Abstract
An overlap of breast-feeding and late pregnancy is associated with decreased intake of human milk and reduced infant growth. We evaluated the association of an overlap with macronutrient and immunological components of milk, infant urinary IgA, and infant and maternal morbidity. On d 2 and 1 mo postpartum, staff measured 24-h intake of breast milk and collected samples from 133 Peruvian women; 68 had breast-fed during the last trimester of pregnancy (BFP) and 65 had not breast-fed during pregnancy (NBFP). Data on maternal and infant anthropometry and health were collected for 1 mo. On d 2, lactose and lysozyme concentrations were higher, total lysozyme intake was higher and concentration and total intake of lactoferrin were lower in the BFP than the NBFP group (P < 0.05). The total 1-mo IgA intake was lower among BFP than NBFP infants (P = 0.01). Urinary IgA concentration was correlated with breast milk IgA concentration (r = 0.29; P = 0.01) but not with breast-feeding during pregnancy. An overlap was not associated with diarrhea but BFP infants were 5 times as likely to have a cough for at least 7 d than NBFP infants (P < 0.05). Reported mastitis was rare and occurred only in the NBFP group (P = 0.05). An overlap of breast-feeding and late pregnancy was associated with changes in milk composition, an increased frequency in symptoms of infant respiratory illness but decreased reported mastitis. Further in-depth studies are warranted to determine the cumulative effects associated with a breast-feeding/pregnancy overlap on infant and maternal outcomes.
Keywords: breast-feeding, pregnancy, breast milk composition, immunology, respiratory illness
Health professionals recommend human milk as the exclusive source of infant nutrition for the first 6 mo of life (1). In addition to meeting nutrient needs, human milk provides immunological factors that directly protect as well as stimulate the infant’s own immune system. The cumulative benefits of breast-feeding will depend on a child’s specific nutrient requirements, environmental and genetic conditions determining the risk of infection and the composition of the mother’s milk. Human milk composition varies dramatically by time postpartum (2). In addition, maternal factors such as diet, nutritional status and disease exposure may influence macronutrient and immunological components of human milk. The influence of some maternal factors, such as the practice of continuing to breast-feed during a new pregnancy, on human milk composition, however, is not well understood.
The effect of an overlap of the physiologic states of lactation and pregnancy on milk composition has been studied extensively in cows but not in humans. Dairy researchers reported milk composition effects of an overlap at least as early as 1916 when Eckles and Palmer (3) reported a reduction in immunoglobulins and total protein. In animals milked until parturition, total nitrogen decreased by 50% and noncasein nitrogen levels were at least one third that of cows that were not milked through pregnancy (4). The degree to which the composition changed was dependent on the length of time the cow was not milked (3). Similar research has not been available for human milk composition although breast-feeding during late pregnancy is practiced in many cultures (5–12).
The present analysis will examine the association between an overlap of breast-feeding and late pregnancy and the macronutrient and immunological factors in human milk at d 2 and 1 mo postpartum. On the basis of the dairy literature, we hypothesized that an overlap during the third trimester of pregnancy would be associated with a colostrum composition that was similar to mature milk in macronutrients (lower total protein; higher lactose and fat) and in immunological factors (lower IgA and lactoferrin, higher lysozyme). Finally, we hypothesized that a lower exposure to immunological factors in human milk would reduce the stimulation of the infant’s immune system, leading to reduced IgA in the infant’s urine and an increased incidence and duration of illness during mo 1 of life.
SUBJECTS AND METHODS
Study site and participants
The study methods and participant characteristics were described elsewhere (12). Briefly, the study was carried out in a periurban shanty-town community in Lima, Peru. Urban communities throughout Peru are experiencing the phenomenon of nutrition transition (13), with malnutrition present in children < 5 y old coexisting with adult overweight and obesity (14).
Based on published data (15–19), a sample size of 44 per group was required to detect a difference of 35% in colostrum and mature milk component concentrations, 10% in mature milk intake and 15% in urinary IgA concentration, with an α of 0.05 and a power of 80%; additional women were enrolled to address other study hypotheses presented elsewhere (12).
Women meeting the following criteria were identified during pregnancy: ≥ 18 y of age; multiparous and lived with a toddler < 4 y old; no indicators for elective cesarean delivery; and either continued to breast-feed into the third trimester or never breast-fed during this pregnancy. After the birth, the mother-infant pair were enrolled if the mother delivered a healthy infant (>2500 g, > 37 wk gestational age, vaginal delivery, and no birth defects or complications that would hinder breast-feeding).
This study was approved by the Human Subjects Research Office at Iowa State University, University of Alabama at Birmingham, and the Ethics Committee at the Instituto de Investigación Nutricional and written informed consent was obtained.
