Abstract
Exocrine pancreatic insufficiency (EPI) and resultant maldigestion occurs in up to 80% of patients following gastric, duodenal or pancreatic surgery. Accurate diagnosis is required to determine the appropriate intervention, but the conventional method of faecal fat quantification is time-consuming and not always readily available. The optimized 13C-mixed triglyceride (13C-MTG) breath test is an accurate alternative post-surgery. Pancreatic enzyme replacement therapy (PERT) is indicated post-surgery in patients with clinically evident steatorrhoea, weight loss or maldigestion-related symptoms. Given its favourable safety profile, PERT is also appropriate in asymptomatic patients with high faecal fat excretion as such patients are at high risk for nutritional deficits. However, published data evaluating PERT in this setting are limited. Uncoated powder preparations may be preferred in cases of low gastric acidity and partial or total gastric resection. In clinical studies, enteric-coated microspheres were associated with greater weight gain after surgery vs. uncoated preparations. This was confirmed in a recent study using the 13C-MTG breath test; fat absorption increased from <40% without therapy to almost 60% with enteric-coated minimicrospheres (40 000 lipase units/meal), with >60% of patients achieving normal breath test results (i.e. normal fat digestion) during PERT. A therapeutic algorithm for the treatment of EPI after surgery is also discussed.
Keywords: exocrine pancreatic insufficiency, gastrointestinal surgical procedure, maldigestion, pancreatic enzyme replacement therapy, pancreatin
Introduction
Exocrine pancreatic insufficiency (EPI) is a common and severe complication of different pancreatic as well as extra-pancreatic diseases. Acute necrotizing pancreatitis, chronic pancreatitis, cystic fibrosis and pancreatic cancer are well-known causes of EPI.1 Measurements of faecal levels of pancreatic elastase have indicated that diabetes mellitus leads to impaired pancreatic secretion and potentially to EPI.2 The possibility of exocrine insufficiency should also be considered in extra-pancreatic conditions, such as coeliac disease and Crohn's disease.3,4 Because they distort normal anatomy and consequently alter digestive physiology, gastric, duodenal and pancreatic surgeries are important causes of maldigestion in themselves. The present article reviews the pathophysiology, clinical consequences, diagnosis and therapy of EPI after gastrointestinal (GI) and pancreatic surgery.
Physiology
Gastric emptying of nutrients is regulated by fundus relaxation, antral motility and the motor activity of the pylorus. These functions are tightly regulated by antro-fundic reflexes (fundus relaxation secondary to the presence of nutrients in the gastric antrum) and duodenogastric reflexes (fundus relaxation and inhibition of antral motility as a consequence of the presence of nutrients within the duodenal lumen) (Fig. 1). In this way, only particles sized <2 mm are able to pass through the pylorus and thus nutrients reach the duodenum in a slow and progressive manner. This allows pancreatic secretions to exert digestive activity at the appropriate site.
Figure 1.
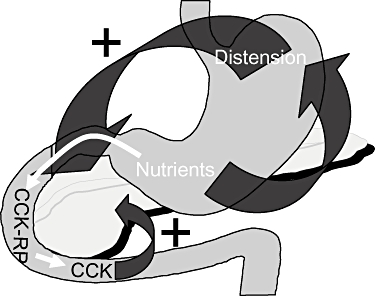
Mechanisms of control of post-prandial gastric and pancreatic function. CCK, cholecystokinin; CCK-RP, cholecystokinin-releasing peptide
Post-prandial pancreatic secretion is first neurally stimulated by fundus relaxation, which triggers a vagal reflex (neurally mediated post-prandial stimulation of exocrine pancreatic secretion). Thereafter, the release of cholecystokinin (CCK) in response to the nutrient-stimulated duodenal secretion of CCK-releasing peptide represents the major hormone-mediated post-prandial stimulation of exocrine pancreatic secretion (Fig. 1).
