Summary
This chapter describes a method by which activating receptor-mediated calcium signaling can be measured in individual murine NK cells using a flow cytometer fitted with a UV laser. One major advantage of this method is that the calcium response of the minority NK cell population and even smaller NK cell sub-populations can be measured simultaneously from a mixture of freshly prepared total splenocytes without resorting to prior cell sorting or expansion in culture. Briefly, cells are harvested and stained to mark the populations of interest, then loaded with indo-1 AM dye and analyzed on the flow cytometer. After an appropriate baseline is established, the cells are treated with a biotinylated antibody to activating receptors, which are subsequently cross-linked by addition of streptavidin. The increase in intracellular calcium is quantified by measuring a shift in the indo-1 emission spectrum that takes place when the dye becomes bound to calcium.
Keywords: Calcium signaling, indo-1, flow cytometry, NK1.1
1. Introduction
Increased levels of cytosolic calcium are critical to numerous lymphocyte functions including proliferation, metabolism, apoptosis, migration, cytotoxicity, and the formation of an immunological synapse (1). This protocol builds on the work of Valittuti and Dessing, who previously described a methodology for measuring calcium signaling in individual cells within a T cell population in response to target cell engagement in this series (2). Here we expand upon the method to demonstrate NK cell calcium mobilization in response to soluble ligands and demonstrate how the relative responsiveness of various developmental sub-populations can be resolved. Cytosolic calcium concentrations are normally tightly regulated in resting cells. The increased intracellular calcium concentration in response to activating receptor engagement takes place in two stages. Calcium is initially released from the endoplasmic reticulum over a short period of time, followed by a sustained influx from the extracellular environment. The data collected is a convolution of these two processes, but since the early time points are dominated by release of intracellular calcium stores and the later time points by influx from the extracellular medium, it should be possible to see a specific effect on either of the two processes. In this method, the cells are first stained with antibodies to cell surface markers that enable the identification of NK cells and any subsets of interest, then loaded with indo-1 AM dye and stimulated through activating receptors. Calcium flux is initiated by cross-linking activating receptors that are first engaged by a biotinylated antibody and subsequently clustered together by addition of streptavidin. Changes in the intracellular calcium concentration are quantified by a shift in the indo-1 emission peak from 485 nm (indo-blue) for unbound dye to 405 nm (indo-violet) when the indo-1 molecule is bound to calcium. Mean intracellular calcium concentration is quantified in terms of the ratio of 405 nm/485 nm indo-1 emission peaks. Measuring the change in emission ratio allows comparisons between individual cells within the population that may not be loaded with equivalent amounts of indo-1 dye.
2. Materials
Erythrocyte lysis buffer: 125 mM NH4Cl, 10 mM KHCO3, 1mM Na2EDTA.
Serum free RPMI-1640 medium (Life Technologies, Rockville, MD).
Complete RPMI medium: RPMI-1640 medium, 10% FBS (Hyclone), 100 μg/ml penicillin/streptomycin, 2 mM L-glutamine, 10 mM HEPES, pH 7.4, 1 mM MEM sodium pyruvate, and 50 μM 2-mercaptoethanol (all from Life Technologies).
Cell permeant indo-1 AM dye (Invitrogen, Eugene OR): prepare a 2.5 mM stock in DMSO. Store protected from light at −20°C (see Note 1).
Pluronic F-127 20% solution in DMSO (Invitrogen)
Conjugated monoclonal antibodies: anti-CD3ε-PerCP/Cy5.5, anti- anti-CD122-FITC [or anti-CD49b-FITC (DX5)], anti-CD11b-APC/Cy7, and biotinylated anti-NK1.1 (PK136) (all from BioLegend, San Diego, CA)
Propidium iodide 1 mg/ml (Invitrogen) (see Note 2).
Purified streptavidin (Sigma)
40 μm cell strainer (Falcon, BD Biosciences)
10 ml syringe
Flow cytometer: Our studies used a BD FacsVantage SE flow cytometer with the FACSDiVa Option fitted with a Coherent Innova Model 302C Krypton Laser. The laser is operated in multiline UV mode with emission lines ranging from 337.5 nm to 356.4 nm and a Chroma 440 DCLP splitter/indo-1 filter set measuring emission peaks at 405 nm and 485 nm.
