Abstract
Exocrine pancreatic insufficiency (EPI) resulting from conditions such as chronic pancreatitis (CP), acute pancreatitis (AP) and upper gastrointestinal (GI) surgery increases risk for malnutrition and metabolic problems. Poor nutrition is associated with more complications and higher mortality. Therefore, effective nutritional management should be a high priority in these patients. In CP, poor nutrition has been shown to significantly affect quality of life and functional status. Clinical study data show that dietary counselling combined with pancreatic enzyme replacement therapy is effective in improving nutritional status and is therefore recommended in these patients. In AP, early enteral nutrition reduces complications and mortality. However, EPI persists in many cases after the resolution of AP; these patients remain at increased risk for malnutrition and require further nutritional support. In patients undergoing surgery, preoperative weight loss is a risk factor for postoperative morbidity and mortality; outcomes can be improved considerably by preoperative screening to identify high-risk patients and by providing appropriate perioperative nutritional support. Pre- and perioperative enteral nutrition are cost-effective interventions that can improve outcomes in patients undergoing GI surgery. In all of these patient populations, nutritional management, including risk assessment and individualized nutritional support, is a key component of an effective multimodal therapeutic approach.
Keywords: acute pancreatitis, chronic pancreatitis, exocrine pancreatic insufficiency, gastrointestinal surgical procedure, malnutrition, nutritional support
Introduction
Malnutrition describes a metabolic state that reduces the capacity of an organism to cope with an insult or stress, such as major surgery. Malnutrition may be static as, for example, in disorders associated with very low body weight such as marasmus and kwashiorkor, or dynamic, such as in anorexia or weight loss or gain as a result of another acute or chronic illness. A focus on only actual body weight or body mass index (BMI) often leads to an underestimation of the latter in the clinical setting. For example, if a patient with slight obesity and a BMI of 30–35 kg/m2 loses 10–15% of his body weight in a short period of time, he is clearly malnourished but still has a BMI within the normal range of 20–25 kg/m2, which may confound the diagnosis of malnutrition.
Exocrine pancreatic insufficiency (EPI) presents in many different clinical settings, such as chronic pancreatitis (CP), cystic fibrosis, pancreatic cancer, acute pancreatitis (AP), severe critical illness and after major upper gastrointestinal (GI) surgery (e.g. gastrectomy and pancreatic resection). The specific situation of patients with EPI resulting from cystic fibrosis will not be discussed in this article. EPI is characterized by inadequate intraluminal pancreatic enzyme activity, resulting in maldigestion of mainly lipids and to a lesser extent of proteins and carbohydrates, which may result in a negative energy balance and malnutrition. The risk for weight loss, malnutrition and deficiency of specific nutrients increases with the severity of EPI. In these patients, available energy is reduced not only by maldigestion, but also by lower food intake caused by symptoms such as pain or postoperative nausea, and risk factors such as alcohol in CP or additional socioeconomic factors. In addition, energy expenditure increases as a result of the underlying disease and associated complications such as diabetes mellitus and chronic inflammation. Together, these factors lead to a negative energy balance and increased risk for micro- and macronutrient deficiency, weight loss, and the development of undernutrition and malnutrition.
Nutrition issues in patients with EPI should be a high priority in the clinical setting but are rarely the focus of attention in the management of such patients. Results from the EuroOOPS prospective cohort study highlight the importance of effective nutritional management in hospitalized patients.1 The aim of this study was to implement nutritional risk screening (NRS-2002) in hospitalized patients and to assess the association between nutritional risk and clinical outcome. A total of 5051 patients were screened in 26 European hospitals and data were collected on complications, mortality and length of hospital stay. Overall, 32.6% of patients were defined as ‘at-risk’ by NRS-2002. This patient group had more complications (30.6% vs. 11.3%), higher mortality (12% vs. 1%) and longer hospital stay (9 days vs. 6 days) compared with ‘not at-risk’ patients (Fig. 1).1
Figure 1.
