis, it is crucial to refine clinically applicable imaging modalities in relevant pre-clinical tumor metastasis models. As noted in many clinical studies, PET and PET–CT are particularly amenable for reporting molecular information in vivo. Hence, in assessing the ability of PET–CT to detect prostate cancer LN metastasis, the F18-fluoro-L-thymidine (FLT) tracer was used to monitor cellular proliferation in a preclinical treatment model [49] (Figure 3). Although the regrowth of primary tumor after resection and the dissemination in the LN can be clearly visualized in the PET–CT scans, the sensitivity of this FLT metabolic tracer for prostate cancer is low in this pre-clinical setting, reflecting similar results with FDG–PET in the clinical scenarios of prostate cancer noted previously. Hence, to increase the accuracy of molecular imaging techniques, we have developed methods to amplify a detectable signal, to specifically deliver the imaging reporter gene expressed under a tumor-specific promoter and to better differentiate non-specific benign signals from tumor-specific signals. In the past ten years, our laboratory has developed molecular tools to increase accuracy of imaging, including the two-step transcriptional amplification (TSTA) system, which is discussed later and which has been reviewed in Ref. [50].
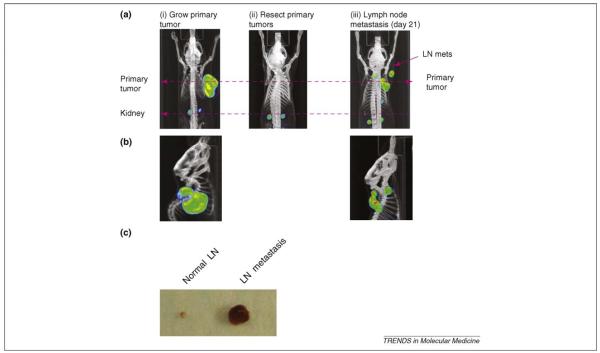
Figure 3.
Monitoring prostate cancer LN metastasis using 18FLT PET–CT imaging. (a,b) LAPC9 prostate tumor cells were grown as xenografts and imaged with PET–CT after intravenous injection of mice with the radioactive tracer 18FLT. 18FLT is phosphorylated by endogenous thymidine kinase and this reflects cell proliferation. When tumors were resected and mice were imaged again, no signal was detected. 21 days after tumor resection, the mice were imaged again and showed local recurrence and LN metastasis. (a) Coronal view. (b) Sagittal view. (c) The discrepancy in size of LN between LN metastasis and normal nodes illustrates the low sensitivity of FLT PET-scan imaging. Image adapted, with permission, from Ref. [49].
Increasing the sensitivity and specificity of molecular imaging
As mentioned earlier, most imaging techniques are based on the expression of an endogenous protein that is restricted to the cancer of interest. The imaging agent is a molecule that specifically interacts with the endogenous protein. For instance, agents developed based on this strategy to detect prostate cancer include the antibody against PSMA and the compound F18–DHT described earlier. A second strategy to achieve a distinct imaging signal based on the molecular signature of the cancerous tissue is to use cell-specific transcription to control the expression of exogenous imaging reporter genes. For example, the expression of the prostate-specific antigen (PSA) gene is known to be active in prostate cancer cells but it is inactive in non-prostate cells such as those constituting normal LNs [49]. To increase the specificity and potency of the PSA promoter, our group has created a chimeric PSA promoter (designated as PSE-BC) that shows 20-fold increased activity over the native PSA promoter in prostate tumor cells while maintaining its androgen responsiveness [51] and its specificity to detect systemic metastases [52]. Despite having stronger activity than the PSA promoter, the PSE–BC promoter still only exhibited approximately 5% of the activity of the strong constitutive cytomegalovirus (CMV) promoter, which limited its use for in vivo imaging [53]. To further increase the potency of the PSA promoter to express sufficient levels of an imaging reporter gene or a therapeutic gene, a TSTA was created [53] (Figure 4a). This system is based on the ability of the Gal4VP16 protein to strongly activate transcription through the VP16 transactivation domain while directing the transcriptional activity to a specific reporter through the Gal4 DNA-binding domain (Gal4DBD). When Gal4-responsive elements (Gal4RE) are placed in a promoter upstream of a reporter gene that can be detected by imaging, Gal4VP16 binds to Gal4RE to strongly transactivate the promoter. The specificity of the TSTA system for tumor cells is obtained by placing the Gal4VP16 expression under the PSE–BC. This PSA–TSTA system has been incorporated into a replication-deficient type 5 adenovirus genome to enable transduction into mammalian cells and testing of transcriptional-targeted gene imaging and gene therapy in living animals (see later).
