Abstract
Background
There is much evidence that the sedative component of anesthesia is mediated by γ-aminobutyric acid A receptors on hypothalamic neurons responsible for arousal, notably in the tuberomammillary nucleus. These γ-aminobutyric acid A receptors are targeted by GABAergic neurons in the ventrolateral preoptic area (VLPO): when these neurons become active, they inhibit the arousal-producing nuclei and induce sleep. According to recent studies, propofol induces sedation by enhancing VLPO-induced synaptic inhibition, making the target cells more responsive to γ-aminobutyric acid A. We explored the possibility that propofol also promotes sedation less directly by facilitating excitatory inputs to the VLPO GABAergic neurons.
Methods
Spontaneous excitatory postsynaptic currents were recorded from VLPO cells – principally mechanically isolated, but also in slices from rats.
Results
In isolated VLPO GABAergic neurons, propofol increased the frequency of glutamatergic spontaneous excitatory postsynaptic currents without affecting their mean amplitude. Propofol’s action was mimicked by muscimol and prevented by gabazine, respectively a specific agonist and antagonist at γ-aminobutyric acid type-A receptors. It was also suppressed by bumetanide, a blocker of Na+-K+-Cl− cotransporter-mediated inward Cl− transport. In slices, propofol also increased the frequency of spontaneous excitatory postsynaptic currents and, at low doses, accelerated firing of VLPO cells.
Conclusion
Propofol induces sedation, at least in part, by increasing firing of GABAergic neurons in ventrolateral preoptic area, indirectly by activation of γ-aminobutyric acid type-A receptors on glutamatergic afferents: because these axons/terminals have a relatively high internal Cl− concentration, they are depolarized by GABAergic agents such as propofol which thus enhance glutamate release.
Introduction
THE CELLULAR and molecular mechanisms underlying the effects of general anesthetics are not well understood. The sedative component of anesthesia appears to be mediated by type A γ-aminobutyric acid receptors (GABAARs) in an endogenous sleep pathway1,2, the relevant GABAergic neurons being located in the ventrolateral preoptic area (VLPO)3–8. Recently, Zecharia et al.2 showed that GABAergic agents such as muscimol and propofol potentiate the GABA-mediated inhibition of orexinergic perifornical9 and histaminergic tuberomammillary neurons6. Together with other monoaminergic and cholinergic projections to cortex, the neurons in tuberomammillary nuclei directly promote cortical arousal10,11; and all are activated by orexinergic neurons12. Thus, their inhibition by VLPO neurons is pivotal in the initiation of sleep13,14. Although the activity of VLPO neurons is strongly influenced by circadian fluctuations in input from suprachiasmatic cells15, the detailed mechanisms underlying activation of VLPO neurons are largely unexplored16.
General anesthetics are known to have marked effects on synaptic transmission. Most previous studies found that propofol enhances the function of inhibitory GABAARs17–20 and may depress the release of glutamate, the major excitatory neurotransmitter21. However, in vitro electrophysiological studies have shown that, in several brain areas, activation of presynaptic GABAARs have a depolarizing effect which enhances glutamatergic transmission22–25. We hypothesized that by potentiating the depolarizing action of presynaptic GABAARs, propofol may increase glutamatergic transmission in the VLPO, and thus increase the activity of VLPO GABAergic neurons. To test this hypothesis, we recorded spontaneous excitatory postsynaptic currents (sEPSCs) in the rat VLPO GABAergic neurons, either in slices, or isolated by a mechanical, enzyme-free procedure. These neurons preserve some functional glutamate-releasing terminals after isolation.
Materials and Methods
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and they were approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey (Newark, NJ). The experiments were done on brains from Sprague-Dawley rats.
Slice Preparation and Mechanical Dissociation
The hypothalamic slices were prepared as described previously26,27. Briefly, Sprague–Dawley rats (aged 10–28 postnatal days) were anesthetized and then decapitated. Coronal slices (300 µm thick) were cut using a VF-200 slicer (Precisionary Instruments Inc., Greenville, NC). They were prepared in an ice-cold glycerol-based artificial cerebrospinal fluid containing 250 mM glycerol, 1.6 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.4 mM CaCl2, 25 mM NaHCO3, and 11 mM glucose, and saturated with 95%O2/5%CO2 (carbogen)28. Slices were allowed to recover for at least 1 h in a holding chamber at 32°C in carbogen-saturated regular artificial cerebrospinal fluid, which has the same composition as glycerol-based artificial cerebrospinal fluid, except that glycerol was replaced by 125 mM NaCl.
Neurons with functional presynaptic terminals attached were obtained by mechanical dissociation (Fig. 1A), as previously described29, with some modifications28,30. A slice containing the VLPO was transferred to a 35 mm culture dish (Falcon, Rutherford, NJ) and held down with a flat U-shape wire. The dish was filled with standard external solution containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose (320 mOsm, pH adjusted to 7.3 with Tris base). Under an inverted microscope (Nikon, Tokyo, Japan), we identified VLPO by its stereotaxic coordinates31, and located the VLPO cells that project to the noradrenergic locus coeruleus and the histaminergic tuberomammillary nucleus3,6,32. A fire-polished pipette, held by a micromanipulator, lightly touched the surface of the VLPO region and vibrated horizontally at 15–20 Hz for 2–5 min. After 20 min, isolated neurons that adhered to the bottom of the dish were kept for electrophysiological recordings at room temperature (21–22 °C). These mechanically dissociated neurons often preserve functional nerve terminals, including some that release glutamate29,30.
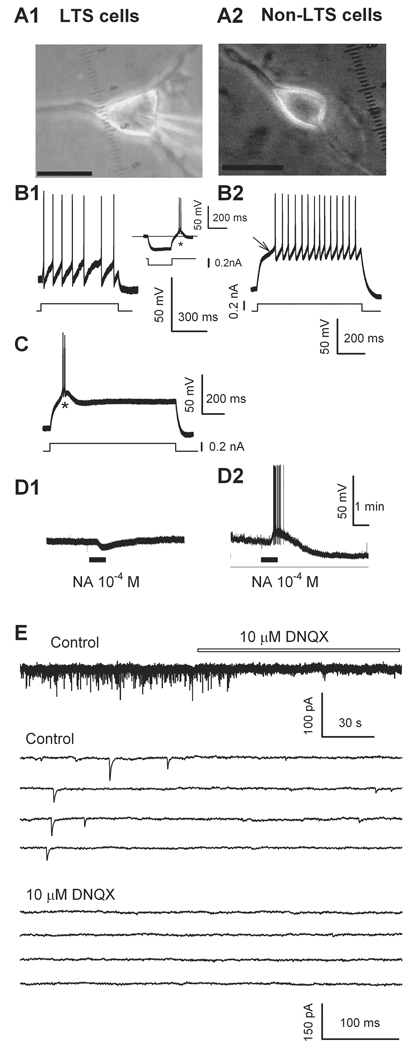
Fig. 1.