Pathophysiology
Anatomical changes secondary to GI and pancreatic surgery lead to important physiological alterations that frequently cause maldigestion. Total or partial resections of the stomach, with or without duodenal resection (e.g. in the context of the Whipple procedure), as well as partial pancreatic resection, are associated with the following events: (i) disturbance of fundus relaxation caused by the disappearance of antro-fundic and duodeno-fundic reflexes; (ii) absence of neurally stimulated pancreatic secretion caused by the lack of fundus relaxation; (iii) reduction in CCK-mediated stimulation of pancreatic secretion secondary to duodenal resection; (iv) large and hard-to-digest nutrient particles reaching the jejunal lumen because of resection of the distal stomach; (v) reduction in exocrine pancreatic secretion in cases of pancreatic resection; and (vi) asynchrony between the gastric emptying of nutrients and bilio-pancreatic secretion as a result of anatomical reconstruction.
A previous study by Ito supported these findings by demonstrating an abnormally low post-prandial CCK release after duodenopancreatectomy, but not after duodenum-preserving pancreatic head resection.5 Low CCK release is in turn associated with abnormal exocrine pancreatic function, as measured by the N-benzoyl-l-tyrosyl-p-aminobenzoic acid (BT-PABA) test.5 All of the alterations mentioned above are responsible for primary and secondary EPI. As a consequence, maldigestion develops in up to 80% of patients who have been operated upon for gastric or pancreatic diseases.6–8
Diagnosis
Diagnosis of maldigestion is highly relevant in patients after gastroduodenal or pancreatic resection in order to evaluate the need for oral pancreatic enzyme replacement therapy (PERT). Faecal elastase has been used frequently to evaluate pancreatic exocrine function after pancreatic surgery, mainly pancreatoduodenectomy.9 However, that faecal elastase is a measure of pancreatic secretion must be taken into account, whereas other factors like the asynchrony between the gastric emptying of nutrients and pancreatic secretion, which cannot be evaluated by quantification of faecal elastase, also play a key role in maldigestion after surgery. Faecal chymotrypsin has also been used by other authors10 to evaluate the frequency of EPI after different pancreatic surgical procedures, but again faecal chymotrypsin reflects only pancreatic secretion and not digestion.
Fat digestion in this setting is best evaluated by the quantification of the coefficient of fat absorption by assessing faecal fat, using either the standard Van de Kamer test or the more recent near infrared analysis. An exact evaluation of the fat ingested is required for this purpose, which frequently requires the patient to be admitted to hospital for 5 days for the test to be performed. As an alternative, maldigestion secondary to gastroduodenal and pancreatic surgery can be accurately evaluated by means of the optimized 13C-mixed triglyceride (13C-MTG) breath test.11 In this context, it is critical that the test is performed as previously optimized, mainly in terms of substrate dose, fat content of the test meal, pre-test oral administration of metoclopramide, and timing and duration of breath sample collection.12–14 Using this test, we have recently demonstrated that up to 82% of patients suffer from fat maldigestion after the Whipple procedure or pylorus-preserving pancreatoduodenectomy (Domínguez-Muñoz et al., unpublished data, 2009).
Ideally, the ability to routinely assess the coefficient of fat absorption by faecal fat quantification or to perform the 13C-MTG breath test should be widely available in clinical practice. This is important not only in testing for EPI, but also in objectively evaluating the efficacy of oral PERT.14 However, as the vast majority of patients develop fat maldigestion after duodenopancreatectomy, the prescription of oral pancreatic enzyme supplements in all these patients should be recommended in centres where the evaluation of fat digestion is not readily available.
Therapy
PERT is indicated in patients who have undergone GI surgery with clinically evident steatorrhoea, weight loss or maldigestion-related symptoms. Whether asymptomatic patients with an abnormally high daily faecal fat excretion are candidates for substitution therapy is debatable. The fact that these patients are at high risk for developing nutritional deficits represents an indication for replacement therapy in this situation, which is supported by evidence of the safety of oral pancreatic enzyme preparations. Thus, the vast majority of patients undergoing any type of GI and pancreatic resection will require oral PERT.