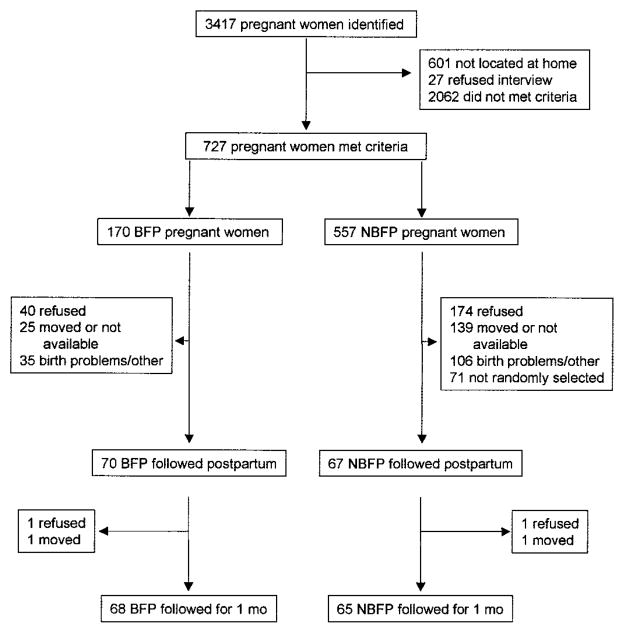
Between July 1998 and January 2000, 3417 pregnant women were identified through a community census, local prenatal health service registers and referrals (Fig. 1). Prenatal clinic records often provided inaccurate addresses and many women could not be located for the initial interview. Field workers identified 727 women who met all of the inclusion criteria: 170 women breast-fed their toddler during the third trimester of pregnancy (BFP)4 and 557 women had not breast-fed at all during the present pregnancy (NBFP). Staff confirmed breast-feeding among BFP women by direct observation if weaning had not occurred. Of the 727 women contacted prenatally, 70 BFP and 67 NBFP infants were followed after birth. For each BFP mother, a pool of NBFP women was randomly chosen, matched by toddler’s age (<24 mo; 24 – 48 mo) and expected date of delivery. The first NBFP woman from the pool who gave birth was included in the postpartum follow-up. There was a significant group difference in the month that enrollment occurred. Twice as many BFP infants were enrolled during the summer compared with NBFP infants (32.5 vs. 15.4%, P < 0.001). The refusal rate was not unexpected given the time commitment required. There were few socioeconomic and demographic differences between the included and excluded families as described previously (12).
FIGURE 1.
Trial profile demonstrating the number of identified pregnant Peruvian mothers, reasons for exclusion, and the number of mother-child pairs followed for 1 mo. BFP, mothers who had an overlap of breast-feeding and late pregnancy; NBFP, mothers who weaned their toddler before they became pregnant.
The reasons for no follow-up after birth included refusal, unavailable, not selected for randomization (NBFP only), birth problems and other. Of the 137 infants who were studied on the d 2, two BFP and two NBFP infants were not repeated at 1 mo because of refusal and out-migration. This analysis is based on 133 infants with observations for both days.
Data collection
Breast-feeding behavior
Toddler
BFP mothers recorded monthly their toddlers’ 24-h feeding frequency. The date of weaning was noted for children weaned during the last trimester.
Newborn
Twenty-four hour breast milk intakes were measured on d 2 (initiated at 41.7 ± 0.5 h after birth) and 1 mo postpartum (33.1 ± 0.3 d). There was no significant study time difference between groups. Milk intake was measured by a standard test weighing method (20), using an electronic digital balance sensitive to 1 g (Mettler Toledo Model SB/16000, Columbus, OH). Total milk intake was corrected for a 3% insensible water loss. On both d 2 and 1 mo, 3 of 2902 (0.1%) and 3 of 2279 (0.1%) breast-feeds, respectively, were not weighed because the mother was not at home when feeding. The missed values were imputed using multiple regression equations that estimated intake per feed by time since last feed, duration of the missed feed and child identifier code (12). Milk intake was expressed as total milk/24 h.
Anthropometry
Birth weight and length and gestational age were extracted from clinical records for infants born at a health facility (78.2%). For home births, a first weight and length was obtained within the first 3 d. Infants were weighed and measured at 1 mo without clothing using a digital electronic infant scale (Soehnle-Waagen GmbH and Co, KG, Murrhardt, Germany) sensitive to 10 g and a locally made length board with a precision of a 0.1 cm. Field workers were standardized (21); measurements were made in triplicate and means were recorded.
Maternal mid-arm and calf circumferences were measured prenatally and 1 mo postpartum to a precision of 1 mm with a nonstretchable tape measure (Lasso, Child Growth Foundation, London, UK). Weight and height were measured at 1 mo postpartum, using an adult beam balance (Seca, Hanover, MD) with a precision of 100 g and a locally made stadiometer with a precision of a 0.1 cm. BMI (kg/m2) was calculated.
Morbidity and diet
Field workers visited families twice weekly over the first 30 d to document illness and diet. Mothers recalled for each day since the last visit whether they had been ill, had symptoms of mastitis (red, hardened, hot, painful areas of the breast; fever) and had taken any medications. They reported for their infant the number and consistency of stools, fever, cough, and other symptoms as well as medications given and health clinic visits. Field workers documented for each day any nonbreast milk liquids and foods that were given to the infant. There were few missing data (1% of days for NBFP infants and 2% of days for BFP infants).
Household data
Socioeconomic data were collected twice: at enrollment and d 2 postpartum. Information on family composition, housing, hygiene, possessions, and education and employment of family members was reported by mothers and verified during the home visit.
Breast milk samples
Day-2 and 1-mo milk samples were collected at 50.9 ± 0.8 h (range: 26 –76 h) and 33.1 ± 0.3 d (range: 27–50 d) postpartum, respectively. Almost all (98%) of the samples were collected in the morning between 0700 and 1200 h (median 1000 h), with no group difference in the time postpartum nor the time of day. Collection occurred by completely emptying one breast from which the infant had not fed for 2 h, using an electronic breast pump (Medela Lactina Select advanced version 1997, Medela, McHenry, IL). The sample was stored immediately in a thermos with a cold pack at 4 – 6°C, refrigerated within 2 h, and sent to the laboratory each day where it was stored at −20°C until analysis.
Urine samples
Urine samples were collected between 0700 and 1300 h (median 1000 h) using adhesive urine collector plastic bags. There was no group difference in the time postpartum nor the time of day at which the samples were collected. Immediately after urination, urine was decanted into a sterile container, stored in a thermos with a cold pack at 4 – 6°C, and transported to the laboratory within 2 h where it was added to antibody-coated plates.