Mechanically dissociated two types of ventrolateral preoptic nucleus (VLPO) neurons and glutamatergic excitatory postsynaptic currents (sEPSCs). (A) Typical mechanically dissociated VLPO neuron: note triangular shape characteristic of GABAergic cells with (LTS) low-threshold spikes3 (A1, with patch pipette in place), scale bar: 20 µm, and bipolar fusiform non-LTS neuron (A2), scale bar: 15 µm. Low-threshold spikes occurred when the multipolar cell was depolarized from a hyperpolarizing level (at * in inset in B1, and in trace C). This same neuron was hyperpolarized by 100 µM noradrenaline (NA, D1). (B2), a slow voltage response toward the first action potential (arrow) recorded from a bipolar, fusiform non-LTS neuron (A2). This neuron was depolarized, and firing was induced by 100 µM noradrenaline (NA, D2). (E) sEPSCs recorded in a multipolar neuron were completely blocked by 2,3-dihydroxy-6,7-dinitroquinoxaline (DNQX, 10 µM), indicating these events were mediated by α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) /Kainate-type glutamate receptors. Some traces are shown on an expanded time scale. For all figures, horizontal bars indicate period of drug applications.
Electrophysiological Recordings
Electrical signals were obtained in whole-cell configurations with an Axon 200B amplifier or a MultiClamp 700A (Molecular Devices Co., Union City, CA), a Digidata 1320A A/D converter (Molecular Devices Co.), and pCLAMP 9.2 software (Molecular Devices Co.). Data were filtered at 1 kHz and sampled at 5 kHz. The patch electrodes had a resistance of 2–5 MΩ when filled with the pipette solution containing: 135 mM CsF, 5 mM KCl, 2 mM MgCl2, 10 mM HEPES, 2 mM Mg Adenosine-5'-triphosphate, and 0.2 mM Guanosine-5'-triphosphate (for voltage clamp). For current clamp recordings, the CsF in the above pipette solution was replaced by K-gluconate. The pH of the pipette solution was adjusted to 7.2 with Tris base. Neurons were voltage clamped at −60 mV to record α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor-mediated spontaneous and miniature excitatory postsynaptic currents (sEPSCs and mEPSCs).
In several experiments, a single coronal slice was transferred into a 0.4 ml recording chamber where it was held down by a platinum ring. Warm carbogenated artificial cerebrospinal fluid flowed through the bath (1.5–2.0 ml/min). Under infrared video microscopy (E600FN; Nikon), we recorded from neurons located in the core of VLPO, where a cluster of sleep-active neurons were originally identified, as illustrated in Fig. 15,6. Cells were deemed of the sleep promoting type according to previously established criteria3: first, they were triangular in shape (Fig. 1A1), which were in contrast to the fusiform, non-low-threshold spike (non-LTS) type (Fig. 1A2); second, when recorded in current-clamp mode, they generated characteristic low-threshold spikes in response to a depolarizing pulse or after a hyperpolarizing current step (Fig. 1B1, C) (Fig. 1B2 shows the high-threshold spikes of non-LTS cells); and finally, they were inhibited by noradrenaline (Fig. 1D1). Triangular-shaped cells inhibited by noradrenaline always displayed low-threshold spikes. By contrast, noradrenaline excited a non-LTS neuron (Fig. 1D2). The series resistance (15–30 MΩ) or input resistance (300–500 MΩ) was monitored throughout the whole-cell recording, and data were discarded if the resistance changed by more than 20%. All these recordings were made at 32°C, maintained by an automatic temperature controller (Warner Instruments, Hamden, CT).
Chemicals and Applications
The chemicals, including 2, 6-diisopropylphenol (propofol), gabazine (SR 95531), 2,3-dihydroxy-6,7-dinitroquinoxaline, tetrodotoxin, bicuculline and bumetanide were obtained from Sigma-Aldrich Chemical Company (St Louis, MO). Drugs were added to the superfusate or applied to a cell by a fast perfusion system (Y tube). Solutions in the vicinity of a neuron could be completely exchanged within 40 ms without damaging the seal33.
Statistical Analysis
sEPSCs and mEPSCs were counted and analyzed with Clampfit 9.2 (Molecular Devices Co.); sEPSCs and mEPSCs were screened automatically (6 pA amplitude threshold), checked visually, and accepted or rejected according to their rise and decay times. The frequency and amplitude of all events, during and after drug application, were compared to the mean of the values observed during the initial control period. Cumulative probability plots of the incidence of various interevent intervals and amplitudes, recorded under different conditions from the same neuron, were subjected to the Kolmogorov–Smirnov (K-S) test. For other plots, data obtained over one min periods at the peak of a drug response were compared to the average values of frequency (or amplitude) of the sEPSC (or mEPSC) recorded during the initial control period (1–4 min). Data are expressed as means (± SEM). sEPSCs were fitted with a standard single exponential equation (Clampfit 9.2, Molecular Devices Co.) to determine the time constant of decay from the EPSC peak. The statistical significance of drug effects was assessed by a paired two-tailed t-test on normalized data with Sigma Plot (Systat Software Inc., San Jose, CA). Values of P < 0.05 were considered significant.
