Abstract
Exocrine pancreatic insufficiency (EPI) is a common problem after surgery of the pancreas and stomach. It is usually caused by inadequate pancreatic enzyme activity resulting from insufficient enzyme production, insufficient enzyme activation or disturbed enzyme deactivation. A variety of direct and indirect pancreatic function tests such as the secretin–cerulein test, the faecal elastase test and the 13C-mixed triglyceride breath test are used to assess exocrine pancreatic function. Few studies have addressed pancreatic enzyme replacement therapy (PERT) following pancreatic surgery. These studies suggest beneficial effects of enzyme replacement after pancreatic resections. A number of studies have been performed to assess post-gastrectomy maldigestion and PERT. The treatment options remain controversial, although the published evidence is in favour of PERT leading to an overall improvement of symptoms. In conclusion, EPI following pancreatic surgery and total or partial gastrectomy remains a common clinical challenge. As a result of the lack of solid evidence, more clinical trials, particularly randomized, controlled clinical trials, are urgently needed.
Keywords: exocrine pancreatic insufficiency, pancreatic function tests, maldigestion, pancreatic enzyme replacement therapy, pancreatin
Introduction
Exocrine pancreatic insufficiency (EPI) is a common clinical problem after pancreatic or gastric surgery.1 It is defined as inadequate pancreatic enzyme activity for digestion, caused by insufficient enzyme production, insufficient enzyme activation or disturbed enzyme deactivation.2 Other than post-surgery patients, EPI mostly affects infants and children suffering from cystic fibrosis, Shwachman–Diamond syndrome or pancreatic agenesis. In adults, the aetiology is diverse, with the underlying diseases being acute or chronic pancreatitis, pancreatic neoplasms, pancreatic resections (partial or total pancreatectomy), short bowel syndromes, hereditary haemochromatosis and partial or total gastrectomy. Importantly, elderly patients newly diagnosed with symptoms of EPI should undergo an immediate check-up for pancreatic cancer, particularly if co-morbid diabetes mellitus has also been newly diagnosed.3 The symptoms of EPI are maldigestion or malabsorption and, specifically, malnutrition with weight loss, steatorrhoea, vitamin deficiencies (especially of vitamins A, D, E, K) and, of course, diabetes mellitus, which in turn may also be a cause of EPI.
Pancreatic function tests
The diagnosis of EPI is made by either direct or indirect pancreatic function tests (Table 1).4,5 The cholecystokinin (CCK) test, the secretin test, the secretin–CCK test, the endoscopic pancreatic function test and the Lundh test are direct tests potentially available in clinical practice.6 In the CCK test, the patient receives CCK or an analogue, and enzymes are collected using a duodenal tube during an 18-min test period.7 The disadvantage of this test is that the determination of perfusion markers requires a specialized laboratory. The secretin test also makes use of a double-lumen gastroduodenal tube with which samples are collected at 15-min intervals during a 1-h period after secretin simulation.4,8 This test is relatively easy to perform and constitutes one of the most frequently used tests in the clinical setting. In addition, the secretin–CCK test can be performed; however, the secretin-induced rapid water flow may result in diluted and unreliable enzyme collections. In addition to these classical tests, the endoscopic pancreatic function test is sometimes used, with endoscopic aspirations at 0, 15, 30, 45 and 60 min. This test has recently attracted considerable clinical attention because it is preferred by many patients for the greater comfort with which it can be performed compared with other tests, and the availability of standardized endoscopy.9 The Lundh test, in which a duodenal tube is necessary, is usually confounded in patients with small bowel mucosal diseases and is more complicated to carry out because it requires a liquid test meal as a stimulus.10–12 However, these direct pancreatic function tests are less comfortable for the patient and therefore indirect pancreatic function tests are considered to be more acceptable to patients and their doctors.
Table 1.
