Abstract
Coccidioidomycosis is a fungal infection endemic in the southwestern United States that is increasing in incidence. While cellular immunity correlates with protection from clinical illness, the precise elements of that response are undefined. Using the coccidioidal antigen preparation T27K and multiparametric flow cytometry, the in vitro frequency of polyfunctional T lymphocytes in the peripheral blood of naturally immune healthy donors and those who were nonimmune was determined. Polyfunctional CD4 lymphocytes, defined as producing intracellular interleukin 2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha simultaneously, had a frequency of 137 per 400,000 events among peripheral blood mononuclear cells (PBMC) of immune donors compared to 11 per 400,000 PBMC from nonimmune donors (P = 0.03). When monocyte-derived mature dendritic cells pulsed with T27K (mDCT27K) were used for antigen presentation, the frequency of polyfunctional CD4 T lymphocytes did not significantly increase for either group, although mDCT27K did significantly increase the concentrations of IL-2 and IFN-γ released by PBMC from nonimmune donors (P = 0.02). After in vitro stimulation with T27K, polyfunctional CD4 and CD8 lymphocytes of PBMC from immune donors had a mixture of low- and high-expression CCR7 cells, suggesting both effector and central memory, compared with predominantly high-expression CCR7 cells when PBMC were incubated with the mitogen phytohemagglutinin (P = 0.03). These data demonstrate the presence of polyfunctional T lymphocytes in the peripheral blood of individuals with coccidioidal immunity and suggest a model for the in vitro testing of vaccine candidates for coccidioidomycosis.
Coccidioidomycosis is a major mycosis endemic in the southwestern United States whose incidence is increasing (7, 10). Infection usually occurs when airborne coccidioidal arthroconidia are inhaled and lodge in the terminal alveoli of the lung (20). In approximately 60% of cases, infection is completely asymptomatic and is manifested only by a cellular immune response, such as a delayed-type hypersensitivity (DTH) reaction to coccidioidal antigens. Smith and colleagues initially demonstrated that DTH is the hallmark of coccidioidal infection and that a lack of this response in otherwise healthy individuals indicates an absence of infection (30). Moreover, this cellular immune response appears to be long-lived and protective (15). In vitro studies have suggested that control of infection is associated with the release of T-helper type 1 (Th1) cytokines, such as interleukin-2 (IL-2) and gamma interferon (IFN-γ), from peripheral blood mononuclear cells (PBMC) (1-5, 13).
Beyond the measurement of DTH and the release of Th1 cytokines by PBMC, little is known of the precise elements of immune protection in human coccidioidomycosis. Defining these elements is critical for the development of a protective vaccine (28). Using multiparametric flow cytometry, Darrah and colleagues (14) have presented murine data demonstrating that immunization with a live Leishmania vaccine results not only in strong protection against rechallenge but also in the production of lung, lymph node, and splenic CD4 T lymphocytes containing intracellular IL-2, IFN-γ and tumor necrosis factor alpha (TNF-α). The majority of these polyfunctional CD4 T lymphocytes also express small amounts of surface CCR7, suggesting that they are of the effector memory phenotype. These results have been interpreted to suggest that the quality of the T-lymphocyte response, as manifested by a polyfunctional cytokine expression, is a marker for protective cellular immunity. Since publication of this work, polyfunctional T lymphocytes have been identified in the peripheral blood of humans with Mycobacterium tuberculosis infection (9), as well as a variety of viral infections (11, 17, 18, 22).
In work previously performed, mature monocyte-derived dendritic cells pulsed with coccidioidal antigen were shown to activate lymphocytes from nonimmune healthy donors as well as lymphocytes from donors with extrathoracic dissemination and anergy (24). In that study, the mature dendritic cells (mDC) generated were functionally and phenotypically normal. Stimulation in this model was accomplished using T27K, an antigen preparation derived from Coccidioides posadasii that specifically induces the release of Th1 cytokines from PBMC from healthy immune donors (3-5) and correlates with DTH responses (6).