Results
Spontaneous EPSCs recorded in neurons mechanically dissociated from VLPO
In VLPO, two thirds of the neurons have a characteristic triangular and multipolar shape and low-threshold-spikes; moreover, as these cells contain the GABA synthesizing enzyme glutamic acid decarboxylase and are inhibited by noradrenaline and carbachol, they correspond to the GABAergic VLPO cells known to be active during sleep3,8. In the current study, we recorded from such multipolar, triangular shaped neurons within VLPO (Fig. 1). Most of the experiments were done on mechanically dissociated neurons (Fig. 1A1), which combine several advantages: good space clamp, preservation of functioning synaptic terminals, including some that release glutamate, as well as better control of the surrounding solution29,30,34,35. The traces in Fig. 1 B-D were recorded under current–clamp. The voltage traces in the Fig. 1B1, Fig. 1C, and Fig. 1D1 were from a triangular-shaped, multipolar VLPO neuron. Depolarization from a relatively hyperpolarized level was followed by the characteristic depolarizing hump and burst of firing (inset in Fig. 1B1, and Fig. 1C), and noradrenaline induced membrane hyperpolarization (Fig. 1D1). Conversely, in the bipolar, fusiform VLPO neuron (Fig. 1A2), in response to a depolarization pulse there was a slow voltage rise towards the first action potential (Fig. 1B2, arrow) and noradrenaline induced membrane depolarization and firing (Fig. 1D2). Under voltage-clamp, sEPSCs were recorded with the CsF-based internal solution at a holding potential of −60 mV. Under these conditions, postsynaptic responses to GABA or glycine are suppressed36, allowing us to monitor changes in frequency and amplitude of the isolated EPSCs30,37,38. 2,3-dihydroxy-6,7-dinitroquinoxaline (10 µM), an antagonist of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) /kainate receptor reversibly blocked the spontaneous synaptic events (Fig. 1E), confirming that these VLPO neurons receive glutamatergic excitatory inputs15.
Propofol raises sEPSC frequency in neurons mechanically dissociated from VLPO
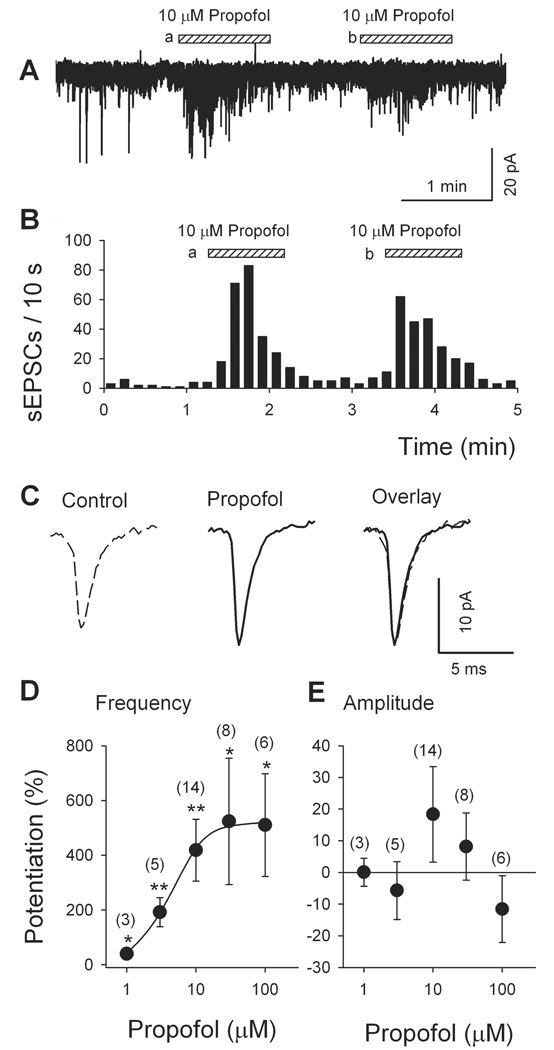
Propofol (1–100 µM) was applied to isolated VLPO neurons by a fast perfusion system (Y-tube)39. While propofol (10 µM) did not significantly change the holding current, it robustly but reversibly increased the frequency of sEPSCs (Fig. 2). A second application of propofol had a comparable effect (88 ± 7%, of first response; n = 8, P > 0.5, Fig. 2A, B). As depicted by averaged traces recorded before and during propofol application (Fig. 2C) and the dose-response data in Fig. 2E, propofol (10 µM) did not significantly alter the mean amplitude of sEPSCs (7.6 ± 1.2 pA in control and 10.3 ± 1.8 pA in presence of propofol, P > 0.05, n = 30 events). The superimposed normalized mean traces at right in Fig. 2C illustrate the absence of any change in sEPSC kinetics: the decay time constant of sEPSCs was not significantly altered (control: 3.9 ± 1.3 ms, propofol: 2.3 ± 0.4 ms, P = 0.13, by t-test). The effect of propofol on sEPSC frequency depended on its concentration, as shown in Fig. 2D. The apparent EC50 for propofol was 4.2 µM. These results are consistent with a presynaptic mechanism of propofol action, enhancing glutamatergic transmission by increasing glutamate release.
Fig. 2.
Propofol increases the frequency (but not amplitude) of spontaneous excitatory postsynaptic currents (sEPSCs) recorded in mechanically dissociated multipolar ventrolateral preoptic nucleus neurons. (A) Increased incidence of sEPSCs during two applications of 10 µM propofol. (B) Time course of propofol-induced enhancement of sEPSC frequency (same data as illustrated in A). (C) Averaged traces show only minor change in amplitude, which was not significant (see E). Overlay of normalized traces indicates no change in sEPSC kinetics in presence of propofol (10 µM) (n = 30 events). Dose-response relationship for propofol-induced changes in sEPSC frequency (D) and amplitude (E). Solid line in D is least square fit of the frequency data to the Michaelis-Menten equation: P = (PMAX * Cn)/(Cn + EC50n), where P, PMAX, C, EC50 and n are percent potentiation, maximal potentiation, propofol concentration, the concentration of propofol at which the potentiation of sEPSC frequency is 50% of maximum, and the Hill coefficient, respectively. The EC50 was 4.2 µM. Each circle represents the mean ± SEM of results from 3 to 14 cells. *, P < 0.05; **, P < 0.01 by paired t test for propofol versus pre-propofol.