Summary of direct and indirect pancreatic function tests
| Tests | Disadvantages |
|---|---|
| Direct tests | |
| CCK | Requires specialized laboratory |
| Secretin | 1-h collection |
| Secretin–CCK | Potential for diluted, unreliable enzyme collections |
| Endoscopic pancreatic function | None |
| Lundh | Confounded in small bowel mucosal diseases |
| Indirect tests | |
| Faecal elastase-1 | Poor sensitivity for early EPI |
| 24-h and 72-h stool fat | Often inadequate patient compliance |
| Secretin-enhanced MRI | Limited assessment |
| Serum/urine pancreolauryl | Limited in bile salt deficiency, coeliac disease, renal failure and post-gastrectomy |
| 13C-mixed triglyceride | Currently under evaluation |
CCK, cholecystokinin; EPI, exocrine pancreatic insufficiency; MRI, magnetic resonance imaging
The faecal elastase-1 test remains one of the most frequently used indirect tests in the clinical setting, although it has a poor sensitivity for early pancreatic insufficiency.13,14 However, it is highly sensitive and specific for advanced EPI and is easily performed. The 24-h and 72-h stool fat tests are often affected by inadequate patient compliance and are therefore rarely used. In addition, there are novel indirect tests that have not yet been extensively studied, such as the secretin-enhanced magnetic resonance imaging test. The classical serum or urine pancreolauryl tests, which measure intraluminal pancreatic enzyme function, are limited in patients with bile salt deficiency, coeliac disease, renal failure and post-gastrectomy symptoms, and are therefore also being abandoned in the clinical setting.15 Most recently, the 13C-mixed triglyceride breath test has attracted considerable clinical attention and is being evaluated in clinical studies. Its results are promising; the test is simple and its findings are reliable.16–19
Treatment options
The therapeutic options for the treatment of EPI involve, firstly, nutritional changes, which include a high-carbohydrate diet, a normal fat diet and, optionally, medium-chain triglycerides, consumed in several (five to seven) small meals.20 These changes are easily implemented and can be recommended for most patients suffering from EPI following surgery of the pancreas or stomach.
In addition, pancreatic enzyme replacement therapy (PERT) may be provided orally; the dose can be adapted to meals and should not fall below 25 000–50 000 units (U) of lipase/meal. As lipase is irreversibly deactivated by gastric acid, patients receiving uncoated enzyme preparations may require simultaneously administered proton pump inhibitors (PPIs).21 Omitting PPIs is common because many patients are not aware of the importance of these drugs in conjunction with uncoated enzyme formulations. Enteric-coated enzyme preparations are resistant to gastric acid and in the majority of patients do not require concomitant PPI administration; however, in patients with EPI who have an incomplete response to PERT, the addition of a PPI may significantly improve and even normalize fat digestion.21 To prevent low vitamin levels, especially in patients with severe diarrhoea, liposoluble vitamins (A, D, E, K) must be parenterally substituted.
Clinical considerations
Acute pancreatitis
Following acute pancreatitis, the incidence of EPI mostly depends on the severity of the attack, but it occurs in up to 86% of patients who have suffered from severe acute necrotizing pancreatitis.22,23 Furthermore, the severity of the disease is not only associated with EPI, but, of course, also with endocrine pancreatic insufficiency.
Post-pancreatectomy EPI
Exocrine pancreatic insufficiency is common following pancreatic resections, including classical or pylorus-preserving Whipple operations, duodenum-preserving pancreatic head resections, left pancreatic resections and resections for benign tumours.24,25 PERT has been tested in only two randomized clinical trials. In one study, the absorption coefficient for fat was significantly worse in the placebo group, as was the absorption coefficient for energy (Fig. 1).26 In a study by Neoptolemos et al., 39 patients were randomized to standard- or high-dose pancreatin following pancreatic resection.27 Although stool fat excretion and stool volume (P < 0.0001) and stool frequency (P < 0.01) correlated with the pancreatin dose, there were no associations between PERT and abdominal pain and global symptoms. Therefore, current evidence does not generally recommend the use of PERT after pancreatic surgery. However, clinical experience favours enzyme supplementation, including ≤100 000 U pancreatin (CREON®; Solvay Pharmaceuticals GmbH, Hannover, Germany) taken together with meals. If these treatment options are not successful, further diagnostic measures should be taken.
