Abstract
Many species employ chemical signals to convey messages between members of the same species (conspecific), but chemosignals may also provide information to another species (heterospecific). Here, we found that conspecific chemosignals (male, female mouse urine) increased immediate early gene-protein (IEG) expression in both anterior and posterior medial amygdala of male mice, whereas most heterospecific chemosignals (e.g.: hamster vaginal fluid, steer urine) increased expression only in anterior medial amygdala. This categorization of responses in medial amygdala conforms to our previously reported findings in male hamsters. The same characteristic pattern of IEG expression appears in the medial amygdala of each species in response to conspecific stimuli for that species. These results suggest that the amygdala categorizes stimuli according to the biological relevance for the tested species. Thus, a heterospecific predator (cat collar) stimulus, which elicited behavioral avoidance in mice, increased IEG expression in mouse posterior medial amygdala (like conspecific stimuli). Further analysis suggests reproduction related and potentially threatening stimuli produce increased IEG expression in different sub-regions of posterior medial amygdala (dorsal and ventral, respectively). These patterns of IEG expression in medial amygdala may provide glimpses of a tertiary sorting of chemosensory signals beyond the primary-level selectivity of chemosensory neurons and the secondary sorting in main and accessory olfactory bulbs.
Keywords: Pheromones, Medial amygdala, IEG, Fos-related antigens (FRAs), Behavior
1. Introduction
In most mammals, chemosensory stimuli important for communication are processed via the main and/or accessory olfactory systems. Rodents are especially reliant on this form of communication (Hurst and Beynon, 2004). Chemical signals with unlearned (preprogrammed) information may be necessary for efficient reproductive and social behaviors (Lin et al., 2007). Rodents need to discriminate between chemosignals from within their own species (conspecific) and those of other species (heterospecific) (Meredith et al., 2008; Pankevich et al., 2006; Meredith and Westberry 2004; Murphy, 1980; Johnston and Brenner, 1982). Not surprisingly, rodents are easily able to discriminate between different conspecific chemosignals (Kavaliers and Colwell, 1995; Mossman and Drickamer, 1996; Pankevich et al., 2004). Without prior experience, rodents also respond with characteristic behaviors and neural activity to some heterospecific chemosignals. (Gouat et al., 1998; Dielenberg et al., 1999; Dielenberg et al., 2001; Meredith and Westberry 2004; Fendt 2006; Kobayakawa et al., 2007; Takahashi et al., 2005). Similar behavioral and physiological responses to conspecific and heterospecific chemosensory stimuli may be mediated by similarities in their chemical components, but regardless of the stimulus composition and/or the species of origin, behavioral responses (some unlearned) suggest that they transmit information of some importance to rodents.
The amygdala is important for normal behavioral responses to chemical signal information, particularly the medial amygdala (Blanchard et al., 2005; Lehman et al., 1980; Petrulis and Johnston, 1999). Chemosensory information converges in the medial amygdala via the main olfactory and accessory olfactory systems. Volatile and some non-volatile chemosensory stimuli interact with main olfactory sensory neurons contained in the nasal epithelium (Spehr et al., 2006). This “main olfactory” information passes via the main olfactory bulb to piriform cortex and the “olfactory amygdala,” principally the anterior cortical amygdala, which sends major connections to medial amygdala (Shipley and Adamek 1984; Coolen and Wood 1998). Some volatile and non-volatile chemosensory stimuli dissolve in intranasal mucus and are detected by vomeronasal sensory neurons in the vomeronasal epithelium (He et al 08; Chamero et al., 2007). Information from the vomeronasal sensory neurons projects to the accessory olfactory bulb (AOB) and on to the “vomeronasal amygdala,” primarily the medial amygdala (Coolen and Wood 1998; Pitkanen et al., 1997; Meredith 1998; Meredith and Westberry, 2004). The medial amygdala is the first site of olfactory and vomeronasal convergence and it appears to be activated both by stimuli of known biological importance (relevant) and those of no apparent importance (non-relevant). Thus, it is in a position to recognize, analyze and categorize incoming chemosensory information.
Previous work from our laboratory examined the expression of Fos-related antigens (FRAs), a complex of immediate-early gene (IEG) proteins, in the medial amygdala of sexually naive male hamsters upon first exposure to a range of conspecific and heterospecific chemosignals (Meredith and Westberry 2004). Conspecific reproductive signals, such as hamster vaginal fluid (HVF), and conspecific competitive/territorial signals such as male and female flank gland secretion, increased FRAs expression in both the anterior (MeA) and posterior (MeP) medial amygdala. Heterospecific stimuli, such as male mouse urine (mMU) (a competitive/territorial signal for male mice) and female mouse urine (fMU) (a reproductive signal for male mice) increased FRAs expression in MeA, but not in MeP. Thus, the hamster medial amygdala exhibited differential IEG expression patterns depending on the category (conspecific or heterospecific) of the stimulus. Artificial stimulation of vomeronasal chemosensory input produced patterns similar to heterospecific chemosignals, suggesting that the actual categorization was between biologically relevant (e.g. conspecific) and non-relevant (heterospecific or non-relevant) signals.
We reasoned that the male mouse medial amygdala should exhibit similar characteristic IEG expression patterns to chemical signals if categorization were a general operating principle of the rodent medial amygdala. However, the same mouse and hamster stimuli would occupy reversed conspecific and heterospecific (or relevant/non-relevant) categories in the two species.