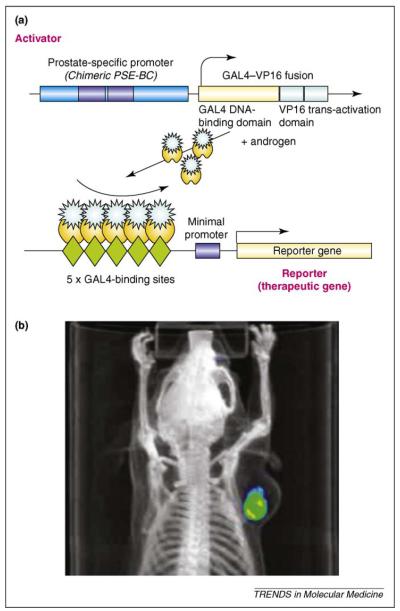
Figure 4.
Schematic description of molecular systems used to increase the sensitivity and specificity of molecular imaging. (a) The PSA–TSTA reporter system. The PSA promoter is a promoter that is transcriptionally active specifically in prostate cells. A PSA-modified chimeric promoter (‘PSE-BC’) can regulate the expression of the transcription factor Gal4VP16 only in cells that can activate the PSA promoter. Gal4VP16 is therefore expressed only in cells that can activate the PSE-BC promoter. When Gal4VP16 is expressed, it binds to Gal4 DNA-binding sites in the same adenovirus genome through the Gal4 DNA-binding domain. By placing the Gal4 DNA-binding sites upstream of a reporter gene (encoding the thymidine kinase sr39tk), the VP16 transactivation domain then strongly amplifies the original PSE-BC signal. (b) In vivo imaging of prostate cancer cells by the PSA–TSTA–sr39tk adenovirus system by PET–CT. When the PSA–TSTA reporter system is integrated into an adenovirus genome, it can be delivered to different cell types (mouse and human cells have been studied) according to the infection capabilities of the adenovirus. This system has been incorporated into adenovirus type 5 using the HSV1-sr39tk enzyme as a reporter gene. HSV1-sr39tk can phosphorylate the radioactive tracer 18F-FHBG and its specific expression can be detected by PET–CT. The PSA–TSTA-sr39tk adenovirus was injected into LAPC9 tumor cells and imaged by PET–CT [56].
Delivery systems to LNs
Adenoviruses are DNA viruses that can be isolated from many species including humans. Among the >30 serotypes of human adenovirus, serotype 5 is one of the most common and is efficient at infecting many cell types, including cancerous cells. Using recombinant DNA technology, it is now straightforward to create recombinant adenovirus as a gene-delivery vector expressing a therapeutic gene for studies aimed at treating the transfected cancer cells. As noted earlier, two strategies to refine such gene therapy approaches have been undertaken for targeting prostate cancer. First, the PSA–TSTA promoter was incorporated into adenovirus expressing the gene(s) of interest (Ad–PSA–TSTA). Second, an imaging reporter gene was incorporated into the prostate-specific adenoviral vector. This enables molecular imaging technology to monitor the gene therapy process in the treated subjects. Adenovirus possesses properties that make it a favorable vector to deliver genes into nodal metastasis – it has a diameter of ~100 nanometers and is negatively charged. These characteristics help its uptake into and migration through the lymphatic vessels and to the nodes. Once in the node, the PSA–TSTA adenovirus can infect prostate tumor cells present there and activate the prostate-restricted expression of an imaging reporter gene, which enables the detection of LN metastases. Using this method, adenovirus was shown to efficiently image LN metastasis in a mouse model [49]. By implanting a LAPC9 prostate tumor xenograft, expressing high levels of pro-lymphangiogenic growth factor VEGF-C in mice, LN metastasis to brachial and axillary nodes was induced. To interrogate these axillary LNs, an adenovirus that expresses an imaging reporter gene under the control of the prostate specific PSA–TSTA system was injected into mouse forepaws. This enabled imaging of prostate cancer LN metastasis by both bioluminescence imaging and PET–CT scan [49]. Therefore, by using an amplification system, the issue of low transcriptional activity of most tumor-specific promoters can be overcome to enable imaging of cancer cells in vivo. Furthermore, imaging of tumor cells is possible even if the reporter gene is not delivered to every cancer cell.