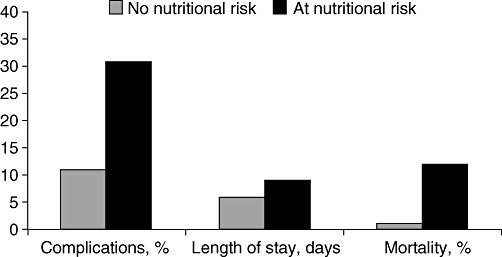
Association between nutritional risk (according to Nutritional Risk Screening [NRS] 2002) and clinical outcome in hospitalized patients. A prospective survey in 26 European hospitals (n= 5051)1
Malnutrition in chronic pancreatitis
It is widely acknowledged that patients with CP are often undernourished, but there have been few well-conducted clinical studies assessing this. In a study conducted in a medical rehabilitation clinic setting (cognitive treatment) assessing 155 patients with CP (74% alcoholic pancreatitis), low BMI and ongoing maldigestion were prevalent: 32% of patients had a BMI of <20 kg/m2; 57% had continued diarrhoea, and 24% had steatorrhoea of >30 g/day.2 In a further study evaluating patients with CP scheduled for elective surgery (n= 224), the median preoperative BMI was 22 kg/m2 (23% of patients had a BMI of <20 kg/m2). Following surgery, the median weight gain was only 2 kg (range −31 kg to +37 kg). These results suggest that many patients are malnourished prior to surgery and that the problem remains after surgery in a substantial proportion of patients with CP.3
Body weight can be divided into lean body mass (functional capacity) and fat mass (energy store). Haaber and colleagues investigated body composition in 32 patients with CP and residual pancreatic function (no enzyme supplementation) and in 26 patients with CP and severe EPI and steatorrhoea (receiving enzyme supplementation).4 Both groups showed a decrease in lean body mass, suggesting reduced functional capacity. Fat mass was also reduced in both groups, but to a significantly greater extent in patients with severe EPI compared with patients with residual pancreatic function.
What do these findings mean in terms of patient quality of life and functional capacity? A study investigating quality of life in patients with CP (n= 66) indicated that poor nutritional status negatively affects quality of life; using the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire for pancreatic cancer, this study found that 34% of patients had moderate to severe weight loss, which affected their quality of life.5 The study also found that fatigue had a major impact on quality of life in these patients, with 46% reporting moderate to severe fatigue. These findings raise questions about what we can do to improve quality of life in our patients. In a randomized controlled trial (RCT) comparing dietary counselling (n= 29) with commercial dietary supplementation (n= 31) in undernourished patients with CP (BMI of <18.5 kg/m2 or loss of >10% of body weight in the previous 6 months) receiving pancreatic enzyme replacement therapy (PERT), BMI and body weight increased and faecal fat decreased to a similar extent in both groups (Fig. 2).6 Energy intake also increased in both study groups. These data clearly show that adequate nutritional management together with PERT has the potential to improve nutritional status and maldigestion. It should be noted that impairment of digestive capacity persists in patients with EPI even with clinically adequate PERT.7 Dietary counselling together with PERT should be recommended initially for CP patients with EPI. The principals of PERT are described elsewhere in this supplement.8
Figure 2.