Experiment one examines how the mouse medial amygdala IEG expression patterns differ depending on the category (conspecific/heterospecific, or relevant/non-relevant) of the stimuli presented. In the hamster study, there were also differences in the patterns of FRAs expression within MeP between the different conspecific stimuli (Meredith et al., 2008), generally corresponding with the division of labor between reproductive and defensive functions proposed by Canteras (Canteras 2002, Choi et al., 2005). Therefore, we also divided MeP (and MeA) into dorsal and ventral subdivisions and looked for possible differences in distribution of FRAs expression between different kinds of biologically relevant chemical signals (reproductive, territorial/competitive or predator). We assess the biological relevance of the stimuli based on known behavioral responses or those seen here. We measured FRAs expression in the male mouse medial amygdala upon exposure to two conspecific and two heterospecific chemical signals. The conspecific (mouse) stimuli are reproductive (fMU) and competitive/territorial (mMU) stimuli. We classify the two heterospecific signals as rodent/neutral (HVF) and non-rodent/neutral (steer urine [SU]). These stimuli have no obvious biological importance for mice, but they serve biologically relevant functions within their own species. HVF is used by female hamsters to signal reproductive readiness (Johnston, 1977). Urine from stressed bovines has been shown to elicit avoidance in other conspecific bovines (Boissy et al., 1998) and was chosen as a non-rodent heterospecific stimulus.
Experiment two examines medial amygdala response upon exposure to a behaviorally relevant heterospecific chemical signal: that of a potential predator (cat-collar). Worn cat-collar (CC) stimulus has been shown to be particularly salient for other rodents, producing fear-responses in rats (Dielenberg et al., 1999; Dielenberg et al., 2001; Takahashi et al., 2007). This experiment confirms that categorization of stimuli by the medial amygdala extends beyond an exclusively conspecific/ heterospecific classification.
2. Results
Experiment 1
As in the previous hamster experiments, conspecific stimuli increased FRAs expression in both MeA and MeP in male mice. Heterospecific stimuli increased FRAs expression only in MeA. When the data for MeA as a whole and MeP as a whole were analyzed together by two-way RM ANOVA there were significant main effects of stimulus (F (4,67) = 7.9, p<0.001) and area (F(1,67), p<0.001) and a significant interaction (F(4,67) = 11.2, p<0.001). This analysis indicates that the medial amygdala IEG expression was different across stimuli and area with a significant interaction between stimulus and area. Within this analysis, response to male mouse urine in posterior medial amygdala as a whole was not significantly different from control. However, further analysis revealed a significant response within the ventral subdivision (MePv).
Responses to female mouse urine (fMU)
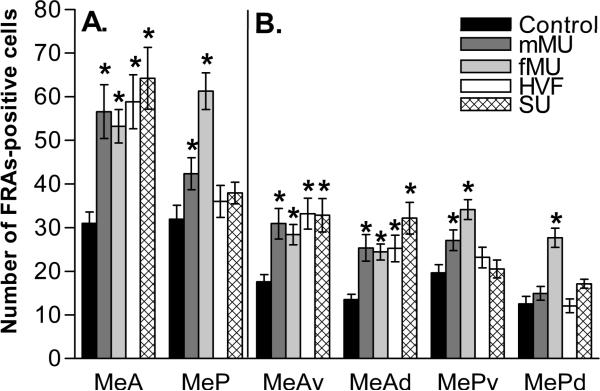
As seen in Figure 1, male mice exposed to the conspecific stimulus fMU exhibited increased FRAs expression in all medial amygdala subdivisions compared to clean-swab controls. ANOVA results for each area were as follows: ventral anterior medial amygdala (MeAv), F(4, 67) = 6.3, p<0.0011, dorsal anterior medial amygdala (MeAd), F(4, 67) = 9.2, p<0.001, ventral posterior medial amygdala (MePv), F(4, 67) = 6.9, p<0.001, and dorsal posterior medial amygdala (MePd), F(4, 67) = 13.611.7, p<0.001. The overall measurements of anterior medial amygdala (MeA), F(4, 67) = 8.3, p<0.001, and posterior medial amygdala (MeP), F(4, 67) = 9.4, p<0.001 also had significantly higher FRAs expression than control.
Figure 1.
Categorical response to chemical signals in the medial amygdala. Compared with controls, (panel A) all stimuli elicited increased FRAs expression (mean number of nuclei ± SEM) in total MeA, but only conspecific stimuli (mMU and fMU) increased FRAS expression in total MeP. (Panel B) The subdivisions of anterior medial amygdala, MeAv and MeAd, exhibit increase FRAs expression to all stimuli. Each conspecific chemical signal increases FRAs expression in the posterior medial amygdala subdivision MePv, and only fMU increases FRAS expression in MePd. * Significantly different from control. Refer to the results section for p values.