In the present study, we wished to determine if polyfunctional T lymphocytes occur in human coccidioidomycosis. We used individuals who had acquired their infection naturally and compared them to donors without infection who were not immune. Infection and immunity were determined based on the in vitro release of Th1 cytokines by PBMC in response to incubation with the coccidioidal antigen preparation T27K. As noted above, the expression of specific coccidioidal cellular immunity indicates infection and long-lived immunity, while the absence of this in a healthy individual indicates no prior infection and no immunity. We have previously demonstrated that in vitro measurement of cellular immunity using T27K correlates significantly with DTH to coccidioidin (6).
In addition, we wished to see if the lymphocyte activation that occurs with incubation with mDC pulsed with coccidioidal antigen is similar to the cytokine profile observed among cells obtained from naturally immune donors. Finally, we attempted to ascertain the memory phenotype of antigen-stimulated lymphocytes from naturally immune donors. In all of this work, we used the antigen preparation T27K, since it has consistently been shown to induce specific Th1 responses from coccidioidal antigen immune individuals (3-5).
MATERIALS AND METHODS
Human subjects.
Healthy volunteers without active coccidioidomycosis or known underlying illnesses were recruited at the Southern Arizona Veterans Affairs Health Care System (SAVAHCS). The Human Subjects Protection Committee at the University of Arizona and the Research and Development Committee of SAVAHCS approved all work.
Antigen preparation.
T27K was prepared as previously described (3, 33). Briefly, a continuous culture of Silveira strain Coccidioides posadasii spherules was mechanically disrupted and centrifuged at 27,000 × g. The soluble portion was collected, preserved with thimerosal, and lyophilized. Prior to use in experiments, the lyophilate was weighed and diluted into phosphate-buffered saline (PBS) (Invitrogen Life Sciences, Carlsbad, CA) and sterilized through a 0.2-μm filter (Millipore, Billerica, MA). T27K was used at a concentration of 20 μg/ml in all experiments.
Incubation of PBMC for cytokine release assay.
Approximately 30 ml of peripheral blood was drawn by venipuncture from volunteer healthy donors, collected into sodium heparin, and separated by density gradient centrifugation (Ficoll-Hypaque; Amersham Pharmacia, GE Healthcare, Piscataway, NJ). PBMC were collected from the interface layer, and these were resuspended in AIM-V serum-free medium (Invitrogen, Carlsbad, CA) at a concentration of 2 × 106 cells/ml. One ml was placed in each well of a 24-well culture plate (Falcon; BD Biosciences, San Jose, CA) and incubated at 37°C in 95% air-5% CO2 for 24 to 48 h. After that time, the supernatant was aspirated and stored at −80°C until assayed for cytokine.
Cytokine supernatant concentrations were determined by enzyme-linked immunosorbent assay (ELISA). For interleukin-2 (IL-2) and tumor necrosis factor alpha (TNF-α), the Human OptEIA kits from BD Biosciences (San Jose, CA) were used. For determination of gamma interferon supernatant concentrations, the human ELISA Ready-Set-Go! kit from eBioscience (San Diego, CA) was used. All assays were run according to the manufacturer's instructions. Sample results were compared to a standard curve generated from a recombinant cytokine protein provided with each kit.
Donors were defined as coccidioidal antigen immune or nonimmune based on the ability of their PBMC to release T-helper type 1 cytokines into culture supernatant in response to incubation with T27K for 24 h. Immune donors were defined by the release of more than 150 pg/ml of IL-2, 100 pg/ml of IFN-γ, and 100 pg/ml of TNF-α. Nonimmune donors released less than these concentrations.
Intracellular cytokine assessment.
One ml of 2 × 106/ml PBMC in AIM-V serum-free medium was added to individual wells of 24-well plates. All wells received 1 μg of anti-CD28 (eBioscience) and 1 μg of anti-CD49d (eBioscience) antibodies. Wells either received no stimulus, 20 μg/ml T27K, or 5 μg/ml of phytohemagglutinin (PHA) (Sigma Chemical, St. Louis, MO). The wells were incubated at 37°C in 95% air-5% CO2 for 48 h. During the final 12 h of the incubation, 10 μg/ml brefeldin A (eBioscience) was added to each well. After this time, nonadherent cells were aspirated from the wells and the cells were centrifuged at 500 × g. The pellet was resuspended in Dulbecco's modified phosphate-buffered saline (Invitrogen) and analyzed by flow cytometry.