FlowJo software, version 8.7.1 (TreeStar) and appropriate computer
3. Methods
3.1 Cell preparation
Prepare single cell suspensions of mouse splenocytes by mashing spleens through a 40μm nylon cell strainer with a rubber-tipped 10 ml syringe plunger and rinse the cell strainer with a total of 10 ml of complete RPMI culture medium.
Spin the cells at 500 g for 5 minutes and re-suspend in ice cold erythrocyte lysis buffer. Incubate for 3 minutes on ice, then pellet cells at 500 g for 5 minutes.
Immediately re-suspend in 10 ml of cold complete RPMI medium and count.
Spin down the cells and re-suspend at a dilution of 20 million cells in 1 ml of complete RPMI1640
Add appropriate staining titer of each of the fluorochrome-conjugated antibodies and incubate on ice for 20 minutes. Single color compensation controls and an unstained control should be prepared at this time as well. (see Notes 3 and 4).
While the cells are being stained, add 1 μl of 20% Pluronic F-127 solution to every 9 μl of indo-1 AM stock solution needed and warm at 37°C for 5–10 minutes in a shaking heat block to ensure that it is mixed properly (see Note 1). Protect from light during all preparation procedures.
Rinse cells twice with serum-free RPMI-1640 medium and re-suspend in 4 ml of serum-free RPMI-1640 medium that has been pre-warmed to 37°C and place in a 37°C water bath. Cell concentration should be about 5 million/ml.
Allow 10 minutes for the cells to warm to 37°C, then add 1–1.5 μl of the indo/Pluronic solution prepared in step 5 to each ml of cell suspension and mix thoroughly by inverting the tube several times. If comparing different cell populations, it is critical to assure uniform indo-1 loading between the different cell samples, so exactly the same conditions should be used for each sample and cells should be mixed thoroughly after addition of the dye.
Incubate the cells for 30 minutes at 37°C under foil to protect from light. Resuspend the cells every 10 minutes during this incubation. Then centrifuge the cells at 500 g for 5 minutes and re-suspend in 2 ml of complete RPMI-1640 medium. If propidium iodide is being used to exclude dead cells, then add at a final concentration of 200 ng/ml.
3.2 Flow Cytometry
Maintain labeled cells in the dark at room temperature (not on ice) at all times prior to analysis on the flow cytometer. Do not store labeled cells on ice, since this will diminish the intensity of subsequent calcium responses.
Prewarm a 500 μl cell sample of cell suspension in a 5 ml FACS tube at 37°C for 5 minutes prior to analysis on the flow cytometer.
Run the 500 μl sample through the flow cytometer at about 2000–2500 events/second for 1–2 minutes to establish the baseline indo-1 signal that represents the basal intracellular calcium concentration. The cell sample should be maintained at 37°C throughout the time course of analysis. Although calcium mobilization can be measured at room temperature, the response will be suboptimal as compared to analysis at 37°C.
Remove the sample and add 4 μg of biotinylated anti-NK1.1 (PK136) mAb and return the sample to the instrument (see Note 5).
Allow the cells to run for another minute to determine if the antibody stimulates the cells in the absence of streptavidin. We have not found this to be true for PK136, but it may occur when other receptors or antibodies are used.
Remove the sample once again, add 8 μg of streptavidin, and return the sample to the instrument to collect data for an additional 4–5 minutes (see note 6). An increase in indo violet (405 nm) signal and decrease in indo blue (485 nm) signal is indicative of a stimulation-induced increase in intracellular calcium concentration. If longer analysis is desired, an increased starting volume of cells (750–1000 μl) should be used and antibody/streptavidin concentrations should be adjusted accordingly.
3.3 Data analysis
We have used FlowJo software (version 8.7.1) to analyze our data. It is necessary to create a derived parameter to display the indo violet (405 nm) intensity divided by the indo blue (485 nm) intensity. This is accomplished by navigating through the following menu tabs: Platform -> Derive Parameters -> Define new or change. Here you can create the new parameter and give it a name. Make sure the “display with linear scale” box is checked and start with lower and upper axis limits of 0.4 and 1.2, respectively.