Responses to male mouse urine (mMU)
Male mice exposed to the conspecific stimulus, mMU, exhibited increased FRAs expression compared to control in both MeAv, F(4, 67) = 6.3, p<0.001, MeAd, F(4, 67) = 9.2, p<0.001, and in the overall measurement of MeA, F(4, 67) = 8.3, p<0.001. There was an overall affect of mMU exposure on FRAs expression in MeP, F(4,67) = 9.4, p<0.038, when analyzed within MeP, but not when analyzed by a joint RM ANOVA including data for MeA and MeP. This difference reflects the additional degrees of freedom in the analysis when additional data are included. When MePd and MePv are analyzed separately, there is a clear increase in FRAs response in MePv, F(4, 67) = 6.9, p<0.014 and none in MePd (Figure 1 right).
Responses to Hamster Vaginal Fluid (HVF)
Male mice exposed to the heterospecific stimulus, HVF, exhibited increased FRAs expression in MeAv, F(4, 67) = 6.3, p<0.001, MeAd, F(4, 67) = 9.2, p<0.001 and the overall measurement of MeA, F(4, 67) = 8.3, p<0.001. HVF produced no significant difference from control FRAs expression in the measured regions of the posterior medial amygdala.
Responses to Steer Urine (SU)
As with every stimulus tested, male mice exposed to the heterospecific stimulus, SU, exhibit increased FRAs expression overall in MeA, F(4,67) = 8.3, p<0.001; and in MeAv, F(4,67) = 6.3, p<0.001, and MeAd, F(4,67) = 9.2, p<0.001. Exposure to SU resulted in no significant difference in FRAs expression in the measured regions of posterior medial amygdala.
Behavioral Response to Stimuli
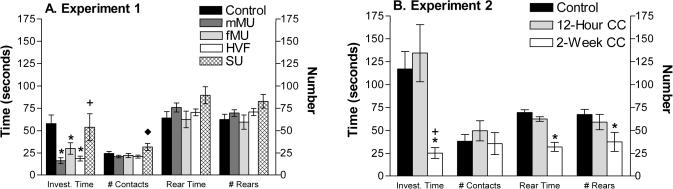
Mice spent significantly less time investigating scented swabs than investigating the control swabs, for MMU, F(4,67) = 5.4, p<0.001, HVF, F(4,67) = 5.4, p<0.002, or FMU F(4,67) = 5.4, p<0.019, but not for SU. Male mice exposed to SU swabs did contact them more frequently than any other stimulus group, F(4,67) = 2.9, p<0.039 (including controls), but the total investigation time was similar to that for controls. Mice shredded clean swabs as though for bedding, but did not systematically shred any type of scented swabs, including SU swabs (discussed below). There were no differences in the other measured behaviors (Figure 2A).
Figure 2.
Mouse behavioral response to conspecific and heterospecific chemical signals. (A) Mice exposed to conspecific and heterospecific stimuli spent less time (mean seconds ± SEM) than control investigating the stimulus swabs, except animals exposed to SU, which were not significantly different from control. * Significantly different from control; + significantly different from mMU and HVF; ◆ significantly different from other stimuli. Refer to the results section for p values. (B) Mice exposed to a 2-week CC spent significantly less time investigating the stimulus compared to both control and 12-hour CC, but contacted them a similar number of times. Also, mice exposed to 2-week CC reared significantly less (mean number ± SEM) and for less time than those exposed to control swabs. * Significantly different from control; + significantly different from 12-hour CC. Refer to the results section for p values.
Experiment 2
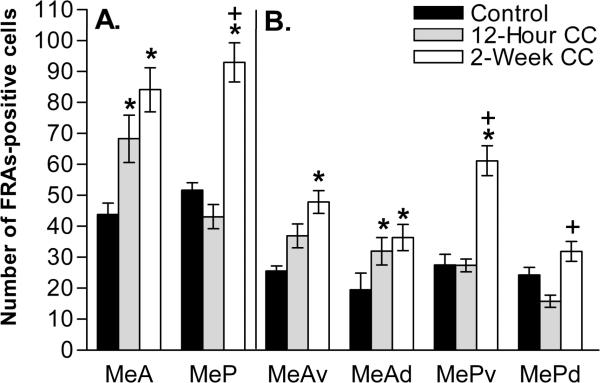
Upon exposure to the proposed biologically-relevant cat-collar stimulus, the pattern of FRAs expression to a collar worn for just 12 hours was similar to other neutral stimuli; with response in MeA, but not MeP. However, collars worn by a cat for 2 weeks did elicit a significant FRAs expression response in MeP (Figure 3). This was true for two-way RM ANOVA analysis including MeA and MeP; and for a one-way ANOVA analysis.
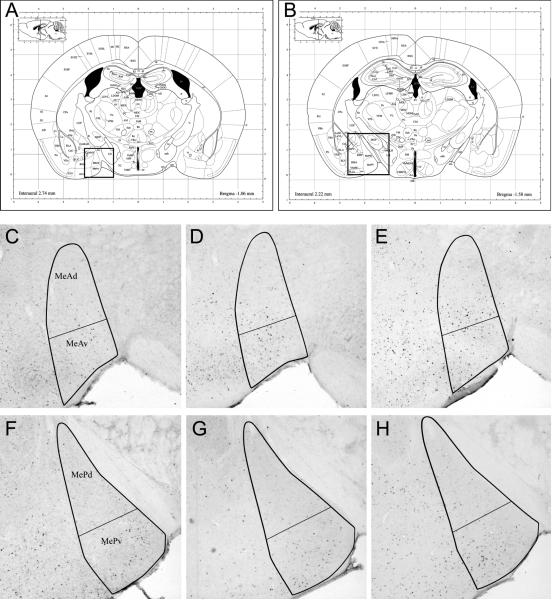
Figure 3.