Propofol acts only on sEPSCs that are suppressed by tetrodotoxin and Cd2+
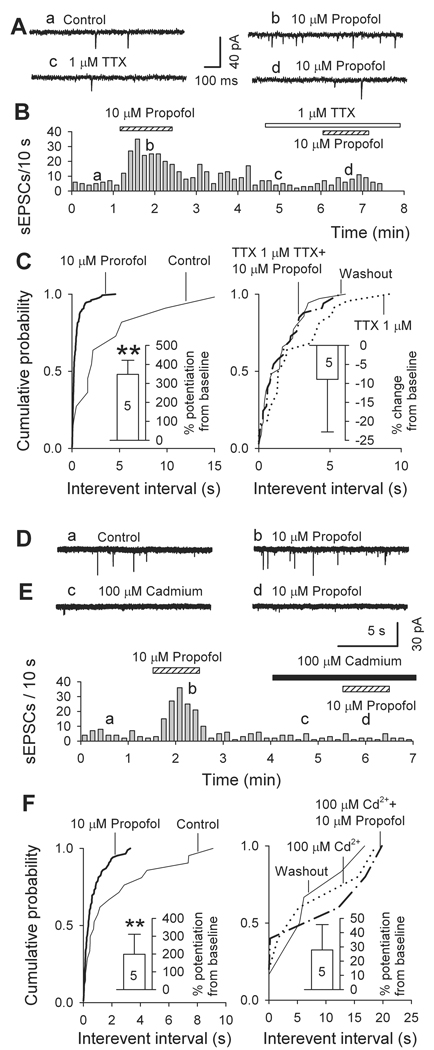
We next examined how block of synaptic transmission affects the propofol-induced facilitation of sEPSC frequency. First, we examined the effect of 1 µM tetrodotoxin, a blocker of voltage-dependent Na+ channels. Tetrodotoxin (1 µM) sharply decreased sEPSC frequency to 38 ± 10% of control (from 3.4 ± 1.2 Hz in control to 1.1 ± 0.6 Hz in tetrodotoxin; n = 5, P < 0.01) but a moderate reduction in sEPSC amplitude was not significant (to 75 ± 7% of control, from 17 ± 5 pA to 13 ± 4 pA; n = 5, P > 0.05). In the presence of 1 µM tetrodotoxin, propofol (10 µM) did not significantly alter either the frequency or the amplitude of the remaining EPSCs (presumably mEPSCs) (Fig. 3A-C). These results indicate that propofol acts only on sEPSCs generated by ongoing activity of tetrodotoxin-sensitive Na+ channels.
Fig. 3.
Miniature excitatory postsynaptic currents, in mechanically isolated ventrolateral preoptic nucleus neurons recorded in presence of tetrodotoxin (TTX) or Cd2+, are not sensitive to propofol. (A-C) effects of propofol on spontaneous excitatory postsynaptic currents (sEPSCs) recorded in absence and presence of 1 µM tetrodotoxin. (A) Original traces of spontaneous EPSCs obtained at times indicated by small letters in B. (B) time course of changes in sEPSC frequency. (C) Cumulative probability plots of intervals between sEPSCs show that sharp effect of propofol disappears in the presence of tetrodotoxin (n = 5). Insets are mean changes (± SEM): **, P < 0.01 by paired t test for propofol versus pre-propofol. (D-F) effects of propofol on sEPSCs recorded in absence and presence of 100 µM Cd2+. (D) Original traces of sEPSCs; (E) time course of changes in sEPSC frequency. (F) Cumulative probability plots of intervals between sEPSCs: as indicated by inset histograms, (means ± SEM, n = 5), effect of propofol was no longer significant in presence of 100 µM Cd2+. **, P < 0.01 by paired t test for propofol versus pre-propofol values.
In further experiments, we applied Cd2+, a general blocker of voltage-dependent Ca2+ channels, which also suppresses synaptic transmission. Indeed, acting somewhat like tetrodotoxin, 100 µM Cd2+ reduced sEPSC frequency by 36 ± 10% (P = 0.03, n = 5) and amplitude by 32 ± 9% (P = 0.02, n = 5); and in its presence, 10 µM propofol did not significantly alter the frequency of the remaining EPSCs (Fig. 3D-F). Judging by these results, propofol targets only EPSCs generated by Na+ channel dependent terminal depolarization and the resulting Ca2+ influx.
The propofol-induced increase in glutamatergic activity is eliminated by a GABAAR antagonist and mimicked by a GABAAR agonist
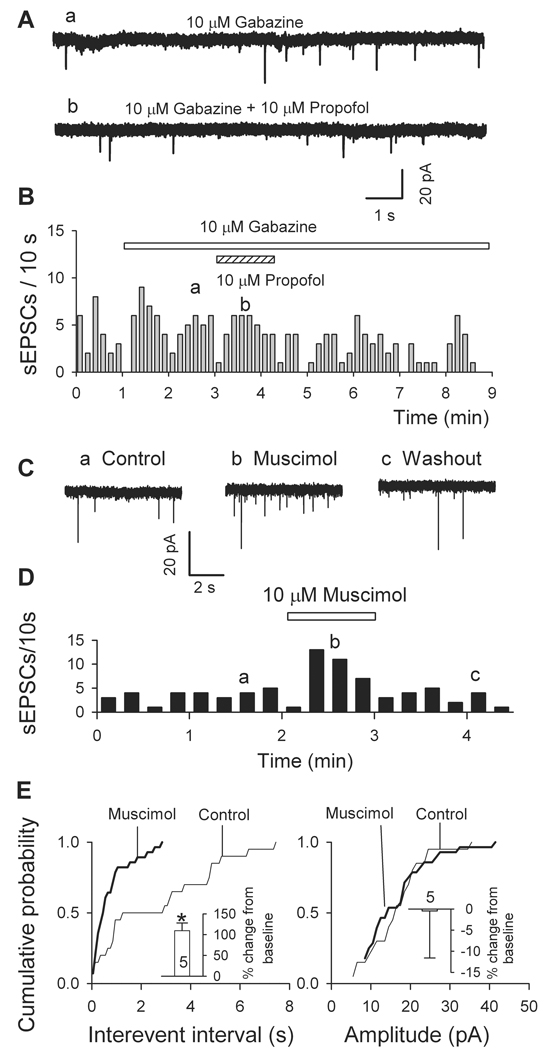
It is well known that propofol enhances the function of GABAARs, in particular those containing β2 and β3 subunits40, and that activation of presynaptic GABAARs enhances glutamate release in several brain areas22–25. To test for a possible involvement of GABAARs in the acceleration of sEPSC frequency, we applied gabazine (10 µM), a specific antagonist of GABAARs. As shown in Fig. 4A-C, in the presence of gabazine, 10 µM propofol caused a mild reduction in sEPSC frequency (−17.9 ± 2.1%, n = 6), presumably mediated by its GABAAR independent depressant effect on glutamate release21. To further confirm the role of GABAARs in the facilitation of sEPSCs, we applied muscimol, the specific GABAARs agonist. In 5 cells tested, 10 µM muscimol significantly increased sEPSC frequency, but not amplitude (Fig. 4C-E).