Despite the relevance of EPI in assessing the nutritional status of patients who have had surgery, the number of studies evaluating the usefulness of PERT in this setting is limited and data regarding the best preparation to be used are scarce.15 Uncoated powder preparations may be preferred in cases of low gastric acidity and partial or total gastric resection, although enteric-coated enzyme microspheres have been shown to be associated with a higher body weight gain compared with uncoated preparations in patients after duodenopancreatectomy.16 In fact, body weight after surgery increases very slowly when uncoated enzymes are used, whereas a much more relevant increase in body weight is obtained by the oral administration of enteric-coated preparations in the form of microspheres (Fig. 2).16 Another study has supported the finding that oral enzyme replacement therapy in the form of enteric-coated microspheres is highly effective in treating post-surgical fat maldigestion.17 We have recently confirmed these data using the 13C-MTG breath test. The proportion of fat digested and absorbed as measured by the 13C-MTG breath test increases from <40% without therapy to almost 60% on PERT in the form of enteric-coated minimicrospheres at a dose of 40 000 units (U) of lipase/meal and 20 000 U lipase/snack (i.e. 160 000 U lipase/day consumed in the course of three main meals plus two snacks). Almost 60% of patients are able to achieve a normal breath test result (i.e. indicating normal fat digestion) during this replacement therapy, whereas higher doses of enzymes (80 000–100 000 U lipase/meal and 40 000–50 000 U lipase/snack for a total of ≤400 000 U lipase/day) may be required in other patients. If gastric acid secretion is preserved, the addition of a proton pump inhibitor improves the therapeutic efficacy of oral pancreatic enzymes in patients with insufficient response to therapy despite the administration of high doses of oral pancreatic enzymes.1 Finally, testing for bacterial overgrowth and treating if positive, and the addition of loperamide, may help in reducing steatorrhoea in patients with abnormal fat digestion despite high doses of enzymes and the addition of a proton pump inhibitor.1 A therapeutic algorithm for the management of EPI after gastroduodenal or pancreatic surgery is summarized in Fig. 3.
Figure 2.
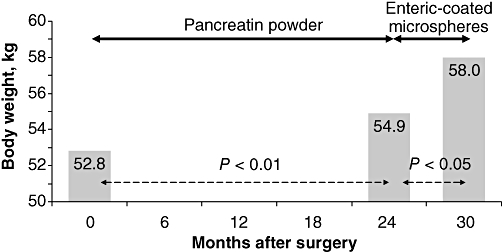
Changes in body weight after duodenopancreatectomy. A mean increase in body weight of only 2 kg is obtained after 2 years of enzyme substitution therapy. A further mean body weight increase of 3 kg is obtained after just 6 months of therapy with pancreatic enzymes in the form of enteric-coated microspheres16
Figure 3.
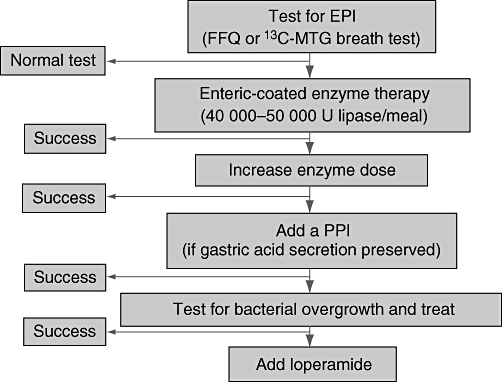
Therapeutic algorithm for exocrine pancreatic insufficiency (EPI) in patients after gastroduodenal or pancreatic surgery. FFQ, faecal fat quantification; 13C-MTG, 13C-mixed triglyceride; U, units; PPI, proton pump inhibitor
Disclosure
This supplement is supported by Solvay Pharmaceuticals Marketing and Licensing AG, Allschwill, Switzerland. Editorial assistance was provided by Helen Varley PhD, Envision Scientific Solutions, Horsham, UK and supported by Solvay Pharmaceuticals Marketing and Licensing AG.
Conflicts of interest
Dr Domínguez-Muñoz has acted as an occasional consultant for Solvay Pharmaceuticals.