Anterior and posterior medial amygdala regions of interest for both experiments, with examples of FRAS expression after cat collar exposure (experiment 2) Coronal plates from the atlas of Paxinos and Franklin (2003) at the level of (A) anterior and (B) posterior medial amygdala (boxed areas). (C–H) Bold outlines show the borders of the amygdala subregions. FRAs expression was estimated by counting all densely-ir nuclei within these borders, averaged over both side of three non adjacent sections, 80 um apart. The figure shows the central section of the three. (C–E) Representative sections showing FRAs expression in MeAd and MeAv to (C) clean control collar, (D) 12-hour CC or (E) 2-week CC. Exposure to either (D) 12-hour CC or (E) 2-week CC significantly increased FRAs expression in both MeAd and MeAv, as compared to (C) clean control collar. (F–H) Representative sections showing FRAs expression in MePd and MePv to the same three stimuli. Exposure to 2-week CC (H) significantly increased FRAs expression only in MePv, as compared to either control (F) or 12-hour CC (G).
Responses to 12-hour and two week Cat-collar (CC)
The 12-hour CC increased FRAs expression overall in the MeA, F(2,21) = 14.7, p<0.01, and the MeAd subdivision, F(2,21) = 9.5, p<0.035, but not the MeAv subdivision or any portion of MeP (Figure 4). The 2-week CC showed an overall increase of FRAs expression in the MeA, F(2,21) = 14.7, p<0.001, and its subdivisions, MeAv, F(2,21) = 13.3, p<0.001, and MeAd, F(2,21) = 9.5, p<0.01. 2-week CC exposure also increased FRAs expression overall in MeP, F(2,21) = 17.5, p<0.001, driven by a large significant increase in MePv, F(2,21) = 22.0, p<0.001. There was no significant difference from 4 control in MePd upon exposure to 2-week CC. The 2-week CC increased FRAs expression compared to 12-hour CC in total MeP, F(2,21) = 17.3, p<0.001, and both of its subdivisions, MePv F(2,21) = 22.0, p<0.001, and MePd, F(2,21) = 6.5, p<0.007 (+ symbols in Figure 4).
Figure 4.
Medial amygdala response to 2-week cat collar (CC), but not 12-hour cat collar (CC). Compared with controls, (panel A) both CC stimuli increased FRAs expression (mean number of nuclei ± SEM) in total MeA, but only 2-week CC increased FRAS expression in MeP. (Panel B) 2-week CC increased FRAs expression in both subdivisions on anterior medial amygdala, MeAv and MeAd, and the ventral portion of posterior medial amygdala (MePv). * Significantly different from control; + significantly different from 12-hour CC. Refer to the results section for p values.
Behavior Response to Cat-collar Stimuli
Mice exposed to clean CC or 12-hour CC pieces did not differ in any measured behavior. However, mice exposed to 2-week CC pieces, while contacting the stimulus an equivalent number of times, spent significantly less time investigating the stimulus than mice in either control, F(2,21) = 5.9, p<0.03 or 12-hour CC, F(2,21) = 5.9, p<0.025 conditions (Figure 2B). Also, mice exposed to 2-week CC reared less, F(2,21) = 4.1, p<0.05, and spent less time in a rearing position, F(2,21) = 4.1, p<0.05, than did mice exposed to a control CC. There were no differences in the number of contacts by the mice with any CC stimuli.
3. Discussion
In this report we provide strong support for the categorical nature of IEG responses in the medial amygdala of male rodents exposed to natural chemosignals from their own or other species. By using additional stimuli and a finer analysis of responses, we also provide evidence that: a) the basis for categorization may be the biological relevance of the stimuli rather than whether conspecific or heterospecific, and b) subdivisions of posterior medial amygdala may respond preferentially to different biologically relevant stimuli.
In our previous report (Meredith and Westberry 2004), we found that the pattern of response of male hamster medial amygdala to social chemosignals used by mice and hamsters was dependant on the species of origin, not the male/female or reproductive/competitive nature of the signal. We interpreted this as a categorization of stimuli by medial amygdala, likely based on the relevance of the stimulus for the responding animal. Conspecific signals increased IEG expression in both anterior (MeA) and posterior medial amygdala (MeP), but heterospecific signals increased IEG expression in only MeA and appeared to suppress MeP. Now, using the same mouse and hamster stimuli, our reciprocal experiment demonstrates a similar categorization of responses in the mouse medial amygdala. The mouse stimuli elicited the conspecific pattern of response (increased IEG expression in both MeA and MeP) in the mouse medial amygdala. The hamster stimulus, which elicits the conspecific pattern in hamster amygdala, now elicits the heterospecific pattern in mouse amygdala; i.e. increased IEG expression only in MeA. Additionally, behavioral responses to cat-collar stimuli suggest that this heterospecific stimulus is salient for mice, as for rats (Dielenberg et al., 1999; Dielenberg et al., 2001; Blanchard et al., 2005; Takahashi et al., 2005; Takahashi et al., 2007). Thus, the increase in FRAs expression in MeP to the cat-collar stimulus suggests that biological relevance is the key to characteristic categorical amygdala responses. We had previously suggested that the conspecific/ heterospecific origin of the stimulus determined the pattern of response (Meredith and Westberry, 2004).