Fig. 4.
The action of propofol is mediated via γ-aminobutyric acid A receptors (GABAAR): propofol-induced increase in frequency of spontaneous excitatory postsynaptic currents (sEPSCs) was abolished by selective GABAAR antagonist gabazine and mimicked by the selective GABAAR agonist muscimol. (A-C) Gabazine effects. (A) Traces from a neuron isolated from ventrolateral preoptic nucleus show that propofol (10 µM) had no effect on sEPSCs in the presence of gabazine (10 µM). (B) Time course of above data. (C-E) like propofol, muscimol increases sEPSC frequency but not amplitude. (C) Traces recorded before (a, control), during (b, 10 µM Muscimol) and after washout (c). (D) Time course of effect of muscimol on one cell. (E) Cumulative plots and the insets (means ± SEM from five cells) show increase in sEPSC frequency (left panel) but not amplitude (right panel). *, P < 0.05 by paired t test for muscimol versus control (n = 5).
The propofol-induced increase in glutamatergic activity is attenuated by an antagonist of Na+-K+-2Cl− cotransporters (NKCC)
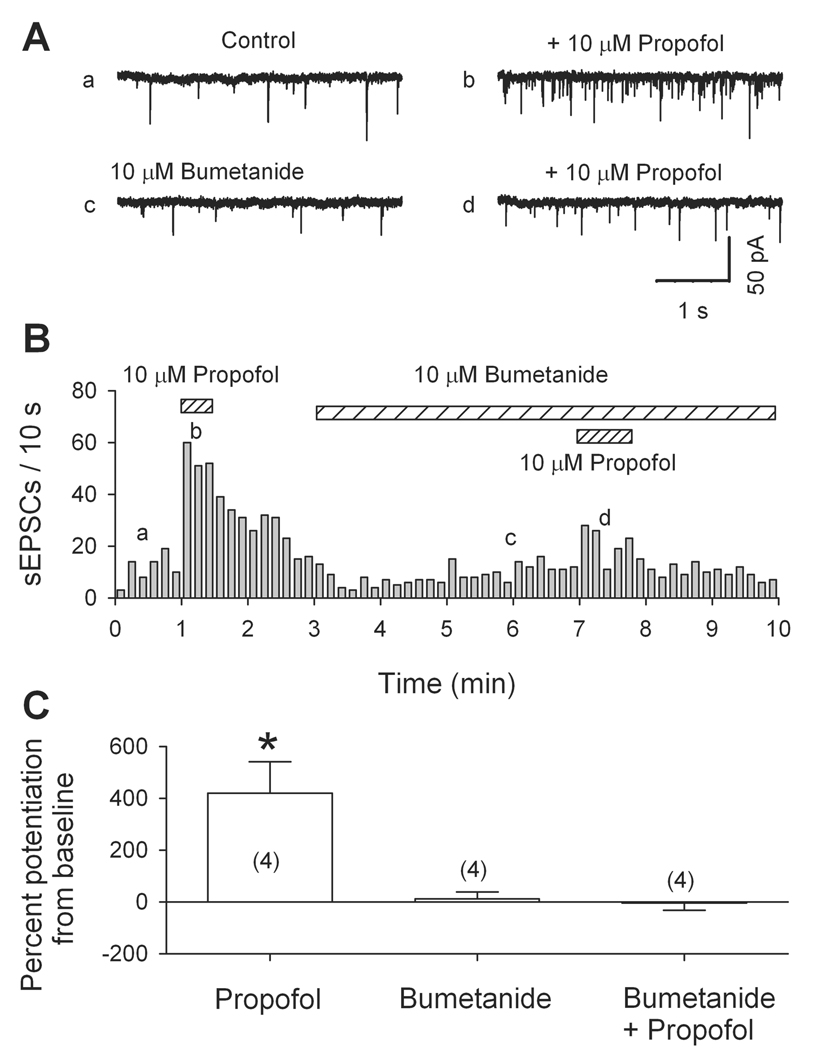
We have shown that voltage-dependent Na+ and Ca2+ channels, as well as GABAARs, are involved in the propofol-induced facilitation of sEPSC frequency. These data suggest that the activation of presynaptic GABAARs depolarizes some glutamatergic nerve terminals by generating Cl− efflux. This implies that the intraterminal Cl− concentration ([Cl−]) is relatively high, perhaps owing to inwardly directed Cl− transport systems via NKCC and/or the Cl−-HCO3− exchanger41–43. In peripheral sensory axons, and immature or injured CNS neurons, NKCC raises intracellular [Cl−]; hence, in these cells GABA induces depolarization43. Indeed, in some other hypothalamic neurons NKCC is responsible for a relatively high [Cl−] in glutamatergic nerve terminals44,45. To determine whether a high internal [Cl−], maintained by NKCC, is essential for the propofol-induced facilitation of sEPSC frequency, we tested propofol in the presence of 10 µM bumetanide, which selectively blocks NKCC43,46 (but not GABA receptors47). As illustrated in Fig. 5 by original traces in A, the plot in B and mean data in C, after 5 min of 10 µM bumetanide application, 10 µM propofol did not significantly alter the frequency of sEPSCs. Propofol’s effect slowly recovered to control values after washing out bumetanide (data not shown). This result suggests that functional NKCC are present on the juxta-terminal portion of glutamatergic axons, where they maintain a relatively high internal [Cl−].
Fig. 5.
Suppression of outward Cl− gradient eliminates excitatory action of propofol. (A) Traces from a dissociated ventrolateral preoptic nucleus neuron show much smaller effect of propofol (10 µM) on the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) in presence of 10 µM bumetanide, a blocker of Na+-K+-Cl− co-transporter. (B) Time course of changes in frequency. (C) Means ± SEM from four cells. *, P < 0.05 by paired t test for propofol versus pre-propofol data.