Generation of dendritic cells.
Generation of dendritic cells was performed as previously described (24). Briefly, 80 ml of blood was obtained by venipuncture and collected in sodium heparin and PBMC obtained by density gradient centrifugation as described above. All collected PBMC were plated in T-75 flasks (Corning, Corning, NY) in AIM-V serum-free medium (Invitrogen) in a volume of 15 ml and allowed to adhere for 2 h at 37°C in 95% air-5% CO2. After this time, the nonadherent cells were removed by washing the flask twice with sterile Dulbecco's modified PBS and then resuspended in 70% heat-inactivated fetal bovine serum (Omega Scientific, Tarzana, CA), 20% RPMI 1640 (Invitrogen), and 10% dimethyl sulfoxide (Sigma Chemical) and stored at −80°C. The adherent cells were further cultivated in 15 ml of X-Vivo serum-free medium (BioWhittaker, Basel, Switzerland) supplemented with 1,000 IU/ml granulocyte-macrophage colony-stimulating factor (Berlex, Seattle, WA) and 500 IU/ml IL-4 (Peprotech, Rocky Hill, NJ) for 5 days at 37°C in 95% air-5% CO2.
Subsequently, all adherent cells were removed using a sterile cell scraper and transferred to 3 wells of a 6-well plate (Falcon, BD Biosciences) in 2.0 ml of X-vivo medium per well. These wells were incubated with 20 μg/ml T27K for 24 h and then incubated with 500 IU/ml TNF-α (Peprotech) and 2 μg/ml prostaglandin E2 (Sigma Chemical) for an additional 48 h to produce mature dendritic cells pulsed with T27K (mDCT27K).
The mDCT27K were then removed from the 6-well plates, counted and assessed for viability, and added to wells of a 24-well plate at a concentration of 2 × 105 in AIM-V medium in 1.0 ml. Then, the frozen nonadherent autologous PBMC previously collected were thawed and 2 × 106 were added to mDCT27K (10:1) from the same donor. The cocultivated PBMC/mDCT27K were incubated at 37°C in 95% air-5% CO2 for 48 h and further assessed either for cytokine released into the supernatant or for intracellular flow cytometry, as described above.
Flow cytometric analysis of lymphocytes.
For intracellular cytokine analysis, nonadherent cells were incubated with fluorochrome-conjugated antibodies specific for the surface antigens CD3 (peridinin chlorophyll protein [PerCP] conjugated), CD4 (allophycocyanin [APC]-Cy7 conjugated), CD8 (APC-Cy7 conjugated), and CD69 (phycoerythrin [PE]-Cy7 conjugated) (BD Bioscience) in PBS for 30 min at 4°C and then fixed and permeabilized using the Fix and Perm kit (Caltag, Burlingame, CA) according to the manufacturer's instructions. After permeabilization, cells were incubated with fluorescently labeled antibodies directed against IL-2 (fluorescein isothiocyanate [FITC] labeled), IFN-γ (PE labeled), and TNF-α (APC labeled) for 30 min at 4°C. Flow cytometry was performed using a FACSCanto II instrument (BD Biosciences) with acquisition of 400,000 events. A polyfunctional lymphocyte was defined as one that met the criteria of a lymphocyte, based on forward- and side-scatter morphology, surface antigen expression, and the intracellular expression of IL-2, IFN-γ, and TNF-α simultaneously.
For memory phenotype analysis, cells were collected and stained with fluorescent antibodies directed against CD3 (APC-Cy7), CD45RO (PE), CD4 or CD8 (Per CP), IFN-γ (APC), IL-2 (FITC), and CCR7 (PE-Cy7) (BD Biosciences). Cells were gated on lymphocytes based on forward- and side-scatter characteristics and on the expression of CD3. A total of 400,000 events were collected. Expression or lack of expression of CCR7 was identified on CD3 lymphocytes expressing either CD4 or CD8. For this analysis, a polyfunctional lymphocyte was defined as one that simultaneously expressed IL-2 and IFN-γ.
Statistical analysis.
Results are expressed as median values with minimum and maximum values in parentheses. Since data were not normally distributed, they were analyzed using either the Mann-Whitney U test, for unpaired results between immune and nonimmune donors, or the Wilcoxon sign test, for paired data of experiments comparing results from the same donor. Statistical analyses were performed using the GraphPad Prism software program (La Jolla, CA).