Molecular reporter genes
Once a delivery system, a specific promoter and an amplification system are developed, it is important to use a reporter gene that is suitable for imaging the metastasis in humans. The ideal reporter gene characteristics have been described recently by Min and Gambhir [54]. The gene has to be specific, non-immunogenic and non-toxic for normal cells and its expression should be detected by a probe that is specific for the expression of the reporter, not toxic and that can reach the tissue of interest. The different reporter genes that are available have been reviewed [54,55]. One of these promising reporters is the modified form of the herpes simplex type 1 thymidine kinase gene (HSV1-tk): HSV1-sr39tk. The protein sr39tk is a mutated form of the HSV-tk gene that can phosphorylate more than ten different substrates including 18F-FHBG. When 18F-FHBG is phosphorylated by sr39tk protein, the product is trapped in the cells and can be detected by PET–CT through its 18F positron-emitting isotope. Moreover, HSV1-sr39tk can also be cytotoxic to cells in which it is expressed when cells are exposed to pharmacological doses of the pro-drug gancyclovir. Indeed, using adenovirus expressing sr39tk, it has been possible to image and treat prostate cancer cells in vivo [56] (Figures 4b and 5). Moreover, by using an adenovirus expressing sr39tk, prostate cancer LN metastasis has been detected in vivo [49] (Figure 5).
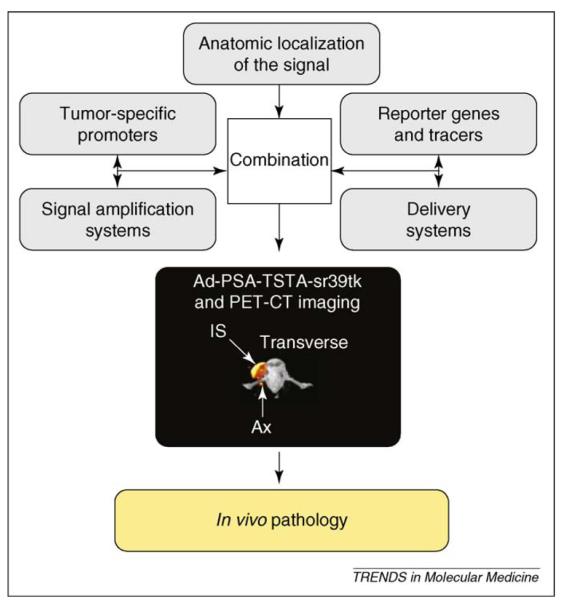
Figure 5.
In vivo pathology. In vivo pathology is the concept of combining molecular and non-molecular imaging tools to ascertain a pathological diagnosis based solely on imaging rather than invasive procedures such as biopsies. By using the combination of a specific promoter (modified chimeric PSA promoter [PSE-BC]), an amplification system (TSTA system), a reporter (gene encoding the thymidine kinase sr39tk), a tracer (18F-FHBG), a gene-delivery system to the nodes (adenovirus type 5) and an anatomic location of the signal (PET–CT), prostate cancer cells were imaged in LNs with high sensitivity and specificity. This is an example of the concept of in vivo pathology, which could be applied to any type of cancer for which there is a tumor-specific gene that is transcriptionally overexpressed in cancer cells and not in expressed in normal nodes.
Molecular imaging of LN metastasis using adenoviruses is still early in its development. Therefore, this technology might encounter the same problems that have previously arisen using adenovirus-mediated gene therapy approaches, such as incomplete gene delivery and the host immune reaction against infection. In fact, we have reported that only 0.1% of adenoviruses are delivered to the lymph nodes after paw injection. Therefore, it will be important to compare the sensitivity of this technology with the current tracers that can be imaged by PET-CT. However, amplification systems such as the TSTA system might overcome gene delivery problems owing to their abilities to lower the number of cells needed to be infected to reach the PET–CT detection threshold.