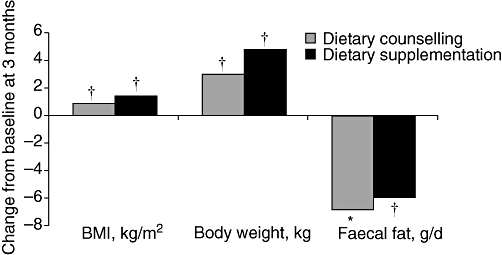
Effect of dietary counselling (n= 29) or commercial dietary supplementation (n= 31) for 3 months on body mass index (BMI), body weight and faecal fat in undernourished patients with chronic pancreatitis receiving pancreatic enzyme replacement therapy. *P= 0.007 and †P= 0.001 vs. baseline6
Nutritional support in acute pancreatitis
In patients suffering an attack of AP, enteral nutrition has been shown to be highly beneficial in reducing morbidity and multi-organ failure.9 Early enteral nutrition (within 24 h of admission to hospital) is recommended in patients with AP as it has been shown to reduce complications, length of hospital stay and mortality,9 thereby improving the disease course. A small number of studies have shown that patients who undergo surgical procedures for AP also benefit from early postoperative enteral nutrition in terms of reduced morbidity and mortality rates compared with conventional support or total parenteral nutrition.10–12
The problem of EPI should also be considered in the AP patient population. Experimental data from animal studies carried out 20 years ago showed that exocrine pancreatic secretion is impaired in AP.13 Several clinical studies have established that EPI is present during and after severe AP attacks,14–16 although the clinical relevance of this is not yet clear and RCTs are needed to assess this further. A recently published study confirmed that EPI not only occurs during AP, but persists once the attack has resolved.17 Of 86 patients initially surviving transluminal endoscopic removal of (peri) pancreatic necroses after an attack of severe AP (with or without further surgery), more than one-third (36%) were still taking PERT at a mean follow-up of 44 months (range 4–96 months). Longterm follow-up of 79 patients (median 50 months, range 15–96 months) indicated that 13% had significant weight loss and more than half (58%) had to change their diets owing to digestive problems. These data highlight the fact that many patients with EPI after severe AP still have an increased risk for malnutrition. Further evaluation of PERT and nutritional management is therefore essential after the resolution of AP in this patient population.
Nutrition following upper gastrointestinal surgery
At the time of diagnosis, patients with GI carcinomas, particularly of the upper GI tract, frequently have existing nutritional problems in the form of weight loss and increased prevalence of malnutrition, as determined by the NRS-2002. Surgery for their underlying condition is likely to increase their nutritional problems.18 It is essential that nutrition is effectively managed before, during and after surgery, as highlighted by an analysis of data from the National Veteran's Affairs Surgical Quality Improvement Program database, which includes records of 417 944 major surgical procedures performed between 1991 and 1997.19 Preoperative decreases in serum albumin and weight loss (>10% in 6 months), among others, were found to be independent risk factors for postoperative morbidity and mortality in patients undergoing non-cardiac surgery.
A meta-analysis of 27 randomized trials in patients undergoing surgery indicated that perioperative total parenteral nutrition may decrease the complication rate, especially in those with already established malnutrition (relative risk 0.52, 95% confidence interval 0.30–0.91), although individual study results appeared to be influenced by method quality and year of publication.20 No influence on mortality rate was observed in this analysis. Recent guidelines from the European Society of Enteral and Parenteral Nutrition (ESPEN) clearly recommend using enteral nutrition wherever possible.21,22 A prospective study by Gianotti et al. indicated that the timing of nutritional support has a major impact on outcome, with preoperative support being crucial.23 In this study of 305 patients with preoperative weight loss of <10% and cancer of the GI tract, preoperative oral dietary supplementation for 5 days before surgery was as effective as combined preoperative and perioperative supplementation in reducing infection rate and hospital stay compared with no nutritional support (Fig. 3), suggesting that nutritional support does not have to be longterm to be effective. Cost–benefit analysis of these data indicated preoperative nutritional supplementation can improve cost-effectiveness by reducing postoperative complication rates: cost-effectiveness values for preoperative and conventional support were €2985 and €6245, respectively.24
Figure 3.