Propofol raises the frequency both of sEPSCs and of ongoing firing of VLPO neurons in brain slices
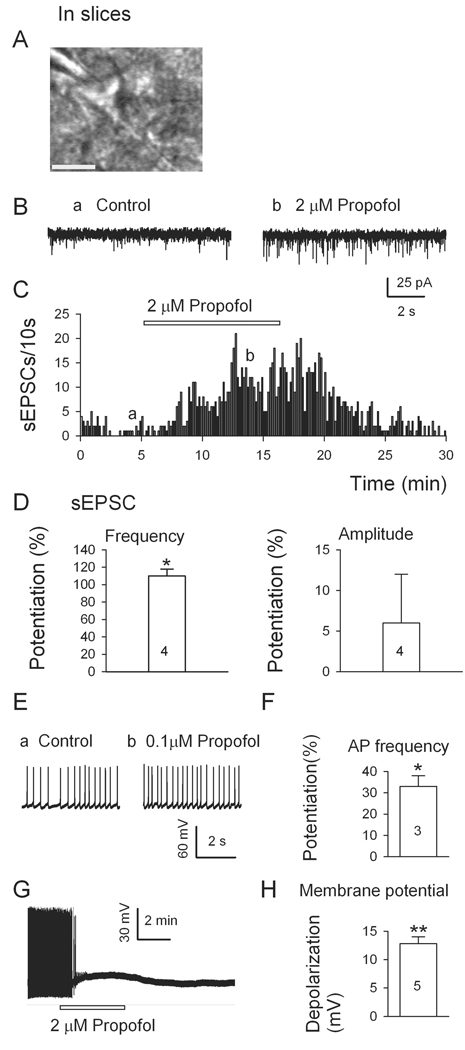
We confirmed that propofol modulates glutamate release onto VLPO neurons under more physiological conditions in slices (Fig. 6). Propofol was tested on multipolar VLPO neurons, identified by the standard criteria (Fig. 6A). As illustrated by the traces (Fig. 6B), plot (Fig. 6C) and histograms (Fig. 6D), 2 µM propofol robustly increased the frequency (but not amplitude) of glutamatergic sEPSCs in these VLPO neurons. Having established that propofol (2 µM) facilitates glutamatergic transmission in slices, we next assessed the physiological consequences of propofol’s action by examining the ongoing discharge of VLPO neurons. As shown in Fig. 6E and Fig. 6F, 0.1 µM propofol significantly raised the frequency of spontaneous firing of VLPO neurons in brain slices. Interestingly, as illustrated in Fig. 6G and Fig. 6H, at a higher concentration (2 µM) propofol had a sharp depolarizing action, which inactivated firing.
Fig. 6.
Propofol increases the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) and firings in multipolar neurons in ventrolateral preoptic nucleus in slices. (A) A triangular-shaped neuron in ventrolateral preoptic nucleus with patch pipette in place. Scale bar, 20 µm. (B) 2 µM propofol significantly increased the frequency of sEPSCs in such a multipolar neuron in ventrolateral preoptic nucleus. (C), Time course of propofol-induced enhancement of sEPSC frequency. (D) Mean effects (± SEM, n = 4 cells) of 2 µM propofol on frequency and amplitude of sEPSCs. (E) Typical current traces and mean effects (± SEM, n = 3 cells) (F) of spontaneous action potential (AP) frequency of neurons in ventrolateral preoptic nucleus show that 0.1 µM propofol significantly increases the firing rate. (G) Typical current traces and mean effects (± SEM, n = 5 cells, H) of neurons in ventrolateral preoptic nucleus show that 2 µM propofol significantly depolarizes the neuron. *, P < 0.05, **, P < 0.01, respectively, by paired t test for propofol versus pre-propofol data.
Discussion
The central finding of this study is that propofol – at concentrations (1–10 µM) similar to those found effective in potentiating GABAARs in previous studies (e.g. 48–51) - raises the frequency of spontaneous glutamatergic transmission to VLPO neurons, both isolated and in slices. This is the first electrophysiological demonstration of the potentiating effect of propofol on glutamatergic transmission at the single cell level. An interesting parallel observation is that propofol, over a range of moderate concentrations (10–13 µM), selectively increases Ca-dependent glutamate release from cortical synaptosomes52. The VLPO neurons under study were multipolar in shape, generated low threshold spikes and were inhibited by noradrenaline, and therefore belonged to the GABAergic population3 that projects to and inhibits the principal deep nuclei responsible for cortical and behavioural arousal8. According to previous studies2,20, the sedative action of propofol is mediated especially by enhanced GABAergic inhibition of the cells of the tuberomammillary nuclei. Specifically, Zecharia and colleagues2 recently found that propofol enhances GABAergic inhibition of these cells by increasing the duration of inhibitory synaptic responses. This did not exclude the possibility that propofol also increases the excitability of VLPO neurons in some indirect manner, for example by disinhibition1. The present findings show that propofol increases ongoing excitation of the GABAergic VLPO neurons by enhancing glutamate release. Clearly, this mechanism could be a significant component of the sedative action of propofol.
Our recordings of AMPA receptor-mediated glutamatergic sEPSCs in the VLPO neurons are in keeping with a previous report15. A presynaptic target for propofol’s action was indicated by the increase in sEPSC frequency and insignificant changes in the mean amplitude of sEPSCs and mEPSCs. The presence of GABAARs on or near the glutamatergic terminals was supported by the demonstration that propofol’s effect was mimicked by muscimol, a GABAAR agonist, and blocked by gabazine, a GABAAR antagonist. The fact that activation of these GABAARs enhances glutamate release is readily explained if the Cl− concentration in these glutamatergic axons/terminals is relatively high; in which case, opening the presynaptic Cl− channels would induce Cl− efflux and thus membrane depolarization. The higher internal Cl− concentration appears to be the result of the activity of axonal NKCC-mediated inward Cl− transport, since bumetanide, - a selective inhibitor of Cl− transport that does not block GABAA receptors47 - prevented the action of propofol. On the other hand, in view of the block of propofol’s effect by tetrodotoxin, the depolarization directly caused by presynaptic GABAAR –induced Cl− efflux was unable to induce glutamate release; either because it was of insufficient magnitude to activate Ca2+ influx into the terminal or because the operative GABAARs are situated at some distance, requiring the conduction of tetrodotoxin-sensitive action potentials: in either case, the participation of voltage-dependent Na+ channels was crucial.