The concept of in vivo pathology
Diagnosis of tumors and/or metastases has classically been based on tissue extraction by biopsy or surgery followed by a histologic analysis by a pathologist. When tumor histology does not provide a differential diagnosis, the pathologist has to make the diagnosis based on tissue-specific protein expression, usually by immunohistochemistry. Indeed, the final diagnosis is made after the pathologist considers the location of the biopsy, the proteins expressed in the tissue and the previous pathology and clinical information of the patient to confirm the tissue of origin for a metastasis. Therefore, in the context of a metastasis, the diagnosis is based mainly on tumor-specific gene expression. By using a PSA–TSTA-sr39tk adenovirus and 18F-FHBG PET–CT, it is possible to image cells that are transcriptionally active via the PSA promoter in the draining or sentinel LN in vivo. In the context of a diagnosed primary prostate cancer after prostate biopsy, a PSA-promoter-dependent positive signal in an ectopic site such as the LN would be highly indicative of the presence of prostate cancer cells at that site. By combining the advances made in: (i) gene delivery using adenovirus; (ii) specificity of the promoter by chimeric construction; (iii) the sensitivity of molecular imaging using amplification systems; and (iv) the ability to correlate a positive signal by PET to an anatomic structure such as a node, we propose that the direct visualization of in vivo pathology is now possible (Figure 5). This technology could be applied to other cancer types because specific histological markers (or combinations) are known for many cancers. By changing the cell- or tumor-specific promoter controlling the TSTA-reporter system, it is theoretically possible to image in vivo pathology without biopsy for any type of cancer for which a specific transcriptionally regulated gene is known. Besides prostate cancer, the feasibility for wider application of this direct method for visualization of tumor lesions in vivo has been demonstrated in breast cancer models using the promoter for the gene encoding mucin 1 and in colon cancer using the promoter for the gene encoding survivin [57,58].
Another useful application of an in vivo pathology approach is to interrogate the real-time activity of an oncogenic pathway in each patient’s tumor or metastasis to provide a tumor-specific therapy. For instance, the androgen receptor (AR) has a crucial role in prostate oncogenesis and cancer progression and regulates the expression of PSA. Exploiting the tight AR regulation of PSA transcription, it has been possible to employ the Ad–PSA–TSTA system to image the transcriptional activity of the androgen receptor in growing tumors in mice [59]. Indeed, PET and bioluminescence signals produced by Ad-PSA-TSTA were able to reflect the functional status of the endogenous androgen receptor and the emergence of an androgen-independent state or the response to androgen-deprivation therapy in vivo [60,61]. However, this model would have limited clinical application for prostate cancer because the levels of serum PSA can also indicate the response to therapy in prostate cancer. Because there is not a serum marker for every cancer and the real-time readout of functional activity at the pathological site (i.e. the visualization of in vivo pathology) could guide clinicians to the most appropriate treatment according to the response in each patient’s cancer.
Concluding remarks
The challenge of molecular imaging is now to increase the accuracy of current clinical imaging techniques. The ability to image a tumor is a major step but the importance of new imaging technologies will be judged on their ability to modify and improve the management of cancers. Improvements can be provided by their ability to detect LN or systemic metastasis earlier in the course of the disease, to predict response to treatments earlier or to detect recurrence when a recurrence-directed treatment is still possible. Presently, management in oncology is based mainly on a pathology report and the gross anatomical staging using a CT-scan, a bone scan and/or an MRI. By increasing the detection accuracy of tumor cells by molecular imaging, it is possible to increase the accuracy of clinical algorithms and better personalize treatments for each patient, limiting the uncertainties of current algorithms. Prospective studies designed to enable imaging and histopathological correlations and, more importantly, to evaluate the impact of imaging on disease-specific and overall survival are needed.
Acknowledgements
We thank NCI (RO1 CA101904 and R21 CA122693) and Prostate Cancer Foundation for their support to Lily Wu. Frédéric Pouliot is a urologic oncology fellow at UCLA, supported in part by the McLaughlin and the Conseil des Médecins Dentistes et Pharmaciens fellowship scholarships, both from Laval University, the Québec Urological Association and the Canadian Institutes of Health Research fellowship scholarships. Mai Johnson is supported by the UCLA Tumor Biology Program (USHHS Ruth L. Kirschstein Institutional National Research Service Award # T32 CA009056).
Footnotes
MRI images can be analysed in different phases. The main ones are T1 and T2.