The conspecific stimuli (mMU and fMU) came from animals with which the test subjects had no prior experience, so individual recognition cannot play a role in the responses. However, the stimuli were not necessarily unfamiliar as representatives of a category. Although single housed a week prior to testing, the male mice had had contact with other mature-males while group housed and with their mother prior to weaning. Thus, the “relevant” category of responses might be due to recognition of previously experienced categories of stimuli. Test subjects had no experience with any of the species supplying heterospecific stimuli (HVF, SU and CC), so the similarity between responses to the conspecific stimuli and to the cat collar stimuli (increased FRAs expression in MeP) could not be due to recognition of a learned signal. A significant response in MeP to some stimuli and not others could simply be due to the presence of particular chemicals in several of the complex stimulus materials. However, the mouse and hamster stimuli consistently elicited significant responses in MeP of the conspecific and not the heterospecific test animals. This characteristic response of conspecific MeP to conspecific stimuli may be dependent upon the precise combination of chemical components and complementary differences in the specificity of each species chemosensory receptors. These data are also consistent with our proposal that the medial amygdala processes information about chemical stimuli in terms of their relevance. This chemosensory circuit appears to be selective for conspecific and other biologically relevant stimuli, such as predator stimuli.
In the context of an amygdala interpretation of information about chemosensory stimuli, we can examine the possible meanings of stimuli used here based on known functions or reasonable properties. Of the two conspecific stimuli, male mouse urine is territorial/competitive and female mouse urine is reproductive. Of the three heterospecific stimuli, cat-collar indicates a potential predator, hamster vaginal fluid and steer urine may be neutral. All stimuli regardless of origin, or “meaning”, increased IEG expression in anterior medial amygdala, in both ventral and dorsal portions (except 12-hour CC; no MeAv). Female mouse urine exposure increased overall IEG expression in MeP and in both MePd and MePv. Male mouse urine and cat collars worn for two weeks significantly increased IEG expression only in MePv, not MePd. Hamster vaginal fluid, steer urine or a collar that had been worn by a cat for only 12 hours, did not elicit any increase in any measured area of MeP. Presentation of two week cat-collar increased IEG expression in MeP overall, driven by a robust expression only in MePv. The striking differences in IEG expression of MeP by stimuli of different putative meanings suggest a possible regional specialization of MeP for the analysis or relaying of specific information. Dorsal MeP (MePd) has been proposed as an area relaying sensory information to reproductive circuits in the hypothalamus (Swanson 2000; Canteras 2002). Ventral MeP (MePv) has been proposed as a component of hypothalamic circuits concerned with defensive behavior (Canteras 2002).
Female mouse urine is clearly a salient stimulus for male mice and conveys reproductive information. It is preferred by males over male mouse urine (Pankevich et al., 2006) and estrous urine is preferred over non-estrous urine (Pankevich et al., 2004). Species-specific reproductive chemical signals increase IEG expression in medial amygdala, especially in MePd, in a variety of rodents (Fernandez-Fewell and Meredith 1994; Kollack-Walker and Newman 1997; Bressler and Baum 1996; Choi et al., 2005). In our experiments, fMU exposure results in increased IEG expression in MeP overall and in both the dorsal and ventral MeP. The IEG expression in both MeA and MeP is similar to that evoked in male hamster medial amygdala by the hamster reproductive stimulus, HVF (Meredith and Westberry 2004). The increased IEG expression in MePv elicited by female stimuli is not immediately consistent with Canteras' proposed division of labor in the amygdala, but Choi et al., (2005) have already shown an overlap in projections from MePv to reproductive and defensive regions of the hypothalamus. Their suggestion that defense-related input might access reproductive areas in order to coordinate powerful competing behaviors is reasonable, but it is also possible that IEG expression in MePv by reproductive stimuli reflects activation important for reproductive behaviors.
Male mouse urine is a signal of potential social competitors and should indicate a need for defensive vigilance. Male mice occupy territory purposefully to defend resources and attract potential mates, although urine from a territory holder is ineffective in keeping out intruder male mice (Hurst and Beynon 2004). Male mice attack an unfamiliar intruder male more aggressively than cage-mates and when cage-mate urine is placed on an intruder, they exhibit decreased aggression (Nakamura et al., 2007). To avoid the possibility of some unusual response to one individual's urine, the mMU used in our experiments was obtained from several males and mixed. Test animals had no prior contact with any of the donors, which would have been neither a resident nor an intruder in the neutral environment of the experimental arena. In the present experiment, exposure to mMU increased IEG expression in the ventral portion of posterior medial amygdala (MePv). This increase is consistent with activation of the defensive circuit proposed by Canteras (2002), raising the question whether unfamiliar mMU is potentially threatening to male mice.
Like FMU for male mice, HVF is an important reproductive chemical signal for male hamsters. However, it may only represent a neutral stimulus for mice. There is no suggestion in the pattern of amygdala IEG expression that the female stimuli used by mice and hamsters have a common female IEG expression pattern. In our previous experiments examining medial amygdala responses to conspecific and heterospecific chemosensory information in the Syrian hamster, the heterospecific mouse stimuli, MMU and FMU, failed to increase alter IEG expression in MeP. In mice, the reciprocal experiment also resulted in no response in MeP to the heterospecific hamster stimulus. This non-response may depend upon the irrelevance of the stimuli as opposed to the heterospecific nature of the chemical signals.