Laboratory analyses
Frozen milk samples were thawed in a water bath at 37°C for analysis. Lactose content was determined according to the AOAC method #984–15 (22). Milk protein was assayed by the method described by Smith et al. (23), using BCA Protein Assay Kit (Pierce 23225, Rockford, IL). Fat content in breast milk was determined by the crematocrit method (24) using a locally designed backlit display screen to facilitate measure and a vernier caliper (Mitutoyo, Kawasaki, Japan), and by measurement of esterified fatty acids as described by Stern and Shapiro (25).
Lactoferrin, lysozyme and IgA in milk and IgA in urine were determined by sandwich ELISA (26). Secretory IgA was not measured. The 96-well plates were coated with rabbit anti-human lactoferrin polyclonal antibody (DAKO, Carpinteria, CA, A0186, diluted 1:4000), rabbit anti-human lysozyme polyclonal antibody (DAKO A0094, diluted 1:1000) or goat anti-human IgA-unlabeled antibody (Southern Biotechnology Associates, Birmingham, AL, Cat # 2050 –01, diluted 1:500) for lactoferrin, lysozyme, and IgA assays, respectively. Standards were applied at 24.5 ng for lactoferrin (Sigma, St. Louis, MO, L0520), 1 ng for lysozyme (Sigma L6394), and 2.5 ng for IgA (The Binding Site, Birmingham, U.K., BP062) and titrated by doubling dilutions (1:2). Plate serial dilutions were 1:3 for lactoferrin and 1:2 for lysozyme and IgA. Breast milk (diluted 1:100,000 for lactoferrin, 1:20,000 for lysozyme and 1:66,666 for IgA) was added and reacted at 37°C for 1 h. Urine was serially diluted 1:2. Detection antibodies were alkaline phosphatase (AP)-conjugated rabbit antihuman lactoferrin (DAKO PE 696, diluted 1:2000), rabbit antihuman lysozyme-AP (DAKO PE 1023, diluted 1:1000), and goat anti-human IgA-AP (Southern Biotechnology Associates, # 2050 – 04, diluted 1:1000) for lactoferrin, lysozyme, and IgA assays, respectively. All the samples, controls, blanks, and standards were assayed in duplicate.
A sufficient quantity of milk samples was not available for all assays at each collection point; Table 2 reflects the sample size for each analysis. Urine was available for 83 (62.4%) infants [33 (49.4%) BFP and 50 (79.4%) NBFP]. The interassay CV for macronutrients was 1.2–1.6%, lysozyme 5– 8%, and lactoferrin and Ig A 22–30%.
TABLE 2.
Concentration and 24-h intakes of macronutrients and immunological factors in colostrum and mature milk of Peruvian women, by presence of a breast-feeding/pregnancy overlap, unadjusted for time of sample1
| Colostrum (d 2) |
Mature milk (1 mo) |
|||||
|---|---|---|---|---|---|---|
| Breast-feeding/pregnancy overlap | No breast-feeding/pregnancy overlap | P-value2 | Breast-feeding/pregnancy overlap | No breast-feeding/pregnancy overlap | P-value2 | |
| Macronutrients, g/L | ||||||
| Total protein | 27.38 ± 1.08 (25.02, 54) | 30.49 ± 1.80 (25.73, 62) | 0.470 | 16.15 ± 0.22 (15.91, 50) | 15.96 ± 0.17 (15.93, 59) | 0.631 |
| Fat | 23.45 ± 1.18 (21.16, 68) | 24.89 ± 1.07 (24.47, 65) | 0.222 | 33.16 ± 1.39 (31.70, 68) | 34.64 ± 1.59 (31.66, 64) | 0.664 |
| Lactose | 53.20 ± 0.87 (54.23, 68) | 51.13 ± 0.87 (52.56, 65) | 0.048 | 62.44 ± 0.59 (63.33, 68) | 63.75 ± 0.37 (64.04, 63) | 0.107 |
| Macronutrients, g/24 h | ||||||
| Total protein | 4.99 ± 0.41 (4.24, 53) | 4.70 ± 0.33 (4.22, 62) | 0.862 | 11.73 ± 0.47 (12.13, 50) | 12.33 ± 0.32 (12.21, 59) | 0.496 |
| Fat | 4.90 ± 0.52 (3.82, 67) | 4.50 ± 0.45 (3.80, 65) | 0.836 | 25.09 ± 1.50 (23.23, 68) | 26.87 ± 1.37 (25.18, 64) | 0.236 |
| Lactose | 10.87 ± 0.95 (8.56, 67) | 8.60 ± 0.64 (8.03, 65) | 0.179 | 46.52 ± 1.64 (49.23, 68) | 49.97 ± 1.35 (49.66, 63) | 0.300 |
| Immunological factors, g/L | ||||||
| IgA | 2.83 ± 0.31 (2.42, 68) | 2.93 ± 0.25 (2.99, 65) | 0.504 | 0.27 ± 0.02 (0.25, 68) | 0.28 ± 0.01 (0.29, 64) | 0.216 |
| Lactoferrin | 2.65 ± 0.20 (2.18, 68) | 3.32 ± 0.20 (3.34, 65) | 0.001 | 0.92 ± 0.07 (0.97, 68) | 0.98 ± 0.06 (0.97, 64) | 0.579 |
| Lysozyme | 0.05 ± 0.007 (0.04, 68) | 0.04 ± 0.004 (0.03, 65) | 0.013 | 0.02 ± 0.002 (0.02, 68) | 0.02 ± 0.003 (0.02, 64) | 0.582 |
| Immunological factors, mg/24 h | ||||||
| IgA | 453.80 ± 46.91 (364.86, 68) | 435.31 ± 36.62 (391.38, 65) | 0.832 | 198.13 ± 13.09 (174.10, 68) | 221.62 ± 9.43 (219.22, 64) | 0.014 |
| Lactoferrin | 457.78 ± 41.55 (368.23, 68) | 517.68 ± 38.91 (481.32, 65) | 0.081 | 700.58 ± 56.35 (668.90, 68) | 778.32 ± 45.50 (809.81, 64) | 0.276 |
| Lysozyme | 10.34 ± 1.62 (7.92, 68) | 6.73 ± 1.28 (4.21, 65) | 0.020 | 14.64 ± 1.70 (11.90, 68) | 16.32 ± 2.03 (12.59, 64) | 0.219 |
Values are means ± SEM (median; n).