References
- 1.Briganti A, et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur. Urol. doi: 10.1016/j.eururo.2008.09.043. (in press) [DOI] [PubMed] [Google Scholar]
- 2.D’Amico AV, et al. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J. Clin. Oncol. 2003;21:2163–2172. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 3.Shariat SF, et al. Inventory of prostate cancer predictive tools. Curr. Opin. Urol. 2008;18:279–296. doi: 10.1097/MOU.0b013e3282f9b3e5. [DOI] [PubMed] [Google Scholar]
- 4.Bader P, et al. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J. Urol. 2003;169:849–854. doi: 10.1097/01.ju.0000049032.38743.c7. [DOI] [PubMed] [Google Scholar]
- 5.Daneshmand S, et al. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J. Urol. 2004;172:2252–2255. doi: 10.1097/01.ju.0000143448.04161.cc. [DOI] [PubMed] [Google Scholar]
- 6.Heesakkers RA, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008;9:850–856. doi: 10.1016/S1470-2045(08)70203-1. [DOI] [PubMed] [Google Scholar]
- 7.Grubnic S, et al. MR evaluation of normal retroperitoneal and pelvic lymph nodes. Clin. Radiol. 2002;57:193–200. doi: 10.1053/crad.2001.0893. [DOI] [PubMed] [Google Scholar]
- 8.Cserni G. Metastases in axillary sentinel lymph nodes in breast cancer as detected by intensive histopathological work up. J. Clin. Pathol. 1999;52:922–924. doi: 10.1136/jcp.52.12.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harisinghani MG, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 10.Burkhard FC, et al. The role of lymphadenectomy in prostate cancer. Nat. Clin. Pract. Urol. 2005;2:336–342. doi: 10.1038/ncpuro0245. [DOI] [PubMed] [Google Scholar]
- 11.Schoder H, et al. Molecular targeting of the lymphovascular system for imaging and therapy. Cancer Metastasis Rev. 2006;25:185–201. doi: 10.1007/s10555-006-8498-0. [DOI] [PubMed] [Google Scholar]
- 12.Al-Sarraf N, et al. Mediastinal lymph node staging by means of positron emission tomography is less sensitive in elderly patients with non-small-cell lung cancer. Clin. Lung Cancer. 2008;9:39–43. doi: 10.3816/CLC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- 13.Birim O, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann. Thorac. Surg. 2005;79:375–382. doi: 10.1016/j.athoracsur.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Crippa F, et al. FDG-PET for axillary lymph node staging in primary breast cancer. Eur. J. Nucl. Med. Mol. Imaging. 2004;31(Suppl 1):S97–S102. doi: 10.1007/s00259-004-1531-z. [DOI] [PubMed] [Google Scholar]
- 15.Jereczek-Fossa BA, et al. Cervical lymph node metastases of squamous cell carcinoma from an unknown primary. Cancer Treat. Rev. 2004;30:153–164. doi: 10.1016/j.ctrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Kramer H, Groen HJ. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann. Surg. 2003;238:180–188. doi: 10.1097/01.SLA.0000081086.37779.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller-Lisse UG, et al. Functional imaging in penile cancer: PET/computed tomography, MRI, and sentinel lymph node biopsy. Curr. Opin. Urol. 2008;18:105–110. doi: 10.1097/MOU.0b013e3282f151fd. [DOI] [PubMed] [Google Scholar]
- 18.Prichard RS, et al. Positron emission tomography for staging and management of malignant melanoma. Br. J. Surg. 2002;89:389–396. doi: 10.1046/j.0007-1323.2002.02059.x. [DOI] [PubMed] [Google Scholar]
- 19.Rice TW. Clinical staging of esophageal carcinoma. CT, EUS, and PET. Chest Surg. Clin. N. Am. 2000;10:471–485. [PubMed] [Google Scholar]
- 20.Strauss LG. Sensitivity and specificity of positron emission tomography (PET) for the diagnosis of lymph node metastases. Recent Results Cancer Res. 2000;157:12–19. doi: 10.1007/978-3-642-57151-0_2. [DOI] [PubMed] [Google Scholar]
- 21.Vansteenkiste JF. Imaging in lung cancer: positron emission tomography scan. Eur. Respir. J. Suppl. 2002;35:49s–60s. doi: 10.1183/09031936.02.00252402. [DOI] [PubMed] [Google Scholar]
- 22.Wagner JD, et al. FDG-PET sensitivity for melanoma lymph node metastases is dependent on tumor volume. J. Surg. Oncol. 2001;77:237–242. doi: 10.1002/jso.1102. [DOI] [PubMed] [Google Scholar]