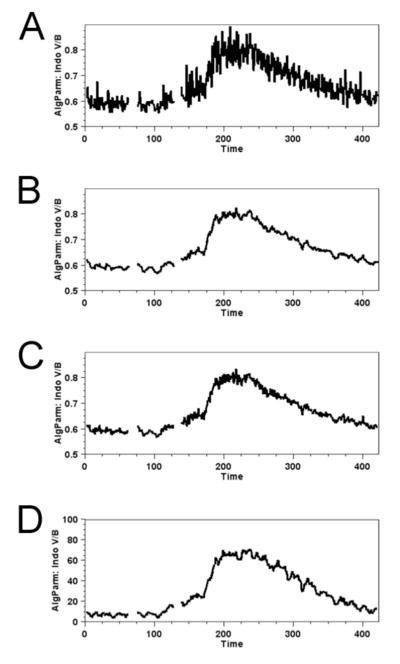
Once the parameter is created you can view the mean relative cytosolic calcium concentration within the gated population by highlighting the desired NK cell gate in the FlowJo work space and choosing Platform -> Kinetics from the menu tabs. In Figure 1A, we set the axis limits to 0.5 and 0.9, while choosing to display the mean fluorescent intensity acquired over the integration time of each data point. Since the actual signal is changing slowly compared with the fluctuations in the data, it is reasonable to increase the signal to noise ratio by averaging adjacent points. Figure 1B shows the same data with a moving average applied. This replaces each data point with the average value of the data point with several points on either side. The Gaussian smoothing shown in Figure 1C is a moving average that weights adjacent points more heavily than those that are further away. This results in less smoothing than the unweighted moving average, but is also less likely to obscure rapidly changing details or distort the actual rates of ascent and decline. Another approach is to plot the percent of cells with an indo-violet to indo-blue ratio above a baseline threshold value. This provides information about what fraction of a given population or sub-population is undergoing calcium flux. Figure 1D shows this with a threshold of 0.65 and moving average smoothing.
Figure 1.
Mean calcium flux data from NK cells stimulated with biotinylated anti-NK1.1 mAb (added at 60 sec) streptavidin (at 120 sec) are visualized by several smoothing methods. Plotting the ratio of mean indo violet/indo blue emission values at individual time points provides values corresponding to relative calcium concentration of cells within the population over the time course of the experiment. A. No smoothing. B. Moving average. C. Gaussian smoothing. D. Plot of indo violet (V)/indo blue (B) events that are above a threshold ratio value of 0.65. Time in seconds is shown in the x-axis.
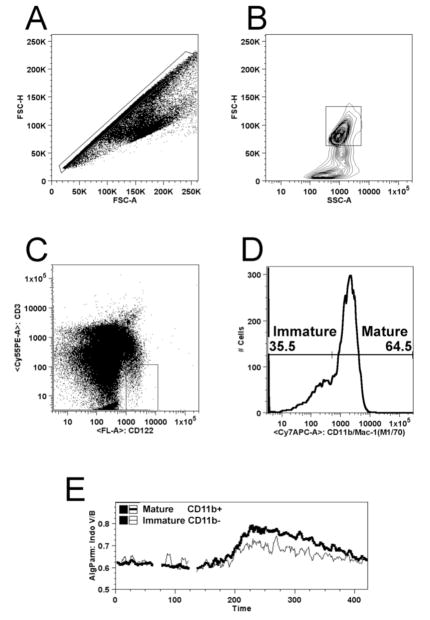
One of the important advantages of this method is the ability to distinguish populations and sub-populations of cells without sorting or other mechanical fractionation. Figure 2 shows how NK cells can be distinguished from other cell types and the different magnitudes of calcium flux within each gated sub-population. The first gate, shown in Figure 1A, is applied to a plot of forward scatter height versus forward scatter area. This is referred to as a singlet gate because it excludes instances where more than one cell is contained in a droplet that is analyzed by the cytometer. Figure 2B shows the lymphocyte gate, which excludes cell fragments, the majority of dead and dying cells, and large aggregates. As shown in Figure 2C, NK cells are gated as CD122+(or CD49b+ if substituted), CD3. Once the NK population has been selected, the cells can be further subdivided according to expression of CD11b into successive maturation stages that are shown in Figure 2D. A comparison of the kinetics of these two sub-populations shown in Figure 2E reveals greater functionality in the mature subset.