Significant differences were tested within each time period with Kruskal-Wallis one-way ANOVA.
Statistical analysis
Mean group differences were tested for significant difference using χ2 for categorical variables, Student’s t test and ANOVA for continuous variables, and Kruskal-Wallis one-way ANOVA for variables with nonnormal distribution. Bonferroni post-hoc tests were used as appropriate. General linear regression models were used to estimate the effect of an overlap on breast milk components and urinary IgA, controlling for confounding factors including characteristics of the mother (e.g., anthropometry, obstetric history and age) and hour sample was collected. Lactoferrin, lysozyme and IgA values were not normally distributed; therefore, a log transformation that improved the distribution was used in regression analyses. Given the group differences of enrollment month and toddler age, these two variables were tested in all models. Month of enrollment was converted to three dichotomous season variables of 3-mo intervals. Illness was measured as the percentage of days with a symptom or sign; missing days were excluded from the denominator. Diarrhea was defined as 3 or more liquid stools in 24 h and used as a continuous variable as well as a dichotomous variable (any day with diarrhea; yes, no). Cough was defined as any day when cough was reported by the caretaker or witnessed by the fieldworker. Risk of frequent cough (>6 d, ≤6 d; 6 d was the value for the 75th percentile) was also determined. Reported maternal mastitis was a dichotomous variable (yes, no). For those illnesses associated with an overlap, further analysis was carried out with logistic linear regression. Other variables reflecting socioeconomic, demographic and health characteristics of the family were entered in the logistic regression models but were ultimately dropped from the model if they were not significant predictors of the outcome. All analyses were carried out with SYSTAT version 10 (SPSS, Chicago, IL). Data are presented as the means ± SEM; significance for all two-tailed probability tests was set at P < 0.05, unless otherwise indicated.
RESULTS
Prepartum breast-feeding practices
Of the 133 participants, 68 of the women breast-fed during the third trimester of pregnancy. Of these 68 women, 16 mothers weaned their toddler before giving birth, 19 weaned at birth, 7 weaned within mo 1, and 26 tandem breast-fed the toddler and the infant beyond 1 mo. For those who weaned during the third trimester, weaning occurred at 45.2 ± 7.2 d (median 45 d; range 1– 85 d) before giving birth. Data on 24-h breast-feeding frequency was obtained from 60 mothers who were still breast-feeding. Toddlers breast-fed 3.9 ± 0.4 feeds/d (median = 3 feeds; range: 1–12 feeds). Breast milk intake by the toddler was not measured.
Maternal, infant and household characteristics
The BFP and NBFP groups did not differ for most maternal, infant and household characteristics (Table 1), including birth weight (3.4 ± 0.1 kg) and length (50.0 ± 0.2 cm). There was a group difference in toddler’s age of ~10 mo resulting in a shorter interbirth interval. The BFP families had better quality housing and more access to water and sewage than NBFP families. BFP mothers tended to have a higher incidence of dystocia (prolonged labor; P = 0.098) (12).
TABLE 1.
Maternal and household environmental characteristics of Peruvian women participants, by presence of a breast-feeding/pregnancy overlap1
| Breast-feeding/pregnancy overlap (n = 68) | No breast-feeding/pregnancy overlap (n = 65) | |
|---|---|---|
| Maternal | ||
| Age, y | 25.9 ± 0.7 | 25.9 ± 0.6 |
| Parity, n | 2.1 ± 0.2 | 2.1 ± 0.1 |
| Calf circumference at 1-mo postpartum, mm | 33.0 ± 0.3 | 32.6 ± 0.4 |
| BMI at 1-mo postpartum, kg/m2 | 26.2 ± 0.5 | 25.9 ± 0.4 |
| Exclusive breastfed during mo 1, % (n) | 66.2 (45) | 70.8 (46) |
| Reported at least 1 d of illness during mo 1, % (n) | 64.7 (44) | 69.2 (45) |
| Reported symptoms of mastitis during mo 1, % (n) | 0.0 (0) | 6.2 (4)* |
| Smoked in pregnancy, % (n) | 2.9 (2) | 3.1 (2) |
| Household environmental | ||
| Children in home, n | 2.4 ± 0.2 | 2.5 ± 0.2 |
| Toddler’s age, y | 1.9 ± 0.1 | 2.7 ± 0.1** |
| Had improved housing quality (brick walls), % (n) | 63.2 (43) | 47.7 (31) |
| Had in-home piped water, % (n) | 73.5 (50) | 49.2 (32)** |
Values are means ± SEM or % (n); n for maternal anthropometry was 64 per group. χ2 goodness-of-fit test was used for comparison of proportions, Student’s t test was used for comparison of means. Different from the overlap group,
P < 0.05;
P < 0.01.
Postpartum breast-feeding practices
Breast-feeding frequency and intake did not differ between BFP and NBFP infants on d 2. Intakes at 1 mo were reported elsewhere (12). There was a 10% group difference for breast milk intake per feed (−4.2 g; 95% CI = −8.7 to 0.2); however, the total 24-h milk intake did not differ between BFP and NBFP infants (−45.0 g; 95% CI = −109.6 to 19.6).