- 23.Antoch G, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. J. Am. Med. Assoc. 2003;290:3199–3206. doi: 10.1001/jama.290.24.3199. [DOI] [PubMed] [Google Scholar]
- 24.Machtens S, et al. Positron emission tomography (PET) in the urooncological evaluation of the small pelvis. World J. Urol. 2007;25:341–349. doi: 10.1007/s00345-007-0194-3. [DOI] [PubMed] [Google Scholar]
- 25.Liu IJ, et al. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology. 2001;57:108–111. doi: 10.1016/s0090-4295(00)00896-7. [DOI] [PubMed] [Google Scholar]
- 26.Oyama N, et al. The increased accumulation of [18F]fluorodeoxyglucose in untreated prostate cancer. Jpn. J. Clin. Oncol. 1999;29:623–629. doi: 10.1093/jjco/29.12.623. [DOI] [PubMed] [Google Scholar]
- 27.Seltzer MA, et al. Comparison of helical computerized tomography, positron emission tomography and monoclonal antibody scans for evaluation of lymph node metastases in patients with prostate specific antigen relapse after treatment for localized prostate cancer. J. Urol. 1999;162:1322–1328. [PubMed] [Google Scholar]
- 28.Sanz G, et al. Positron emission tomography with 18fluorinelabelled deoxyglucose: utility in localized and advanced prostate cancer. BJU Int. 1999;84:1028–1031. doi: 10.1046/j.1464-410x.1999.00349.x. [DOI] [PubMed] [Google Scholar]
- 29.Sung J, et al. Fluorodeoxyglucose positron emission tomography studies in the diagnosis and staging of clinically advanced prostate cancer. BJU Int. 2003;92:24–27. doi: 10.1046/j.1464-410x.2003.04297.x. [DOI] [PubMed] [Google Scholar]
- 30.Scattoni V, et al. Detection of lymph-node metastases with integrated [11C]choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: results confirmed by open pelvicretroperitoneal lymphadenectomy. Eur. Urol. 2007;52:423–429. doi: 10.1016/j.eururo.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 31.de Jong IJ, et al. Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J. Nucl. Med. 2003;44:331–335. [PubMed] [Google Scholar]
- 32.Husarik DB, et al. Evaluation of [(18)F]-choline PET/CT for staging and restaging of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:253–263. doi: 10.1007/s00259-007-0552-9. [DOI] [PubMed] [Google Scholar]
- 33.Heinisch M, et al. Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA < 5 ng/ml? Mol. Imaging Biol. 2006;8:43–48. doi: 10.1007/s11307-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 34.Fricke E, et al. Positron emission tomography with 11C-acetate and 18F-FDG in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:607–611. doi: 10.1007/s00259-002-1104-y. [DOI] [PubMed] [Google Scholar]
- 35.Oyama N, et al. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J. Nucl. Med. 2003;44:549–555. [PubMed] [Google Scholar]
- 36.Kotzerke J, et al. Carbon-11 acetate positron emission tomography can detect local recurrence of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:1380–1384. doi: 10.1007/s00259-002-0882-6. [DOI] [PubMed] [Google Scholar]
- 37.Sandblom G, et al. Positron emission tomography with C11-acetate for tumor detection and localization in patients with prostate-specific antigen relapse after radical prostatectomy. Urology. 2006;67:996–1000. doi: 10.1016/j.urology.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 38.Albrecht S, et al. (11)C-acetate PET in the early evaluation of prostate cancer recurrence. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:185–196. doi: 10.1007/s00259-006-0163-x. [DOI] [PubMed] [Google Scholar]
- 39.Morris MJ, Scher HI. (11)C-acetate PET imaging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:181–184. doi: 10.1007/s00259-006-0281-5. [DOI] [PubMed] [Google Scholar]
- 40.Schuster DM, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J. Nucl. Med. 2007;48:56–63. [PubMed] [Google Scholar]
- 41.Bouchelouche K, Oehr P. Positron emission tomography and positron emission tomography/computerized tomography of urological malignancies: an update review. J. Urol. 2008;179:34–45. doi: 10.1016/j.juro.2007.08.176. [DOI] [PubMed] [Google Scholar]
- 42.Larson SM, Schoder H. Advances in positron emission tomography applications for urologic cancers. Curr. Opin. Urol. 2008;18:65–70. doi: 10.1097/MOU.0b013e3282f19cde. [DOI] [PubMed] [Google Scholar]