Figure 1.
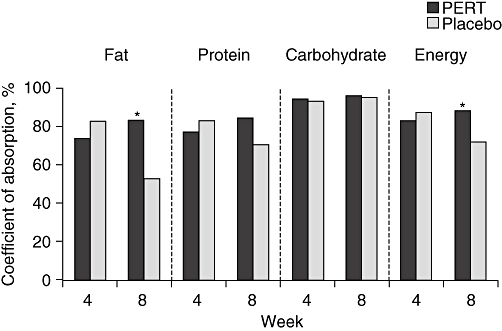
Outcomes after 4 weeks of treatment with pancreatic enzyme replacement therapy (PERT) or placebo in patients who had previously received 4 weeks of individualized PERT following surgery for chronic pancreatitis. *P= 0.02 vs. placebo26
As there is no randomized, controlled trial evidence regarding PERT following pancreatic resections, randomized clinical studies are urgently required.
Post-gastrectomy EPI
Following gastric resections, EPI is a common clinical problem that may reflect a reduced enzyme-release response to endogenous stimulation or to reduced enzyme activation caused by bacterial overgrowth.25 In a study by Friess et al., 15 patients who had undergone total gastrectomy with preservation of the duodenal food passage were tested for pancreatic juice volume, trypsin, chymotrypsin, amylase and bicarbonate secretion after stimulation.28 All of these parameters were found to be reduced following total gastrectomy compared with preoperative levels. Furthermore, these patients had pathological glucose tolerance, with increased plasma insulin and glucagon levels. These findings were associated with significantly lower gastrin and pancreatic polypeptide secretion, although CCK plasma secretion was increased. Armbrecht et al. conducted a double-blind, crossover study of 15 patients who underwent surgery for gastric cancer (gastric resection) and compared PERT with placebo.29 The authors assessed abdominal symptoms, bowel habits, faecal fat excretion and oro-caecal transit time. The median postoperative follow-up was 20 months (range 4–156 months). The number of stools did not differ between the placebo and treatment groups. However, the stool score was significantly lower (better) and the faecal fat excretion significantly reduced in the group of patients who were treated with PERT. Therefore, the authors concluded that PERT after gastric resection for cancer reduced steatorrhoea and improved stool consistency, but had no influence on pain, vomiting, nausea, bloating or dumping. In another study by Brägelmann et al., 52 institutionalized patients with a faecal fat output ≥14 g/day were randomized to receive either pancreatic enzymes or placebo for 14 days.30 All of these patients had undergone total gastrectomy for gastric cancer and their postoperative follow-up ranged between 39 and 869 days. The endpoints of this study were abdominal symptoms, faecal frequency and faecal consistency. Interestingly, an overall improvement in symptoms was found in significantly more enzyme-treated patients than placebo-treated subjects (P < 0.01). However, no differences were found regarding body mass index, bowel habits or fat malassimilation. The authors concluded that PERT after total gastrectomy has a positive effect on overall symptoms.
Conclusions
Exocrine pancreatic insufficiency after partial or total gastrectomy is a main cause of maldigestion and postoperative weight loss and should be treated with adequate PERT. Although the studies conducted so far have included low numbers of patients and have been unable to draw firm conclusions, they seem to justify PERT in those patients who suffer from the classical clinical symptoms of EPI following gastrectomy. Adequate substitution with pancreatic enzymes prevents maldigestion, improves postoperative nutritional status and may improve non-specific symptoms. However, in patients who have undergone pancreatic resections or who suffer from primary pancreatic disorders (such as acute or chronic pancreatitis), the evidence is much less solid. Although PERT seems justified from a clinical viewpoint, no large randomized, controlled clinical trials have yet been performed. Such trials are urgently required to confirm the clinical assumption that PERT is necessary in most patients following pancreatic resection.