Macronutrients and immune factors in colostrum, mature milk, and urine
Both age of infant and an overlap were associated with milk composition. Between d 2 and 1 mo postpartum, the concentration of protein, IgA, lactoferrin and lysozyme decreased, and fat and lactose increased in both groups (P < 0.01). The concentrations of lactose and lysozyme were higher and lactoferrin was lower in the colostrum of BFP compared with those of the NBFP group (Table 2). An overlap was not associated with the concentration of other milk components on d 2 or any milk components at 1 mo.
Total 24-h intake of all milk components except for IgA increased between d 2 and 1 mo in both groups; IgA intake decreased (P < 0.01) (Table 2). The associations between overlap and total intake of macronutrients (except lactose) and immunological factors were consistent with the concentration results. An overlap was associated with an increased d 2 total intake of lysozyme and a tendency for a decreased total intake of lactoferrin (P = 0.081). Although there was about an 11% group difference in the mean 24-h intakes of lysozyme (P = 0.219), lactoferrin (P = 0.276) and IgA at 1 mo, only the IgA difference was significant (Table 2).
Using regression models, we further examined d-2 milk (lactoferrin, lysozyme and IgA) and 1-mo milk and urine (IgA) components, using the log transformed values (Table 3). Milk volume was inversely associated with lactoferrin and IgA concentration. Lactoferrin was lowest when the milk sample was collected during the summer (January–March) and was from older mothers. After controlling for maternal age, higher parity was associated with a higher lactoferrin concentration. Lysozyme and IgA concentrations were not associated with season or any maternal characteristics.
TABLE 3.
Regression coefficients from general linear models for breast-feeding/pregnancy overlap and other predictors of lactoferrin, lysozyme and IgA concentrations in colostrum and 24-h IgA intake from mature milk of Peruvian women
| Colostrum (d 2), g/L |
Mature milk (1 mo), mg/d |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| log (Lactoferrin) (n = 133) |
log (Lysozyme) (n = 132) |
(log (IgA) n = 132) |
log (IgA) (n = 127) |
|||||||||
| b | SEM | P | b | SEM | P | b | SEM | P | b | SEM | P | |
| Breast-feeding/pregnancy overlap, 1 = yes | −0.234 | 0.086 | 0.008 | 0.266 | 0.126 | 0.036 | −0.052 | 0.092 | 0.575 | −0.283 | 0.093 | 0.003 |
| Time of sample collection,1 d | −0.021 | 0.009 | 0.014 | −0.034 | 0.013 | 0.008 | −0.040 | 0.009 | 0.000 | |||
| Milk volume, g | −0.001 | 0.000 | 0.002 | −0.003 | 0.000 | 0.000 | ||||||
| Summer season, 1 = yes | −0.495 | 0.094 | 0.000 | |||||||||
| Maternal 1-mo calf circumference, mm | 0.034 | 0.015 | 0.021 | |||||||||
| Maternal parity, n | 0.120 | 0.042 | 0.005 | |||||||||
| Toddler’s age, y | −0.126 | 0.061 | 0.041 | |||||||||
| Maternal age, y | −0.023 | 0.010 | 0.028 | |||||||||
| Overlap × Time | 0.018 | 0.010 | 0.084 | 0.025 | 0.015 | 0.110 | 0.020 | 0.011 | 0.077 | |||
| Constant | 1.795 | 0.271 | 0.000 | −3.534 | 0.090 | 0.000 | 1.431 | 0.094 | 0.000 | 4.549 | 0.495 | 0.000 |
| Adjusted R2 | 0.308 | 0.079 | 0.364 | 0.100 | ||||||||
Values for time of sample collection were centered at the mean.
At the mean sampling hour (50.9 h postpartum), the d-2 lysozyme concentration among NBFP mothers was almost 30% greater than that of the overlap group (Table 3). The lactoferrin concentration was 23% lower in the BFP than the NBFP group; IgA concentration did not differ between groups. The time of sample collection was inversely associated with the d-2 concentrations of lactoferrin, lysozyme, and IgA; however, the presence of an overlap tended to modify its slope for the three outcomes (P = 0.08; P = 0.11 and P = 0.08, respectively). In the NBFP group, the lactoferrin, lysozyme and IgA concentrations were 2.1, 3.4 and 4% lower, respectively, for each hour increase in time of sample collection. In the BFP group, the rate of change for lactoferrin and lysozyme was <1%/h; for IgA the rate of change was 2%/h.
The group difference in 1-mo IgA 24-h intake that was noted in the bivariate analysis widened when other factors were included in the model; IgA intake of BFP infants was >25% lower than that of infants of NBFP mothers (Table 3). A 1-y difference in the toddler’s age was associated with an ~13% reduction in IgA, whereas maternal anthropometry (calf circumference) was positively associated with intake.
There was no difference in 1-mo urinary IgA concentration between BFP and NBFP infants (0.131 ± 0.015 vs. 0.201 ± 0.015 mg/L; P = 0.20). Urinary IgA concentration was associated only with 1-mo breast milk IgA concentration (r = 0.29; P = 0.01). Other demographic, health, and environmental factors as well as an overlap were not associated with IgA in urine.
One-month infant and maternal morbidity
Mothers reported that their infant had diarrhea on 3.1% of observed days. Diarrheal episodes among infants tended to be more common in the winter (29.0%; P = 0.096) and less common in the spring (7.2%; P = 0.076) compared with other times of the year. The percentage having at least 1 d of diarrhea among exclusively breast-fed infants was half that of nonexclusively breast-fed infants (14.3 vs. 28.6%, respectively; P = 0.05) A breast-feeding/pregnancy overlap was not associated with any of the indicators for diarrheal morbidity; ~20% of both BFP and NBFP mothers reported that their child had one or more days of diarrhea.