Previous studies on spinal cord, hippocampus, thalamus, and neocortex have not reported an excitatory effect of propofol on glutamatergic EPSCs. The simplest explanation could be that in contrast to previous authors, we recorded EPSCs in the absence of GABAAR antagonists – which eliminate this propofol action (as shown by our observations in the presence of gabazine).
Previous studies suggest that some GABAAR agonists promote sleep by inhibiting the histaminergic cells in the tuberomammillary nucleus and weakly activating the VLPO via agonist binding to the α1 subunit of GABAARs; whereas, gaboxadol (THIP; 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol) binds to the α4δ-subunits, potentially promoting sleep by activation of the VLPO16. However, how activation of GABAARs could excite VLPO neurons was not determined. Our study may provide an answer to this question. Future research will define the relevant subunits of GABAARs, and whether the α4δ-subunits in particular mediate the propofol-induced potentiation of sEPSCs of VLPO neurons.
In the current study, we tested 2 µM propofol on both mechanically dissociated neurons and slices. Its effects on sEPSCs (in whole cell voltage clamp recordings) were similar in both preparations; but the facilitation of sEPSCs occurred more quickly in mechanically dissociated neurons than in slices, as might be expected in view of the very slow diffusion of propofol in slices53. On the other hand, in current clamp recordings of ongoing firing in slices, we found that even 0.1 µM propofol significantly raised the firing rate of some VLPO neurons. Interestingly, at 2 µM, propofol induced a sufficiently large depolarization that firing was inactivated. A possible explanation is that the indirect potentiation of glutamate release produced by propofol is especially pronounced in slices, where many more glutamatergic inputs to VLPO neurons are likely to be preserved.
Conclusion
Our results show that chloride channel and GABAA receptors exist on the glutamatergic axons/terminals which make synapses on the VLPO neurons. By enhancing Cl− efflux from these axons, propofol depolarizes these terminals and stimulates the release of glutamate, which increases the activity of VLPO neurons, and thus potentiates GABAergic inhibition of arousal systems. This indirect mode of propofol action can be expected to contribute to its known effectiveness as a general anesthetic.
Acknowledgments
Financial supports: This work is made possible by the Foundation of the University of Medicine and Dentistry of New Jersey, Newark, NJ; and National Institute of Health, Bethesda, MD, grant: AA016964.
Footnotes
Parts of the contents have been presented at the Annual meeting, American Society of Anesthesiology, October 20, 2008, Orlando, FL.
Summary Statement: Propofol enhances glutamate release onto neurons of the ventrolateral preoptic area by potentiating the function of γ-aminobutyric acid A receptors on the glutamatergic afferents. These effects may contribute to its sedative action.
References
- 1.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 2.Zecharia AY, Nelson LE, Gent TC, Schumacher M, Jurd R, Rudolph U, Brickley SG, Maze M, Franks NP. The involvement of hypothalamic sleep pathways in general anesthesia: Testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse. J Neurosci. 2009;29:2177–2187. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Muhlethaler M, Serafin M. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 4.Szymusiak R. Magnocellular nuclei of the basal forebrain: Substrates of sleep and arousal regulation. Sleep. 1995;18:478–500. doi: 10.1093/sleep/18.6.478. [DOI] [PubMed] [Google Scholar]
- 5.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 6.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 8.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 9.Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology. 1988;27:111–122. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- 11.Lin JS, Hou Y, Sakai K, Jouvet M. Histaminergic descending inputs to the mesopontine tegmentum and their role in the control of cortical activation and wakefulness in the cat. J Neurosci. 1996;16:1523–1537. doi: 10.1523/JNEUROSCI.16-04-01523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakurai T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 13.Ericson H, Kohler C, Blomqvist A. GABA-like immunoreactivity in the tuberomammillary nucleus: An electron microscopic study in the rat. J Comp Neurol. 1991;305:462–469. doi: 10.1002/cne.903050309. [DOI] [PubMed] [Google Scholar]
- 14.Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol. 2001;429:638–653. [PubMed] [Google Scholar]
- 15.Sun X, Whitefield S, Rusak B, Semba K. Electrophysiological analysis of suprachiasmatic nucleus projections to the ventrolateral preoptic area in the rat. Eur J Neurosci. 2001;14:1257–1274. doi: 10.1046/j.0953-816x.2001.0001755.x. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Greco MA. Sleep circuitry and the hypnotic mechanism of GABAA drugs. J Clin Sleep Med. 2006;2:S19–S26. [PubMed] [Google Scholar]
- 17.Trapani G, Altomare C, Liso G, Sanna E, Biggio G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem. 2000;7:249–271. doi: 10.2174/0929867003375335. [DOI] [PubMed] [Google Scholar]
- 18.Bieda MC, MacIver MB. Major role for tonic GABAA conductances in anesthetic suppression of intrinsic neuronal excitability. J Neurophysiol. 2004;92:1658–1667. doi: 10.1152/jn.00223.2004. [DOI] [PubMed] [Google Scholar]
- 19.Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008;14:95–106. doi: 10.1111/j.1527-3458.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeller A, Jurd R, Lambert S, Arras M, Drexler B, Grashoff C, Antkowiak B, Rudolph U. Inhibitory ligand-gated ion channels as substrates for general anesthetic actions. Handb Exp Pharmacol. 2008:31–51. doi: 10.1007/978-3-540-74806-9_2. [DOI] [PubMed] [Google Scholar]