To further test heterospecific stimuli, we chose to examine male mouse medial amygdala response to steer urine (SU), a non-rodent heterospecific chemical signal with no clear biological relevance for mice. Mice living in fields or barns with cattle populated by large ungulates would be exposed to their urine. Like most other heterospecific stimuli, including HVF, SU failed to alter IEG expression in mouse posterior medial amygdala. As discussed above, this failure may simply be a consequence of a lack (or presence) of particular component(s) of the stimulus by the tested species. However, the MeA response shows that the amygdala receives information on all these stimuli.
Male mice spent significantly more time “investigating” the control swab than most of the other stimulus swabs (except for SU). The increased investigation of a control stimulus is contrary to most of the published behavioral literature. Usually animals spend significantly more time investigating an overt stimulus than a control. The reason for the increased time spent investigating the control stimulus seems to be the male mouse's “shredding” of the swab. Mice in our animal facility are required to be housed with a Nestlet (a small square of cotton) in addition to corn cob bedding. The mice shred these Nestlets to provide extra bedding for nest building. Mice shred the control swab in the same manner as a Nestlet, but do not shred any swabs containing a stimulus. This increased time shredding the control swabs leads to an overall significant increase in control investigation time, but does not seem to be related to the quality of the stimuli. Despite the increased time contacting and manipulating the control swabs, the FRAs expression in response to control swabs was significantly less in MeA than to any scented swab.
In experiment 2, it is notable that mice spent significantly less time investigating pieces of a cat collar worn for 2 weeks than a clean (control) collar or a collar worn for only 12 hours, but this difference appears to be due to avoidance of the 2-week collar stimulus (2-week CC). Rats exhibit unlearned fear responses to similar cat-collar stimuli, which also increase IEG expression in ventral MeP of rats (Dielenberg et al., 2001, Takahashi et al., 2005, Takahashi et al., 2007) and mice (Choi et al., 2005). Interestingly, a collar worn by a cat for only 12-hours (12-hour CC) increased IEG expression overall in the anterior medial amygdala, but did not produce significant changes in either subdivision of the posterior medial amygdala. Exposure to a 2-week CC increased IEG expression of MeP. This significant increase in overall MeP response to 2-week CC was due to a robust increase in IEG expression in MePv; consistent with its proposed importance in the hypothalamic defensive circuit (Canteras, 2002). There was no increase above control in MePd. Mice exposed to 2-week CC spent significantly less accumulated time investigating the collar as compared to control CC and 12-hour CC, but returned for numerous very brief samples of the stimulus. This behavior is consistent with a wariness that may be expected of a mouse entering an area frequented by a predator. Mice also reared significantly less often in the presence of the “strong” CC stimulus. This decrease in rearing behavior may make the mouse less visible to a predator, an appropriate response for our attributed meaning.
MeA exhibited increased IEG expression to both types of CC stimuli, suggesting both types of CC stimuli are physiologically detectable. However, it is surprising that exposure to a cat collar worn for 12 hours results in different behavioral and IEG expression patterns from one worn for 2 weeks. Concentration may be an unexpected factor affecting biological relevance (independent of any identification). Takahashi (2005) has previously shown a behavioral difference in rat response to cat stimuli depending on concentration. A low concentration exposure results in less stereotypical fear behavior, suggesting it may be less threatening. In hamsters, dilute cat urine also produced a response in MeA, but not MeP (Meredith and Westberry 2004). However, the cat urine used in the hamster experiment was diluted 1:10, which may have reduced its biological relevance below some critical level, as we suggest here for the difference between responses to the 12-hour and 2-week cat-collars. Blanchard et al., (2003) have also suggested that cat urine and predator feces are less potent “predator” stimuli for rats than strong cat fur odorants.
The medial amygdala is positioned appropriately to receive chemosensory information about specific chemosignals from vomeronasal sensory neurons, some of which are highly selective and narrowly tuned (Leinders-Zufall et al., 2000; He et al., 2008). It receives direct input from the accessory olfactory bulb (AOB) and may also receive complementary information via a small projection to rostral MeA from the main olfactory bulb (MOB) directly (Lehman and Winans, 1982; see Martel and Baum, 2008). The medial amygdala also receives indirect input via anterior cortical amygdala and piriform cortex (Shipley and Adamek 1984; Coolen and Wood 1998; Pitkanen et al., 1997; Meredith 1998). The MOB may also provide information about specific chemicals (as opposed to its action as a substrate for learned associations). Recent publications suggest some specificity for reproductive and “danger” signals in ventral and dorsal MOB response, respectively (Lin et al., 2007; Kobayakawa et al., 2007), and behavioral data indicate main olfactory contributions to reproductive behaviors in mice (Pankevich et al., 2004; Pankevich et al., 2006). Regardless of the source of input or whether some stimuli may belong to classes that were learned, the IEG expression patterns in MeP of mice and hamsters confirms the categorical nature of the amygdala response to biologically relevant stimuli.