A significantly higher proportion of BFP infants had a cough for at least 7 d compared with NBFP infants (35.3 vs. 20.0%). An overlap remained a strong predictor of risk of frequent cough in the general linear model (OR = 4.96; 95% CI = 1.75 to 14.06). The only other factor associated with frequent cough was the age of the toddler (OR = 2.48; 95% CI = 1.26 to 4.88). Cough was associated with other symptoms of illness. Compared with days with no cough, on days with cough, mothers reported that their infants were at least threefold more likely to have fever, irritability, general malaise and to have been given medicine (P < 0.001).
Mothers infrequently reported symptoms of mastitis. No BFP women reported mastitis whereas 6.2% of NBFP women reported symptoms (P = 0.05).
DISCUSSION
The effects of a breast-feeding/pregnancy overlap have been studied extensively by dairy researchers for over a century because of the large economic implications of a change in milk composition or production (3). Cost-benefit analyses have determined the optimal initiation of a dry (nonmilking) period for cows to be between 50 and 70 d before parturition (27). Milking cows in late pregnancy decreases milk production by 13% (28), similar to the 10% decrease per feed we reported (12). Dairy researchers also have related the dry period to milk composition (3), as was reported here. The presence of an overlap tended to alter the typical postpartum decline in the concentration of milk immunological factors (29) such that time postpartum was associated with a more rapid decrease in these components for the NBFP than the BFP group. Wheelock et al. (30) associated these differences with the degree of milk extraction before parturition and suggested that continued prepartum milking may interrupt the selective resorption of lactose that would normally leave higher concentrations of protein in prepartum milk and colostrum. Prosser et al. (31) demonstrated that Australian toddlers may extract large volumes of milk late in pregnancy (e.g., up to 300 g/24 h until 2 wk before birth). Very late milk reduction was assumed to be regulated by hormonal changes and the reduced availability of glucose to mammary cells.
The present study had several limitations. First, no pregnancy registrar existed from which a random sample of women could have been chosen. We identified participants using as many census-type recruitment approaches as possible to access women throughout the study area. Our efforts were limited by inaccurate addresses on the health post logs and excluded women working full-time and women breast-feeding only during early pregnancy. A second limitation is the self-selection of the breast-feeding behavior itself. Mothers who chose to breast-feed during the last trimester of pregnancy may have had other childcare behaviors or a socioeconomic status (SES) that comprised confounding factors for the outcomes. However, poorer SES was associated with the NBFP families and did not support the concept that greater poverty was responsible for limited milk intake or increased illness (12). Finally, the presence of an overlap was known to the field worker, providing the opportunity for information bias. To minimize this, experienced field workers were hired, all home visits were supervised and standardization in weighing and data collection was carried out.
A breast-feeding/pregnancy overlap was hypothesized to decrease milk immunological factors, decrease milk production, increase use of human milk substitutes and, therefore, increase diarrheal illnesses. The absence of an overlap-diarrhea association may reflect the difficulty of detecting diarrhea in this age group, inadequate sample size to detect differences where there is a low prevalence or a true lack of effect of an overlap on diarrheal morbidity. In contrast, one symptom of respiratory illness (cough) was affected by overlap. The etiology of this cough is unclear. Cough and rhinitis are very common in the study site and among older Peruvian children have been noted to be rarely associated with other signs of infection (32). However, in this young population, cough was associated with a number of other symptoms of illness, supporting the conclusion that BFP infants experienced more illness than NBFP infants.
An overlap was associated with reduced reported symptoms of mastitis. The rate of reported mastitis among mothers was 3%, similar to the 2.6% reported in studies among Gambian women (33). Breast problems may be reduced among BFP mothers because tandem breast-feeding was common among this group. The more complete emptying of the breast by two children may contribute to a reduction in risk of engorgement and infection (15). Because no NBFP mothers tandem breast-fed for mo 1 postpartum, it is not possible to separate the effects of the overlap during pregnancy from those of tandem breast-feeding postpartum. Future studies are needed to examine in depth the implications of an overlap for other aspects of maternal health.
Human milk stimulates the development of the secretory immune response in infants as demonstrated in the production of mucosal IgA (34,35). Prentice (36) reported that breast-fed English infants had a threefold higher production of urinary sIgA at 6 wk of age than nonbreast-fed infants; urinary IgA was not associated with milk IgA. In contrast to Prentice’s results, in the present study, urinary IgA was positively associated with total milk IgA intake at 1 mo. Total intake is a result of two factors, i.e., concentration of IgA and volume of milk. An overlap was negatively associated with milk volume but not significantly with concentration (P = 0.216). Total milk IgA intake may be a proxy variable for the physiological phenomenon of a breast-feeding/pregnancy overlap, thus explaining the difference in the results of Prentice and this study.
Although the study demonstrated an association between an overlap and milk composition, other factors influence immunological components and may be confounding variables. Miranda et al. (37) reported that maternal malnutrition (defined as weight/height ratio < 0.35 in addition to other clinical criteria) was associated with a significant reduction in IgA concentration in colostrum. However, the mothers in our study were not undernourished (mean weight/height ratio was 0.39 ± 0.01), and more than half of them were overweight. In addition, maternal anthropometry was not associated with a breast-feeding/pregnancy overlap. Maternal morbidity also has been associated with changes in milk composition. Lönnerdal et al. (38) reported that Peruvian mothers with acute, febrile infection during labor or early postpartum had significantly lower lactoferrin levels during the first 2 wk postpartum compared with healthy controls. However, we found no group difference in general prevalence of illness, and the incidence of mastitis reported was lower among BFP mothers.