- 43.Haseman MK, et al. Capromab Pendetide imaging of prostate cancer. Cancer Biother. Radiopharm. 2000;15:131–140. doi: 10.1089/cbr.2000.15.131. [DOI] [PubMed] [Google Scholar]
- 44.Polascik TJ, et al. Comparison of clinical staging algorithms and 111indium-capromab pendetide immunoscintigraphy in the prediction of lymph node involvement in high risk prostate carcinoma patients. Cancer. 1999;85:1586–1592. [PubMed] [Google Scholar]
- 45.Zanzi I, Stark R. Detection of a non-Hodgkin’s lymphoma by capromab pendetide scintigraphy (ProstaScint) in a patient with prostate carcinoma. Urology. 2002;60:514. doi: 10.1016/s0090-4295(02)01835-6. [DOI] [PubMed] [Google Scholar]
- 46.Chang SS, et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 47.Pandit-Taskar N, et al. Antibody mass escalation study in patients with castration-resistant prostate cancer using 111In-J591: lesion detectability and dosimetric projections for 90Y radioimmunotherapy. J. Nucl. Med. 2008;49:1066–1074. doi: 10.2967/jnumed.107.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leyton JV, et al. Humanized radioiodinated minibody for imaging of prostate stem cell antigen-expressing tumors. Clin. Cancer Res. 2008;14:7488–7496. doi: 10.1158/1078-0432.CCR-07-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burton JB, et al. Adenovirus-mediated gene expression imaging to directly detect sentinel lymph node metastasis of prostate cancer. Nat. Med. 2008;14:882–888. doi: 10.1038/nm.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu L, Sato M. Integrated, molecular engineering approaches to develop prostate cancer gene therapy. Curr. Gene Ther. 2003;3:452–467. doi: 10.2174/1566523034578230. [DOI] [PubMed] [Google Scholar]
- 51.Wu L, et al. Chimeric PSA enhancers exhibit augmented activity in prostate cancer gene therapy vectors. Gene Ther. 2001;8:1416–1426. doi: 10.1038/sj.gt.3301549. [DOI] [PubMed] [Google Scholar]
- 52.Adams JY, et al. Visualization of advanced human prostate cancer lesions in living mice by a targeted gene transfer vector and optical imaging. Nat. Med. 2002;8:891–897. doi: 10.1038/nm743. [DOI] [PubMed] [Google Scholar]
- 53.Iyer M, et al. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14595–14600. doi: 10.1073/pnas.251551098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min JJ, Gambhir SS. Molecular imaging of PET reporter gene expression. Handb Exp Pharmacol. 2008;185:277–303. doi: 10.1007/978-3-540-77496-9_12. [DOI] [PubMed] [Google Scholar]
- 55.Serganova I, et al. Human reporter genes: potential use in clinical studies. Nucl. Med. Biol. 2007;34:791–807. doi: 10.1016/j.nucmedbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Johnson M, et al. Micro-PET/CT monitoring of herpes thymidine kinase suicide gene therapy in a prostate cancer xenograft: the advantage of a cell-specific transcriptional targeting approach. Mol. Imaging. 2005;4:463–472. doi: 10.2310/7290.2005.05154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huyn S, et al. A potent imaging adenoviral vector driven by the cancer selective mucin-1 promoter that targets breast cancer metastasis. Clin. Cancer Res. doi: 10.1158/1078-0432.CCR-08-2666. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ray S, et al. Noninvasive imaging of therapeutic gene expression using a bidirectional transcriptional amplification strategy. Mol. Ther. 2008;16:1848–1856. doi: 10.1038/mt.2008.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato M, et al. Functionality of androgen receptor-based gene expression imaging in hormone refractory prostate cancer. Clin. Cancer Res. 2005;11:3743–3749. doi: 10.1158/1078-0432.CCR-04-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L, et al. Interrogating androgen receptor function in recurrent prostate cancer. Cancer Res. 2003;63:4552–4560. [PubMed] [Google Scholar]
- 61.Ilagan R, et al. Imaging mitogen-activated protein kinase function in xenograft models of prostate cancer. Cancer Res. 2006;66:10778–10785. doi: 10.1158/0008-5472.CAN-05-3577. [DOI] [PubMed] [Google Scholar]
- 62.Thompson I, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J. Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]