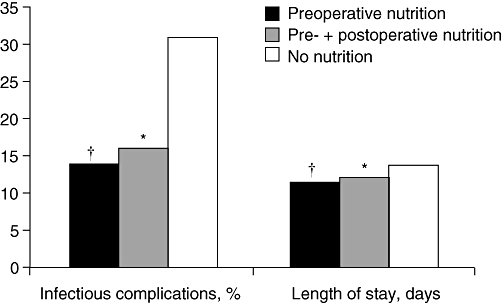
Comparison of preoperative oral supplementation (arginine, omega-3 fatty acids and ribonucleotide) for 5 days (n= 102) with the same preoperative therapy plus postoperative jejunal infusion (n= 101) and no artificial nutrition before or after surgery (n= 102). *P < 0.05 and †P < 0.01 vs. no nutrition23
In summary, nutrition needs to be managed effectively at all three stages of surgery in patients with EPI, including preoperatively (e.g. in anorexia or weight loss), perioperatively (e.g. in patients with an inflammatory response or metabolic risk) and postoperatively (e.g. in convalescence or functional improvement). Patient care can be improved considerably by preoperative screening to identify patients with high risk for malnutrition (weight loss of >10% in 3–6 months, BMI of <18 kg/m2, albumin <30 g/l) and by providing nutritional support before surgery. In the perioperative period, metabolic control can be increased by providing early enteral nutrition in patients who are not able to ingest a normal diet. Postoperatively, a significant proportion of patients still require nutritional support, including counselling and PERT as appropriate.
Conclusions
Patients with EPI have an increased risk for malnutrition, which in turn increases morbidity and mortality and negatively impacts on quality of life. Along with PERT, individually tailored nutritional management, including counselling and oral supplements as appropriate, can improve nutritional status, reduce morbidity and improve functional status and quality of life in patients with CP (Fig. 4). Exocrine pancreatic function is impaired in severe AP and remains impaired after the resolution of the attack; patients with AP are therefore at continued risk for malnutrition and should be monitored appropriately. For patients undergoing upper GI tract surgery, pre- and perioperative nutritional support – mainly by enteral nutrition – are cost-effective interventions that improve outcomes. Nutritional management, including nutritional risk assessment and individualized nutritional support, is therefore a key component of an effective multimodal therapeutic approach in diseases associated with EPI, such as CP and AP, and in patients undergoing GI surgery.
Figure 4.
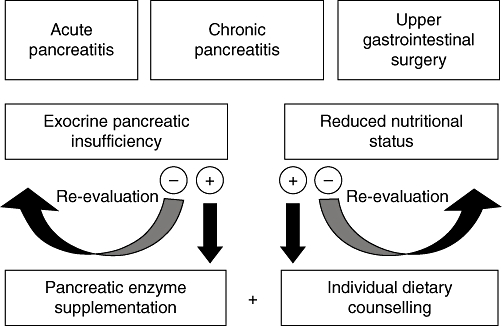
Role of nutritional management in diseases with exocrine pancreatic insufficiency
Disclosure
This supplement is supported by Solvay Pharmaceuticals Marketing and Licensing AG, Allschwill, Switzerland. Writing and editorial assistance was provided for this article by Helen Varley PhD, Envision Scientific Solutions, Horsham, UK and funded by Solvay Pharmaceuticals Marketing and Licensing AG.
Conflicts of interest
Johann Ockenga is a consultant and speaker for Solvay Pharmaceuticals GmbH, Hannover, Germany.