Figure 2.
Electronic gating to distinguish NK cells and sub-sets of NK cells from other lymphocytes and cellular debris. A. To assure a single cell analysis, events containing one cell are selected based on linear correlation between forward scatter area (FSC-A) and forward scatter height (FSC-H). B. Predominantly viable cells are distinguished from dead cells and small particles based on their forward scatter height and side scatter area (SSC). C. NK Cells are gated as CD3 (TCR)−, CD122+. D. Sub-populations of NK cells are defined by their expression levels of CD11b immature (CD11blow) and mature (CD11bhigh). E. Calcium flux measurements of the subsets defined in D. above with the thin line representing immature NK cells and the thicker line representing mature NK cells.
Acknowledgments
We would like to thank Dr. Richard (Randy) Hardy for assistance in setup of the flow cytometry equipment and for suggestions to improve the manuscript. Supported by National Institutes of Health grants R01-CA083859, R01-CA100226 (K.S.C.), T32-AI007492 (A.W.M.), and Centers of Research Excellence grant CA06927 (FCCC). The research was also supported in part by the FCCC Blood Cell Development and Cancer Keystone Program and an appropriation from the Commonwealth of Pennsylvania. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
The acetoxymethyl (AM) ester derivative of indo-1 is uncharged and can cross the plasma membrane into cells. Once inside the cells, esterases cleave the side chain to create a charged form of the parent indo-1 molecule that is retained in the cytoplasm. Pluronic F-127 is non-ionic detergent with low cytoxicity that aids solubility of indo-1AM in aqueous solutions for improved loading of cells.
Propidium iodide or 7AAD can be used to exclude dead cells from the analysis, but if the emission channel is needed for another antibody it is also possible to gate populations of cells that have greater than 98% viability by using the forward scatter and side scatter gates. This is possible because dead and dying cells tend to be smaller and more granular, so they exhibit less forward scatter and greater side scatter than viable cells.
Avoid staining with antibodies directed toward NK cell activating receptors that can be triggered by antibody engagement.
One should be cautious if considering the use of Cascade Blue, Alexafluor 405, or other fluorophores in the violet/blue emission range as gating antibodies, because their emission spectra may overlap with that of indo-1.
NK1.1 is a member of the NKR-P1 family of receptors and is only expressed on NK cells from certain mouse strains, such as C57Bl/6, but not 129 or Balb/c.
The balance of biotinylated antibody to streptavidin determines the degree of receptor aggregation, which is critical for the experiment to succeed. Biotinylated antibody and streptavidin concentrations should be titrated to determine the optimal concentrations whenever a new activating receptor is chosen to initiate the calcium flux. This may require significant effort to achieve the proper combination. If not enough streptavidin is used, then the receptors will be poorly cross-linked and fail to stimulate the cells. Using too much streptavidin can also result in poor cross-linking if each biotin moiety binds a single streptavidin molecule, instead of multiple biotin molecules binding to each streptavidin tetramer. Another option is to use fluorophore-conjugated streptavidin as a crosslinking agent to assess degree of crosslinking and surface levels of receptor being engaged. The conditions for biotinylated anti-NK1.1 and streptavidin have been optimized in our hands using this experimental design, in which the NK cells make up on average about 3% of the total splenocytes being analyzed.
References
- 1.Clapham DE. Calcium Signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Valitutti S, Dessing M. Measurement of Calcium Mobilization Responses in Killer Cell/Target Conjugates by FACS Analysis. Meth Mol Biol. 2000;121:305–311. doi: 10.1385/1-59259-044-6:305. [DOI] [PubMed] [Google Scholar]