RESULTS
Immune donors express polyfunctional CD4 T lymphocytes in response to in vitro stimulation with T27K compared to results for nonimmune donors; these are not increased by dendritic cells.
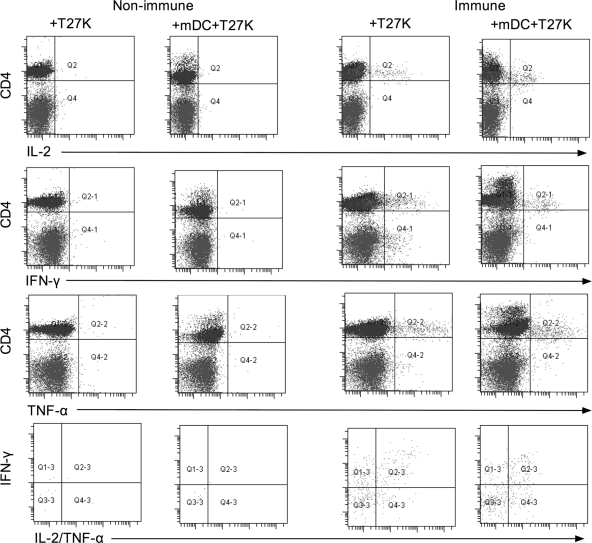
To examine for polyfunctional CD4+ T lymphocytes, PBMC were incubated for 48 h with T27K and then examined by flow cytometry. Cells were selected based on lymphocyte morphology and surface expression of CD4 antigen and analyzed for simultaneous expression of IL-2, IFN-γ, and TNF-α. As shown in Table 1, cells from nonimmune donors had a median of 11 cells per 400,000 events simultaneously expressing all three cytokines. Similarly gated cells from immune donors had a median of 137 cells per 100,000, approximately a 10-fold-greater response (P = 0.02). When PBMC from either donor group were incubated with mature dendritic cells (mDC) pulsed with T27K, the frequency of polyfunctional CD4+ T lymphocytes did not significantly increase from that for PBMC incubated alone with T27K (Table 1). In addition, there was no significant change in the mean fluorescent intensity of intracellular expression of IL-2, IFN-γ, or TNF-α between PBMC incubated alone or those incubated with mDCT27K for either nonimmune or immune donors (data not shown). Results from representative experiments are demonstrated graphically in Fig. 1.
TABLE 1.
Frequencies of single and polyfunctional CD4+ T lymphocytesa
| Intracellular cytokine(s) produced | Condition | Frequency of lymphocyte for donor group |
P value | |
|---|---|---|---|---|
| Nonimmune | Immune | |||
| IL-2 | + T27K | 134 (57-1,193) | 1571 (69-2,831) | 0.05 |
| + mDC, + T27K | 372 (54-1,963) | 2819 (967-6,574) | 0.005 | |
| P value | 0.16 | 0.03 | ||
| IFN-γ | + T27K | 217 (78-333) | 514 (30-3,407) | 0.30 |
| + mDC, + T27K | 220 (52-2,027) | 1354 (308-3,181) | 0.06 | |
| P value | 0.58 | 0.22 | ||
| TNF-α | + T27K | 599 (114-1,887) | 1077 (161-3,816) | 0.63 |
| + mDC, + T27K | 1246 (110-4,209) | 829 (587-4,845) | 1.00 | |
| P value | 0.15 | 0.56 | ||
| IL-2, IFN-γ, TNF-α | + T27K | 11 (0-24) | 137 (11-1,107) | 0.03 |
| + mDC, + T27K | 21 (5-102) | 141 (41-1,136) | 0.02 | |
| P value | 0.30 | 0.40 | ||
Frequencies were determined for PBMC from seven nonimmune donors and six immune donors after incubation with T27K and with mDC pulsed with T27K. Values indicate median (minimum—maximum) frequencies per 400,000 cells counted.
FIG. 1.