We propose that the amygdala responses reported here reflect a categorization of conspecific and heterospecific stimuli, according to their biological relevance in the animal's environment. At least some stimuli are unlearned, suggesting a preprogrammed response. The amygdala, which can reassign value to stimuli on the basis of conditioned association (Aggleton, 2000), may perform a higher level analysis of the chemosensory information from AOB and MOB for the assignment of “meaning” to chemical signals that are important for animals to recognize and respond to appropriately upon first exposure.
4. Experimental Procedures
Animals
Seventy-eight (Exp. 1) and 24 (Exp. 2) sexually naive 3–4 month old male C57 BL/6 mice (Jackson Laboratory) were maintained on a reverse 12/12hr light/dark cycle with food and water ad libitum. All animal procedures were approved by the Florida State University Institutional Animal Care and Use Committee. Animals had no contact with any heterospecific stimuli before the experimental session, no contact with females or female stimuli since weaning and no contact with the male conspecific stimulus donors.
Stimuli
Female mouse urine was collected from 3–4 adult mice placed in a metabolic cage over a 5 day period. Five days of collection were used in order to collect urine from all estrus stages of normally cycling female mice (Champlin and Dorr 1973). Male mouse urine was collected from 3–4 animals and mixed in a similar manner. Hamster vaginal fluid was collected from 2–4 adult female hamsters in estrus using a spatula, mixed together and placed in centrifuge tubes. Steer urine was removed via syringe from the bladder of a recently slaughtered male castrate and frozen until dilution. All liquid stimuli were diluted 1:10 by weight with distilled water (purified by reverse osmosis and polishing with activated carbon) and centrifuged for 30 min (Fisher clinical centrifuge; medium speed). The supernatants were decanted and held at −20°C until presentation. Nylon cat-collars (Puritan Medical Products Company) were unworn-control or were worn for either 12-hours or 2-weeks by a neutered male house cat. The collars were removed, placed in zip-lock plastic bags and held at −20°C until use.
Testing Procedure and Stimulus Presentation
All mice were single housed at least 7 days prior to testing to minimize exposure to other male odorants. On the day of the experiment, mice were placed in a clean cage with clean corn cob bedding and allowed 2 minutes to acclimate to the surroundings. Polyester swab-tips (Puritan Medical Products Company) were used to present the stimuli. Clean swabs (control) or swabs containing ~200μl of liquid stimulus were presented in the middle of the cage and replaced every 3 minutes for a total of 15 minutes; a total of five swabs per trial. For cat-collar presentation, a 2.5cm × 1.27cm piece of collar worn for 12-hours, 2-weeks or unworn clean control collar, was presented in the middle of the clean cage and left for the entire 15 minute trial. Behavior was recorded using a computer program and numbered key pad with each key corresponding to a different behavior. The computer records the latency, the number of presses and total elapsed time each key is depressed. Behaviors recorded were: Investigation time (contact or sniffing at the stimulus; < 1mm), number of discrete investigatory events, number of rears, time spent rearing and general investigation of cage. All animals were tested in the dark phase of the light cycle in a room lit by red light.
Immunocytochemistry
Forty-five minutes after the initial stimulus exposure, mice were perfused with cold 4% paraformaldehyde (PFA). Brains were removed and post-fixed overnight in 4% PFA. The next morning brains were placed in 30% sucrose overnight for cyroprotection. Using a freezing microtome, brains were sliced into 40μm sections. Alternate free-floating coronal sections were washed in 0.1M PBS, blocked in a solution of 5% normal goat serum (30 min) and incubated in rabbit anti-FRAs primary antibody solution (SC253 – detects c-Fos, Fos B, Fra-1 and Fra-2; 1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA) for 20–24 hours at room temperature. The next day, sections were washed in 0.1M PBS and incubated in biotinylated goat anti-rabbit secondary antibody solution (1:400; Vector Laboratories, Burlingame, CA) for 2 hours. Sections were processed in ABC reagent (Vector Laboratories, Burlingame, CA) for 1 hour and stained with diamino benzidine (DAB) (Vector Laboratories, Burlingame, CA). FRAs expression was assessed by averaging numbers of densely labeled cell nuclei within the neuroanatomical nucleus of interest on both sides in three (alternate) sections per anatomical area (80um apart). Numbers are reported as mean and standard error. Areas of interest included: 1) Anterior medial amygdala (MeA), which was divided into ventral anterior medial amygdala (MeAv) and dorsal anterior medial amygdala (MeAd); 2) Posterior medial amygdala (MeP), which was divided into ventral posterior medial amygdala (MePv) and dorsal posterior medial amygdala (MePd); both as indicated in the mouse brain atlas (Paxinos and Franklin, 2003). Image analysis software (ImagePro plus, Media Cybernetics, Inc.) was used to count all densely labeled cell nuclei within the borders of the neuroanatomical nucleus of interest (see figure 3).