References
- 1.Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krähenbühl L, Meier R, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr. 2008;27:340–349. doi: 10.1016/j.clnu.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Armbrecht U. Chronic pancreatitis: weight loss and poor physical performance – Experience from a specialized rehabilitation centre. Rehabilitation (Stuttg) 2001;40:332–336. doi: 10.1055/s-2001-18966. [DOI] [PubMed] [Google Scholar]
- 3.Riediger H, Adam U, Fischer E, Keck T, Pfeffer F, Hopt UT, et al. Long-term outcome after resection for chronic pancreatitis in 224 patients. J Gastrointest Surg. 2007;11:949–959. doi: 10.1007/s11605-007-0155-6. discussion 959–960. [DOI] [PubMed] [Google Scholar]
- 4.Haaber AB, Rosenfalck AM, Hansen B, Hilsted J, Larsen S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int J Pancreatol. 2000;27:21–27. doi: 10.1385/IJGC:27:1:21. [DOI] [PubMed] [Google Scholar]
- 5.Fitzsimmons D, Kahl S, Butturini G, van Wyk M, Bornman P, Bassi C, et al. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol. 2005;100:918–926. doi: 10.1111/j.1572-0241.2005.40859.x. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Midha S, Singh N, Joshi YK, Garg PK. Dietary counseling versus dietary supplements for malnutrition in chronic pancreatitis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2008;6:353–359. doi: 10.1016/j.cgh.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 7.Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54(Suppl 6):vi1–vi28. doi: 10.1136/gut.2005.065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domínguez-Muñoz JE. Pancreatic enzyme replacement therapy: exocrine pancreatic insufficiency after gastrointestinal surgery. HPB. 2009;11(Suppl 3):3–6. doi: 10.1111/j.1477-2574.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marik PE. What is the best way to feed patients with pancreatitis? Curr Opin Crit Care. 2009;15:131–138. doi: 10.1097/MCC.0b013e328319910a. [DOI] [PubMed] [Google Scholar]
- 10.Hernández-Aranda JC, Gallo-Chico B, Ramírez-Barba EJ. [Nutritional support in severe acute pancreatitis. Controlled clinical trial. Nutr Hosp. 1996;11:160–166. [PubMed] [Google Scholar]
- 11.Pupelis G, Austrums E, Jansone A, Sprucs R, Wehbi H. Randomised trial of safety and efficacy of postoperative enteral feeding in patients with severe pancreatitis: preliminary report. Eur J Surg. 2000;166:383–387. doi: 10.1080/110241500750008934. [DOI] [PubMed] [Google Scholar]
- 12.Pupelis G, Selga G, Austrums E, Kaminski A. Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition. 2001;17:91–94. doi: 10.1016/s0899-9007(00)00508-6. [DOI] [PubMed] [Google Scholar]
- 13.Niederau C, Niederau M, Lüthen R, Strohmeyer G, Ferrell LD, Grendell JH. Pancreatic exocrine secretion in acute experimental pancreatitis. Gastroenterology. 1990;99:1120–1127. doi: 10.1016/0016-5085(90)90633-c. [DOI] [PubMed] [Google Scholar]
- 14.Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology. 2003;3:303–308. doi: 10.1159/000071768. [DOI] [PubMed] [Google Scholar]
- 15.Migliori M, Pezzilli R, Tomassetti P, Gullo L. Exocrine pancreatic function after alcoholic or biliary acute pancreatitis. Pancreas. 2004;28:359–363. doi: 10.1097/00006676-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Symersky T, van Hoorn B, Masclee AA. The outcome of a long-term follow-up of pancreatic function after recovery from acute pancreatitis. JOP. 2006;7:447–453. [PubMed] [Google Scholar]
- 17.Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study) Gut. 2009;58:1260–1266. doi: 10.1136/gut.2008.163733. [DOI] [PubMed] [Google Scholar]
- 18.Bozzetti F. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer. 2009;17:279–284. doi: 10.1007/s00520-008-0476-3. [DOI] [PubMed] [Google Scholar]
- 19.Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB, et al. The Department of Veterans Affairs' NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 1998;228:491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyland DK, Montalvo M, MacDonald S, Keefe L, Su XY, Drover JW. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg. 2001;44:102–111. [PubMed] [Google Scholar]
- 21.Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN guidelines on parenteral nutrition: surgery. Clin Nutr. 2009;28:378–386. doi: 10.1016/j.clnu.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, et al. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr. 2006;25:224–244. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122:1763–1770. doi: 10.1053/gast.2002.33587. [DOI] [PubMed] [Google Scholar]
- 24.Braga M, Gianotti L. Preoperative immunonutrition: cost-benefit analysis. JPEN J Parenter Enteral Nutr. 2005;29(Suppl. 1):S57–S61. doi: 10.1177/01486071050290S1S57. [DOI] [PubMed] [Google Scholar]