Dot plot for CD4 versus IL-2, IFN-γ, or TNF-α between a representative nonimmune donor and immune donor where PBMC were stimulated with T27K (+T27K) or by autologous mature DC loaded with T27K (+mDC+T27K). The bottom panel represents cells expressing all three cytokines with IFN-γ versus IL-2/TNF-α.
Polyfunctional CD4 and CD8 T lymphocytes from immune donors are activated after incubation with T27K.
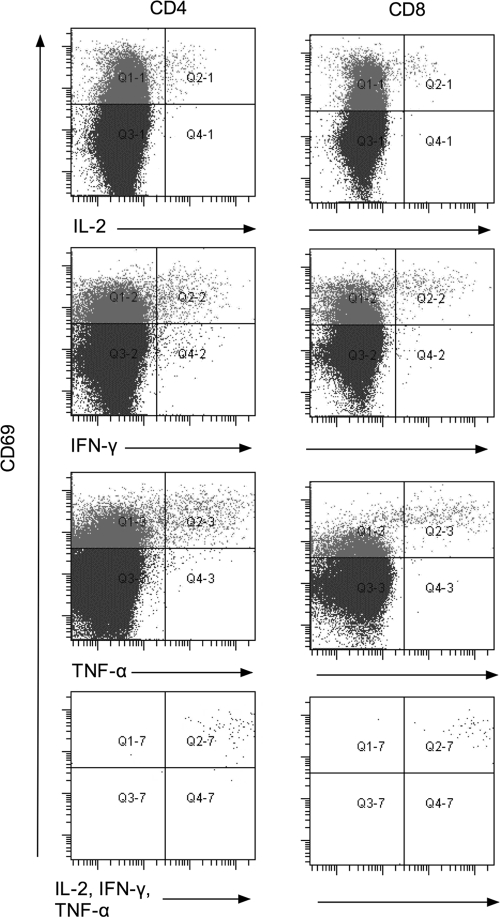
To further examine the nature of polyfunctional T lymphocytes from immune donors, the flow cytometry gating strategy was changed. After a lymphocyte gate was established, cells expressing CD3 were selected and subsequently cells expressing either CD4 or CD8 were examined for intracellular cytokine expression. In addition, staining with antibody directed against CD69, a marker for cellular activation (26), was also performed. In these experiments, using 6 immune donors, the median frequency of polyfunctional CD4 lymphocytes was 46 per 400,000 after incubation with T27K for 48 h, compared to 1 per 400,000 for PBMC incubated alone (P = 0.02). Similarly, the median frequency of polyfunctional CD8+ lymphocytes was 32 per 400,000, compared to 0 per 400,000 for PBMC incubated alone (P = 0.02). In both cases, all polyfunctional cells were also CD69 positive. Representative data of CD69 expression in relation to cytokine expression for CD4 and CD8 lymphocytes from these immune donors are presented as dot plots in Fig. 2.
FIG. 2.
Dot plot comparing expression of CD69 versus individual intracellular expression of IL-2, IFN-γ, or TNF-α or simultaneous expression of all three cytokines among CD4 and CD8 lymphocytes from a representative coccidioidal immune donor after incubation with T27K.
Multiple cytokines are released by PBMC from nonimmune and immune donors after incubation with mature dendritic cells pulsed with T27K mDCT27K).
As shown in Table 2, the concentrations of IL-2, IFN-γ, and TNF-α released into the supernatant by PBMC obtained from nonimmune donors after incubation with T27K were significantly below those for PBMC obtained from immune individuals and incubated with T27K (for all three, P ≤ 0.003).
TABLE 2.
Supernatant cytokines released by PBMCa
| Cytokine and donor group | Concn (pg/ml) of cytokine for: |
P value | |
|---|---|---|---|
| PBMC + T27K | PBMC + mDC + T27K | ||
| IL-2 | |||
| Nonimmune | 41 (0-94) | 526 (221-6,432) | 0.02 |
| Immune | 1,488 (389-12,274) | 4,046 (422-24,583) | 0.09 |
| P value | 0.001 | 0.20 | |
| IFN-γ | |||
| Nonimmune | 11 (0-20) | 49 (9-977) | 0.02 |
| Immune | 517 (196-10,388) | 3,402 (606-10,639) | 0.03 |
| P value | 0.003 | 0.002 | |
| TNF-α | |||
| Nonimmune | 16 (4-171) | 31 (20-399) | 0.22 |
| Immune | 706 (368-6,238) | 244 (37-4,024) | 0.03 |
| P value | 0.001 | 0.05 | |
Supernatant cytokines released by PBMC incubated alone, with 20 μg/ml of T27K, or with mDC pulsed with T27K for 48 h. Data were obtained from seven nonimmune donors and six immune donors. Values represent the median (minimum-maximum) concentrations.