The present results suggest that humans also may benefit from a nonlactating period during the third trimester of pregnancy. The complete effect of an overlap, however, can be assessed only by accounting for all of the benefits and negative consequences for the mother, fetus, the growing infant as well as the sibling toddler. Health professionals require additional information to determine the optimal duration of a nonbreast-feeding period during pregnancy to ensure the health of the next child while protecting the breast-feeding practice of the older sibling.
Acknowledgments
The authors thank the staff at the Instituto de Investigación Nutricional for their outstanding work and the staff at the Instituto Materno-Perinatal, the Hospital Materno-Infantil, Huascar and the Ministry of Health facilities in San Juan de Lurigancho in the study community for facilitating access to mothers giving birth in their units. Medela, McHenry, IL generously loaned the electronic breast pumps that were used in this study.
Footnotes
Presented in part at the following meetings: Experimental Biology 2001, March/April 2001, Orlando, FL [Marquis, G.. S., Penny, M. E., Diaz, J. & Marin, M. (2001) Continued lactation during late pregnancy alters the composition of colostrum for the subsequent newborn. FASEB J. 15: A642 (abs.)]; International Society for Research on Human Milk and Lactation. September 2000, Tucson, AZ [Marquis, G. S. & Penny, M. (2000). Effect of lactation during pregnancy on the subsequent breastfeeding of healthy Peruvian newborns.]; and International Society for Research on Human Milk and Lactation. October, 1999, Irsee, Germany [Marquis, G. S., Penny, M. E. & Díaz J. (1999) Determinants of day two and one month milk intakes of healthy Peruvian infants.].
Supported by a grant from the National Institutes of Health R03 Grant #HD35183–03.
Abbreviations used: AP, alkaline phosphatase; BFP, overlap of breastfeeding during pregnancy; NBFP, no overlap of breastfeeding during pregnancy; SES, socioeconomic status.
LITERATURE CITED
- 1.World Health Organization. The optimal duration of exclusive breastfeeding. [Accessed Jan. 13, 2003];Results of a WHO systematic review . 2003 http://www.who.int/inf-pr-2001/en/note2001-07.html.
- 2.Jensen RG. Handbook of Milk Composition. Academic Press; San Diego, CA: 1995. [Google Scholar]
- 3.Eckles CH, Palmer LS. The influence of parturition on the composition and properties of the milk and milk fat of the cow. J Biol Chem. 1916;27:313–326. [Google Scholar]
- 4.Vanlandingham AH, Weakley CE, Ackerman RA, Hyatt G. The relationship of production of heifers milked prepartum to the composition of colostrum. J Dairy Sci. 1949;32:559–564. [Google Scholar]
- 5.Cantrelle P, Leridon H. Breast feeding, mortality in childhood and fertility in a rural zone of Senegal. Popul Stud. 1971;25:505–533. doi: 10.1080/00324728.1971.10405821. [DOI] [PubMed] [Google Scholar]
- 6.Bøhler E, Bergström S. Child growth during weaning depends on whether mother is pregnant again. J Trop Pediatr. 1996;42:104–109. doi: 10.1093/tropej/42.2.104. [DOI] [PubMed] [Google Scholar]
- 7.Dettwyler KA. Breastfeeding and weaning in Mali: cultural context and hard data. Soc Sci Med. 1987;24:633–644. doi: 10.1016/0277-9536(87)90306-6. [DOI] [PubMed] [Google Scholar]
- 8.Moscone SR, Moore MJ. Breastfeeding during pregnancy. J Hum Lact. 1993;9:83–88. doi: 10.1177/089033449300900219. [DOI] [PubMed] [Google Scholar]
- 9.Oliveros C, Marquis GS, Ormsby G, Rudatsikira E. Maternal Lactation: A Qualitative Analysis of the Breastfeeding Habits and Beliefs of Pregnant Women Living in Lima, Peru. Int Q Community Health Educ. 1999;18:415–434. [Google Scholar]
- 10.Merchant K, Martorell R, Haas J. Maternal and fetal responses to the stresses of lactation concurrent with pregnancy and of short recuperative intervals. Am J Clin Nutr. 1990;52:280–288. doi: 10.1093/ajcn/52.2.280. [DOI] [PubMed] [Google Scholar]
- 11.Huffman SL, Chowdhury AKMA, Charkraborty J, Simpson M. Breastfeeding patterns in rural Bangladesh. Am J Clin Nutr. 1980;33:144–153. doi: 10.1093/ajcn/33.1.144. [DOI] [PubMed] [Google Scholar]
- 12.Marquis GS, Penny ME, Diaz JM, Marín RM. Post-partum consequences of an overlap of breastfeeding and pregnancy: Reduced breast milk intake and growth during early infancy. Pediatrics. 2002;109:1–8. doi: 10.1542/peds.109.4.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popkins BM. The shift in stages of the nutrition transition in the developing world differs from past experiences! Public Health Nutr. 2002;5:205–214. doi: 10.1079/PHN2001295. [DOI] [PubMed] [Google Scholar]
- 14.Reyes J, Ochoa LH. Encuesta Demográfica y de Salud Familiar 2000 (Demographic and Family Health Survey 2000) Instituto Nacional de Estadística e Informática; Lima, Peru: 2001. [Google Scholar]
- 15.Lawrence RA, Lawrence RM. A Guide for the Medical Profession. 5. The CV Mosby Co; St. Louis, MO: 1999. Breastfeeding; pp. 277–281. [Google Scholar]