- 21.Lingamaneni R, Birch ML, Hemmings HC., Jr. Widespread inhibition of sodium channel-dependent glutamate release from isolated nerve terminals by isoflurane and propofol. Anesthesiology. 2001;95:1460–1466. doi: 10.1097/00000542-200112000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Turecek R, Trussell LO. Reciprocal developmental regulation of presynaptic ionotropic receptors. Proc Natl Acad Sci U S A. 2002;99:13884–13889. doi: 10.1073/pnas.212419699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang IS, Nakamura M, Ito Y, Akaike N. Presynaptic GABAA receptors facilitate spontaneous glutamate release from presynaptic terminals on mechanically dissociated rat CA3 pyramidal neurons. Neuroscience. 2006;138:25–35. doi: 10.1016/j.neuroscience.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Stell BM, Rostaing P, Triller A, Marty A. Activation of presynaptic GABA(A) receptors induces glutamate release from parallel fiber synapses. J Neurosci. 2007;27:9022–9031. doi: 10.1523/JNEUROSCI.1954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koga H, Ishibashi H, Shimada H, Jang IS, Nakamura TY, Nabekura J. Activation of presynaptic GABAA receptors increases spontaneous glutamate release onto noradrenergic neurons of the rat locus coeruleus. Brain Res. 2005;1046:24–31. doi: 10.1016/j.brainres.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Ye JH, McArdle JJ. Excitatory amino acid induced currents of isolated murine hypothalamic neurons and their suppression by 2,3-butanedione monoxime. Neuropharmacology. 1995;34:1259–1272. doi: 10.1016/0028-3908(95)00100-k. [DOI] [PubMed] [Google Scholar]
- 27.Dittman JS, Regehr WG. Mechanism and kinetics of heterosynaptic depression at a cerebellar synapse. J Neurosci. 1997;17:9048–9059. doi: 10.1523/JNEUROSCI.17-23-09048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye JH, Zhang J, Xiao C, Kong JQ. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: Glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods. 2006;158:251–259. doi: 10.1016/j.jneumeth.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Akaike N, Moorhouse AJ. Techniques: Applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol Sci. 2003;24:44–47. doi: 10.1016/s0165-6147(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 30.Deng C, Li KY, Zhou C, Ye JH. Ethanol enhances glutamate transmission by retrograde dopamine signaling in a postsynaptic neuron/synaptic bouton preparation from the ventral tegmental area. Neuropsychopharmacology. 2009;34:1233–1244. doi: 10.1038/npp.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. In: The Rat brain in stereotaxic coordinates 6th edition. Paxinos G, Watson C, editors. Academic press; 2007. Publisher: Elsevier Inc., Chapter page range 33–38. [Google Scholar]
- 32.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou C, Xiao C, Commissiong JW, Krnjevic K, Ye JH. Mesencephalic astrocyte-derived neurotrophic factor enhances nigral gamma-aminobutyric acid release. Neuroreport. 2006;17:293–297. doi: 10.1097/01.wnr.0000201504.23255.bc. [DOI] [PubMed] [Google Scholar]
- 34.Ye JH, Wang F, Krnjevic K, Wang W, Xiong ZG, Zhang J. Presynaptic glycine receptors on GABAergic terminals facilitate discharge of dopaminergic neurons in ventral tegmental area. J Neurosci. 2004;24:8961–8974. doi: 10.1523/JNEUROSCI.2016-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuo S, Jang IS, Nabekura J, Akaike N. alpha 2-Adrenoceptor-mediated presynaptic modulation of GABAergic transmission in mechanically dissociated rat ventrolateral preoptic neurons. J Neurophysiol. 2003;89:1640–1648. doi: 10.1152/jn.00491.2002. [DOI] [PubMed] [Google Scholar]
- 36.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- 38.Xiao C, Shao XM, Olive MF, Griffin WC, 3rd, Li KY, Krnjevic K, Zhou C, Ye JH. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2009;34:307–318. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou C, Xiao C, McArdle JJ, Ye JH. Mefloquine enhances nigral gamma-aminobutyric acid release via inhibition of cholinesterase. J Pharmacol Exp Ther. 2006;317:1155–1160. doi: 10.1124/jpet.106.101923. [DOI] [PubMed] [Google Scholar]
- 40.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 41.Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 42.Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na(+)-K(+)-2Cl- cotransporter BSC2 in the nervous system. Am J Physiol. 1997;272:C173–C183. doi: 10.1152/ajpcell.1997.272.1.C173. [DOI] [PubMed] [Google Scholar]
- 43.Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 44.Jang IS, Jeong HJ, Akaike N. Contribution of the Na-K-Cl cotransporter on GABA(A) receptor-mediated presynaptic depolarization in excitatory nerve terminals. J Neurosci. 2001;21:5962–5972. doi: 10.1523/JNEUROSCI.21-16-05962.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim do Y, Son EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haas M, Harrison JH., Jr. Stimulation of K-C1 cotransport in rat red cells by a hemolytic anemia-producing metabolite of dapsone. Am J Physiol. 1989;256:C265–C272. doi: 10.1152/ajpcell.1989.256.2.C265. [DOI] [PubMed] [Google Scholar]
- 47.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 48.Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam DW, Reynolds JN. Modulatory and direct effects of propofol on recombinant GABAA receptors expressed in xenopus oocytes: influence of alpha- and gamma2-subunits. Brain Res. 1998;784:179–187. doi: 10.1016/s0006-8993(97)01334-6. [DOI] [PubMed] [Google Scholar]
- 50.Patten D, Foxon GR, Martin KF, Halliwell RF. An electrophysiological study of the effects of propofol on native neuronal ligand-gated ion channels. Clin Exp Pharmacol Physiol. 2001;28:451–458. doi: 10.1046/j.1440-1681.2001.03469.x. [DOI] [PubMed] [Google Scholar]
- 51.Martella G, De Persis C, Bonsi P, Natoli S, Cuomo D, Bernardi G, Calabresi P, Pisani A. Inhibition of persistent sodium current fraction and voltage-gated L-type calcium current by propofol in cortical neurons: implications for its antiepileptic activity. Epilepsia. 2005;46:624–635. doi: 10.1111/j.1528-1167.2005.34904.x. [DOI] [PubMed] [Google Scholar]
- 52.Westphalen RI, Hemmings HC., Jr. Selective depression by general anesthetics of glutamate versus GABA release from isolated cortical nerve terminals. J Pharmacol Exp Ther. 2003;304:1188–1196. doi: 10.1124/jpet.102.044685. [DOI] [PubMed] [Google Scholar]
- 53.Gredell JA, Turnquist PA, Maciver MB, Pearce RA. Determination of diffusion and partition coefficients of propofol in rat brain tissue: Implications for studies of drug action in vitro. Br J Anaesth. 2004;93:810–817. doi: 10.1093/bja/aeh272. [DOI] [PubMed] [Google Scholar]