Disclosure
This supplement is supported by Solvay Pharmaceuticals Marketing and Licensing AG, Allschwill, Switzerland. Editorial assistance was provided by Helen Varley PhD, Envision Scientific Solutions, Horsham, UK and supported by Solvay Pharmaceuticals Marketing and Licensing AG.
Conflicts of interest
Professor Friess has received a travel grant from Solvay Pharmaceuticals.
References
- 1.Nakamura H, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, et al. Predictive factors for exocrine pancreatic insufficiency after pancreatoduodenectomy with pancreaticogastrostomy. J Gastrointest Surg. 2009;13:1321–1327. doi: 10.1007/s11605-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 2.Domínguez-Muñoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007;9:116–122. doi: 10.1007/s11894-007-0005-4. [DOI] [PubMed] [Google Scholar]
- 3.Rothenbacher D, Löw M, Hardt PD, Klör HU, Ziegler H, Brenner H. Prevalence and determinants of exocrine pancreatic insufficiency among older adults: results of a population-based study. Scand J Gastroenterol. 2005;40:697–704. doi: 10.1080/00365520510023116. [DOI] [PubMed] [Google Scholar]
- 4.Keller J, Aghdassi AA, Lerch MM, Mayerle JV, Layer P. Tests of pancreatic exocrine function – clinical significance in pancreatic and non-pancreatic disorders. Best Pract Res Clin Gastroenterol. 2009;23:425–439. doi: 10.1016/j.bpg.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Pungpapong S, Raimondo M. Endoscopy-based pancreatic function tests. Curr Gastroenterol Rep. 2006;8:127–131. doi: 10.1007/s11894-006-0008-6. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury RS, Forsmark CE. Pancreatic function testing. Aliment Pharmacol Ther. 2003;17:733–750. doi: 10.1046/j.1365-2036.2003.01495.x. [DOI] [PubMed] [Google Scholar]
- 7.Ribet A, Tournut R, Duffaut M, Vaysse N. Use of caerulein with submaximal doses of secretin as a test of pancreatic function in man. Gut. 1976;17:431–434. doi: 10.1136/gut.17.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregg JA, Sharma MM. Endoscopic measurement of pancreatic juice secretory flow rates and pancreatic secretory pressures after secretin administration in human controls and in patients with acute relapsing pancreatitis, chronic pancreatitis, and pancreatic cancer. Am J Surg. 1978;136:569–574. doi: 10.1016/0002-9610(78)90312-4. [DOI] [PubMed] [Google Scholar]
- 9.O'Keefe SJ, Stevens S, Lee R, Zhou W, Zfass A. Physiological evaluation of the severity of pancreatic exocrine dysfunction during endoscopy. Pancreas. 2007;35:30–36. doi: 10.1097/mpa.0b013e3180646775. [DOI] [PubMed] [Google Scholar]
- 10.Rolny P, Jagenburg R. The secretin–CCK test and a modified Lundh test. A comparative study. Scand J Gastroenterol. 1978;13:927–931. doi: 10.3109/00365527809181370. [DOI] [PubMed] [Google Scholar]
- 11.Lundh G. Pancreatic secretion after stimulation by a test meal. Lakartidningen. 1968;65:4575–4580. [PubMed] [Google Scholar]
- 12.Lankisch PG. Function tests in the diagnosis of chronic pancreatitis. Critical evaluation. Int J Pancreatol. 1993;14:9–20. doi: 10.1007/BF02795225. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto J, Traverso LW. Exocrine function following the Whipple operation as assessed by stool elastase. J Gastrointest Surg. 2006;10:1225–1229. doi: 10.1016/j.gassur.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Naruse S, Ishiguro H, Ko SB, Yoshikawa T, Yamamoto T, Yamamoto A, et al. Fecal pancreatic elastase: a reproducible marker for severe exocrine pancreatic insufficiency. J Gastroenterol. 2006;41:901–908. doi: 10.1007/s00535-006-1884-0. [DOI] [PubMed] [Google Scholar]