- 16.Mehta PD, Mehta SP, Issacs CE. Distribution of IgG subclasses in human colostrum and milk. Immunol Lett. 1989;22:235–38. doi: 10.1016/0165-2478(89)90197-1. [DOI] [PubMed] [Google Scholar]
- 17.Reddy V, Bhaskaram C, Raghuramuhi N, Jagadeesan V. Antimicrobial factors in human milk. Acta Pædiatr Scand. 1977;66:229–232. doi: 10.1111/j.1651-2227.1977.tb07838.x. [DOI] [PubMed] [Google Scholar]
- 18.Butte NF, Villalpando S, Wong WW, Flores-Huerta S, Hernandez-Beltran M, O’Brian Smith E, Garza C. Human milk intake and growth faltering or rural Mesoamerindian infants. Am J Clin Nutr. 1992;55:1109–16. doi: 10.1093/ajcn/55.6.1109. [DOI] [PubMed] [Google Scholar]
- 19.Goldblum RM, Schanler RJ, Garza C, Goldman AS. Human milk feeding enhances the urinary excretion of immunologic factors in low birth weight infants. Pediatr Res. 1989;25:184–188. doi: 10.1203/00006450-198902000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Neville MC. Volume and caloric density of human milk. In: Jensen RG, editor. Handbook of Milk Composition. Academic Press; San Diego, CA: 1995. pp. 99–113. [Google Scholar]
- 21.Habicht JP. Estandarización de métodos epidemiológicos cuantitativos sobre el terreno (Quantitative epidemiological standardization methods for the field.) Bol Sanit Panam. 1974;76:375–84. [PubMed] [Google Scholar]
- 22.Association of Official Analytical Chemists. AOAC Official Method 984.15. Lactose in milk Enzymatic method. J Assoc Off Anal Chem. 1984;67:627. [Google Scholar]
- 23.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 24.Lucas A, Gibbs JAH, Lyster RJL, Baum JP. Crematocrit: simple clinical technique for estimating fat concentration and energy value of human milk. Br Med J. 1978;1:1018–1020. doi: 10.1136/bmj.1.6119.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern I, Shapiro B. A rapid and simple method for determination of esterified fatty acids and for total fatty acids in blood. J Clin Pathol. 1953;6:158–160. doi: 10.1136/jcp.6.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kearney JF, Barletta R, Quan ZS, Quintans J. Monoclonal vs. heterogeneous anti-H-8 antibodies in the analysis of the anti-phosphorylcholine response in BALB/c mice. Eur J Immunol. 1981;11:877–883. doi: 10.1002/eji.1830111106. [DOI] [PubMed] [Google Scholar]
- 27.Larson BL. Lactation. The Iowa State University Press; Ames, IA: 1985. [Google Scholar]
- 28.Remond B, Ollier A, Miranda G. Milking of cows in late pregnancy: milk production during this period and during the succeeding lactation. J Dairy Res. 1992;59:233–41. doi: 10.1017/s002202990003051x. [DOI] [PubMed] [Google Scholar]
- 29.Goldman AS, Garza C, Nichols BL, Goldblum RM. Immunological factors in human milk during the first year of lactation. J Pediatr. 1982;100:563–567. doi: 10.1016/s0022-3476(82)80753-1. [DOI] [PubMed] [Google Scholar]
- 30.Wheelock JV, Rook JAF, Dodd FH. The effect of milking throughout the whole of pregnancy on the composition of cow’s milk. J Dairy Res. 1965;32:249–254. [Google Scholar]
- 31.Prosser CG, Saint L, Hartmann PE. Mammary gland function during gradual weaning and early gestation in women. Aust J Exp Biol Med Sci. 1984;62:215–228. doi: 10.1038/icb.1984.22. [DOI] [PubMed] [Google Scholar]
- 32.Lanata CF. Incidencia y evolución de la neumonia en niños a nivel comunitario. (Incidence and evolution of pneumonia in children in the community.) In: Benguigui Y, López Antuñano FJ, Schmunis G, Yunes J, editors. Infecciones respiratorios en Niños (Respiratory Infections in Children) Panamerican Health Organization; Washington, DC: 1997. pp. 65–86. [Google Scholar]
- 33.Prentice A, Prentice AM, Lamb WH. Mastitis in rural Gambian mothers and the protection of the breast by milk antimicrobial factors. Trans R Soc Trop Med Hyg. 1985;79:90–95. doi: 10.1016/0035-9203(85)90245-7. [DOI] [PubMed] [Google Scholar]
- 34.Glass RI, Stoll BJ. The protective effect of human milk against diarrhea. A review of studies from Bangladesh Acta Pædiatr. Scand Suppl. 1989;351:131–136. doi: 10.1111/j.1651-2227.1989.tb11225.x. [DOI] [PubMed] [Google Scholar]
- 35.Slukvin II, Pilipenko VV, Chernyshov VP, Philchenkov AA. B-cell promoting activity of human colostrum. In: Mestecky J, Russell MW, Jackson S, Michalek SM, editors. Advances in Mucosal Immunology. Plenum Press; New York, NY: 1995. pp. 153–157. [DOI] [PubMed] [Google Scholar]
- 36.Prentice A. Breast feeding increases concentrations in IgA in infants’ urine. Arch Dis Child. 1987;62:792–795. doi: 10.1136/adc.62.8.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miranda R, Saravia NG, Ackerman R, Murphy N, Berman S, McMurray DN. Effect of maternal nutritional status on immunological substances in human colostrum and milk. Am J Clin Nutr. 1983;37:632–640. doi: 10.1093/ajcn/37.4.632. [DOI] [PubMed] [Google Scholar]
- 38.Lönnerdal B, Zavaleta N, Kusunoki L, Lanata CL, Peerson JM, Brown KH. Effect of postpartum maternal infection on proteins and trace elements in colostrum and early milk. Acta Pædiatr. 1996;85:537–542. doi: 10.1111/j.1651-2227.1996.tb14081.x. [DOI] [PubMed] [Google Scholar]