References
- 1.Domínguez-Muñoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007;9:116–122. doi: 10.1007/s11894-007-0005-4. [DOI] [PubMed] [Google Scholar]
- 2.Ewald N, Raspe A, Kaufmann C, Bretzel RG, Kloer HU, Hardt PD. Determinants of exocrine pancreatic function as measured by fecal elastase-1 concentrations (FEC) in patients with diabetes mellitus. Eur J Med Res. 2009;14:118–122. doi: 10.1186/2047-783X-14-3-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leeds JS, Hopper AD, Hurlstone DP, Edwards SJ, McAlindon ME, Lobo AJ, et al. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther. 2007;25:265–271. doi: 10.1111/j.1365-2036.2006.03206.x. [DOI] [PubMed] [Google Scholar]
- 4.Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22:202–206. doi: 10.1097/00004836-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Ito K. Duodenum preservation in pancreatic head resection to maintain pancreatic exocrine function (determined by pancreatic function diagnostant test and cholecystokinin secretion) J Hepatobiliary Pancreat Surg. 2005;12:123–128. doi: 10.1007/s00534-004-0954-z. [DOI] [PubMed] [Google Scholar]
- 6.Büchler M, Malfertheiner P, Glasbrenner B, Friess H, Beger HG. Secondary pancreatic insufficiency following partial and total gastrectomy. Nutrition. 1988;4:314–316. [Google Scholar]
- 7.Friess H, Böhm J, Müller MW, Glasbrenner B, Riepl RL, Malfertheiner P, et al. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol. 1996;91:341–347. [PubMed] [Google Scholar]
- 8.Prinz RA, Greenlee HB. Pancreatic duct drainage in 100 patients with chronic pancreatitis. Ann Surg. 1981;194:313–320. doi: 10.1097/00000658-198109000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto J, Traverso LW. Exocrine function following the Whipple operation as assessed by stool elastase. J Gastrointest Surg. 2006;10:1225–1229. doi: 10.1016/j.gassur.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias-García J, Vilariño-Insua M, Iglesias-Rey M, Lourido MV, Domínguez-Muñoz JE. Accuracy of the optimized 13C-mixed triglyceride breath test for the diagnosis of exocrine pancreatic insufficiency in patients after gastrointestinal resection. Pancreatology. 2003;3:457–481. Abstract 43; Third Meeting of Mediterranean Societies of Pancreatology, 11–13 December 2003, Santiago de Compostela, Spain. [Google Scholar]
- 12.Domínguez-Muñoz JE, Iglesias-García J, Iglesias-Rey M, Figueiras A, Vilariño-Insua M. Effect of the administration schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005;21:993–1000. doi: 10.1111/j.1365-2036.2005.02390.x. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez-Muñoz JE, Iglesias-García J, Iglesias-Rey M, Vilariño-Insua M. Optimising the therapy of exocrine pancreatic insufficiency by the association of a proton pump inhibitor to enteric coated pancreatic extracts. Gut. 2006;55:1056–1057. doi: 10.1136/gut.2006.094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domínguez-Muñoz JE, Iglesias-García J, Vilariño-Insua M, Iglesias-Rey M. 13C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2007;5:484–488. doi: 10.1016/j.cgh.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Lankisch PG. Appropriate pancreatic function tests and indication for pancreatic enzyme therapy following surgical procedures on the pancreas. Pancreatology. 2001;1(Suppl 1):14–26. [Google Scholar]
- 16.Braga M, Cristallo M, De Franchis R, Mangiagalli A, Zerbi A, Agape D, et al. Pancreatic enzyme replacement therapy in post-pancreatectomy patients. Int J Pancreatol. 1989;5(Suppl):37–44. [PubMed] [Google Scholar]
- 17.Bruno MJ, Borm JJ, Hoek FJ, Delzenne B, Hofmann AF, de Goeij JJ, et al. Comparative effects of enteric-coated pancreatin microsphere therapy after conventional and pylorus-preserving pancreatoduodenectomy. Br J Surg. 1997;84:952–956. doi: 10.1002/bjs.1800840712. [DOI] [PubMed] [Google Scholar]