- 15.Elphick DA, Kapur K. Comparing the urinary pancreolauryl ratio and faecal elastase-1 as indicators of pancreatic insufficiency in clinical practice. Pancreatology. 2005;5:196–200. doi: 10.1159/000085271. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura H, Morifuji M, Murakami Y, Uemura K, Ohge H, Hayashidani Y, et al. Usefulness of a 13C-labeled mixed triglyceride breath test for assessing pancreatic exocrine function after pancreatic surgery. Surgery. 2009;145:168–175. doi: 10.1016/j.surg.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Herzog DC, Delvin EE, Albert C, Marcotte JE, Pelletier VA, Seidman EG. 13C-labeled mixed triglyceride breath test (13C MTG-BT) in healthy children and children with cystic fibrosis (CF) under pancreatic enzyme replacement therapy (PERT): a pilot study. Clin Biochem. 2008;41:1489–1492. doi: 10.1016/j.clinbiochem.2008.08.087. [DOI] [PubMed] [Google Scholar]
- 18.Braden B, Lembcke B, Kuker W, Caspary WF. 13C-breath tests: current state of the art and future directions. Dig Liver Dis. 2007;39:795–805. doi: 10.1016/j.dld.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Domínguez-Muñoz JE, Iglesias-García J, Vilariño-Insua M, Iglesias-Rey M. 13C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2007;5:484–488. doi: 10.1016/j.cgh.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108:832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Domínguez-Muñoz JE, Iglesias-García J, Iglesias-Rey M, Vilariño-Insua M. Optimising the therapy of exocrine pancreatic insufficiency by the association of a proton pump inhibitor to enteric coated pancreatic extracts. Gut. 2006;55:1056–1057. doi: 10.1136/gut.2006.094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliori M, Pezzilli R, Tomassetti P, Gullo L. Exocrine pancreatic function after alcoholic or biliary acute pancreatitis. Pancreas. 2004;28:359–363. doi: 10.1097/00006676-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology. 2003;3:303–308. doi: 10.1159/000071768. [DOI] [PubMed] [Google Scholar]
- 24.Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 25.Kahl S, Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004;18:947–955. doi: 10.1016/j.bpg.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Van Hoozen CM, Peeke PG, Taubeneck M, Frey CF, Halsted CH. Efficacy of enzyme supplementation after surgery for chronic pancreatitis. Pancreas. 1997;14:174–180. doi: 10.1097/00006676-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Neoptolemos JP, Ghaneh P, Andrén-Sandberg A, Bramhall S, Patankar R, Kleibeuker JH, et al. Treatment of pancreatic exocrine insufficiency after pancreatic resection. Results of a randomized, double-blind, placebo-controlled, crossover study of high vs standard dose pancreatin. Int J Pancreatol. 1999;25:171–180. [PubMed] [Google Scholar]
- 28.Friess H, Böhm J, Müller MW, Glasbrenner B, Riepl RL, Malfertheiner P, et al. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol. 1996;91:341–347. [PubMed] [Google Scholar]
- 29.Armbrecht U, Lundell L, Stockbrügger RW. The benefit of pancreatic enzyme substitution after total gastrectomy. Aliment Pharmacol Ther. 1988;2:493–500. doi: 10.1111/j.1365-2036.1988.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 30.Brägelmann R, Armbrecht U, Rosemeyer D, Schneider B, Zilly W, Stockbrügger RW. The effect of pancreatic enzyme supplementation in patients with steatorrhoea after total gastrectomy. Eur J Gastroenterol Hepatol. 1999;11:231–237. doi: 10.1097/00042737-199903000-00004. [DOI] [PubMed] [Google Scholar]