Statistics
FRAs expression comparisons were analyzed for each experiment initially by two-way repeated measures ANOVA with factors area (MeA, MeP) and stimulus (CON, mMU, fMU, HVF, SU; or CON, 12-hour CC, 2-week CC). A significant main effect of “stimulus” and interaction between “stimulus” and “area” indicate significant differences in response between different stimuli and a significant difference across areas in the pattern of response elicited by the set of stimuli. FRAs expression in MeA and MeP and their dorsal and ventral subdivisions were further analyzed by comparing control FRAs expression levels to stimulus FRAs expression levels (Expt. 1: mMU, fMU, HVF, SU; or Expt. 2: 12-hour CC, 2-week CC) for each brain area using a one-way ANOVA. Post-hoc comparisons were made using the Fisher's least significant difference test. Comparison of numbers of labeled nuclei between areas is not meaningful because areas differ in size and cell packing. The behavioral data were log transformed before analysis. Behaviors were analyzed using One-way ANOVAs, with post-hoc comparisons using the Fisher's least significant difference test. Behavioral responses to the control stimulus were compared to behavioral responses to the test stimuli (mMU, fMU, HVF, SU; or 12-hour CC and 2-week CC). Reported behaviors include number and cumulative duration of close investigation or contact with the stimulus swab/collar, number of rears, and time spent rearing.
Acknowledgements
This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC 005813 and T32 DC00044 (M.M.) and fellowship F31 DC08062 (C.L.S.). We thank Dr. Gerald Mast and Cordelia Samuelsen for technical assistance. We thank the anonymous reviewers for excellent suggestions and comments that have led to a considerable improvement in the paper.
Abbreviations
- mMU
male mouse urine
- fMU
female mouse urine
- HVF
hamster vaginal fluid
- SU
steer urine
- CC
cat collar
- MeAv/d
anterior medial amygdala, ventral/dorsal divisions
- MePv/d
posterior medial amygdala, ventral/dorsal divisions
Literature References
- Aggleton JP. The amygdala. Ed 2. Oxford UP; New York: 2000. [Google Scholar]
- Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav. Neurosci. 2003;117(2):360–8. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: Effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29(8):1243–53. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Boissy A, Terlouw C, Le Neindre P. Presence of Cues from Stressed Conspecifics Increases Reactivity to Aversive Events in Cattle: Evidence for the Existence of Alarm Substances in Urine. Physiol Behav. 1998;63(4):489–95. doi: 10.1016/s0031-9384(97)00466-6. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience. 1996;71:1063–1072. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450(7171):899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46(4):647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Arnold JC, McGregor IS. Low-dose midazolam attenuates predatory odor avoidance in rats. Pharmacol Biochem Behav. 1999;62:197–201. doi: 10.1016/s0091-3057(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Fendt M. Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J Chem Ecol. 2006;32:2617–2627. doi: 10.1007/s10886-006-9186-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. c-fos expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: contributions from vomeronasal sensory input and expression related to mating performance. J Neurosci. 1994;14:3643–3654. doi: 10.1523/JNEUROSCI.14-06-03643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouat P, Patris B, Lalande C. Conspecific and heterospecific behavioural discrimination of individual odours by mound-building mice. C R Acad Sci III. 1998;321:571–575. doi: 10.1016/s0764-4469(98)80459-9. [DOI] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science. 2008;25;320(5875):535–8. doi: 10.1126/science.1154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Johnston RE. The causation of two scent-marking behaviour patterns in female hamsters (Mesocricetus auratus) Animal Behaviour. 1977;25:317–327. doi: 10.1016/0003-3472(77)90007-0. [DOI] [PubMed] [Google Scholar]
- Johnston R, Brenner D. Species specificity of scent marking in hamsters. Behav Neural Biol. 1982;35:46–55. [Google Scholar]
- Kavaliers M, Colwell DD. Discrimination by female mice between the odours of parasitized and non-parasitized males. Proc Biol Sci. 1995;261:31–35. doi: 10.1098/rspb.1995.0113. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997;32(5):481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210(4469):557–60. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240(1):27–41. doi: 10.1016/0006-8993(82)90641-2. [DOI] [PubMed] [Google Scholar]
- Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci U S A. 2007;104:2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur J Neurosci. 2008 doi: 10.1111/j.1460-9568.2008.06564.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann N Y Acad Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M, Samuelsen CL, Blake C, Westberry J. Selective responses of medial amygdala subregions to reproductive and defensive chemosignals from conspecific and heterospecific species. In: Hurst JL, editor. Chemical signals in Vertebrates. Vol. 11. Springer; 2008. pp. 367–378. [Google Scholar]
- Mossman CA, Drickamer LC. Odor preferences of female house mice (Mus domesticus) in seminatural enclosures. J Comp Psychol. 1996;110:131–138. doi: 10.1037/0735-7036.110.2.131. [DOI] [PubMed] [Google Scholar]
- Murphy MR. Sexual preferences of male hamsters: importance of preweaning and adult experience, vaginal secretion, and olfactory or vomeronasal sensation. Behav Neural Biol. 1980;30:323–340. doi: 10.1016/s0163-1047(80)91210-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kikusui T, Takeuchi Y, Mori Y. The critical role of familiar urine odor in diminishing territorial aggression toward a castrated intruder in mice. Physiol Behav. 2007;90:512–517. doi: 10.1016/j.physbeh.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav Neurosci. 2006;120:925–936. doi: 10.1037/0735-7044.120.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. Compact Second Edition Pub. Elsevier Science & Technology Books; 2003. [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav. Neurosci. 1999;13(2):345–57. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Adamek GD. The connections of the mouse olfactory bulb: a study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res Bull. 1984;12:669–688. doi: 10.1016/0361-9230(84)90148-5. [DOI] [PubMed] [Google Scholar]
- Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behav Neurosci. 2007;121:100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]