Incubating PBMC with autologous mDCT27K from nonimmune donors resulted in significant increases in IL-2 and IFN-γ (for both, P = 0.02) compared to results for PBMC alone. However, the concentrations of TNF-α did not significantly change (P = 0.22). Among immune donors, concentrations of IFN-γ and TNF-α increased significantly when PBMC were incubated with autologous mDCT27K compared to results for PBMC plus T27K (for both, P = 0.02). However, the increase in IL-2 concentrations for cultivation with mDCT27K was not significant compared to results for PBMC plus T27K (P = 0.09).
Although IL-2 and IFN-γ concentrations increased significantly when PBMC from nonimmune donors were incubated with autologous mDCT27K, the cytokine concentrations still remained significantly below the levels for immune donor PBMC incubated with T27K. While the median IL-2 concentration for PBMC incubated with mDCT27K from nonimmune donors was 526 pg/ml, it was 1,488 pg/ml for immune donors (P = 0.001). The IFN-γ concentration for nonimmune PBMC incubated with mDCT27K was 49 pg/ml, compared to 517 pg/ml for PBMC from immune donors (P = 0.002). Finally, the levels for TNF-α were 31 pg/ml for nonimmune PBMC incubated with mDCT27K and 706 pg/ml for immune PBMC (P = 0.02).
Both effector memory and central memory are expressed by polyfunctional CD4+ T lymphocytes from immune donors after T27K incubation.
We next wished to examine the memory phenotypes of polyfunctional CD4 and CD8 T lymphocytes obtained from immune donors after stimulation with T27K. To do this, we gated on lymphocytes expressing CD3 and either CD4 or CD8 antigen. A further gate was set on those cells expressing the memory antigen CD45RO. We then examined for the expression of CCR7 among those gated lymphocytes that also simultaneously expressed IL-2 and IFN-γ intracellularly. We compared these results to results for cells from the same donors that were similarly gated but were stimulated in vitro with the mitogen PHA.
As shown in Table 3, after PBMC from immune donors were incubated with T27K, the median number of cells per 400,000 that expressed or did not express CCR7 was equivalent among CD3/CD4 and CD3/CD8 polyfunctional lymphocytes (for both, P ≥ 0.50). On the other hand, there was a significant increase in the median frequency of cells expressing CCR7 for both CD3/CD4 and CD3/CD8 polyfunctional lymphocytes after PBMC were stimulated with PHA (for both, P = 0.03). This increase in CCR7 expression associated with PHA was significantly greater than that seen for PBMC incubated with T27K for both CD3/CD4 and CD3/CD8 lymphocytes (for both, P = 0.03). Representative histograms for CD4+/CD45RO+ lymphocytes from an immune donor are displayed in Fig. 3.
TABLE 3.
Expression of CCR7 among PBMC from six immune donorsa
| Incubation | Frequency of expression among CD4 lymphocytes |
P value for CD4 group | Frequency of expression among CD8 lymphocytes |
P value for CD8 group | ||
|---|---|---|---|---|---|---|
| CCR7− | CCR7+ | CCR7− | CCR7+ | |||
| T27K | 31 (1-295) | 30 (1-151) | 0.50 | 3 (0-138) | 9 (1-70) | 0.59 |
| PHA | 120 (6-367) | 1,513 (238-3,304) | 0.03 | 54 (3-116) | 1,034 (240-1,704) | 0.03 |
| P value | 0.56 | 0.03 | 0.68 | 0.03 | ||
Median (minimum—maximum) frequency per 400,000 events of expression of CCR7 among PBMC from six immune donors incubated with either 20 μg/ml T27K or 5 μg/ml PHA and gated on CD3/CD45RO cells and for simultaneous expression of IL-2 and IFN-γ.
FIG. 3.
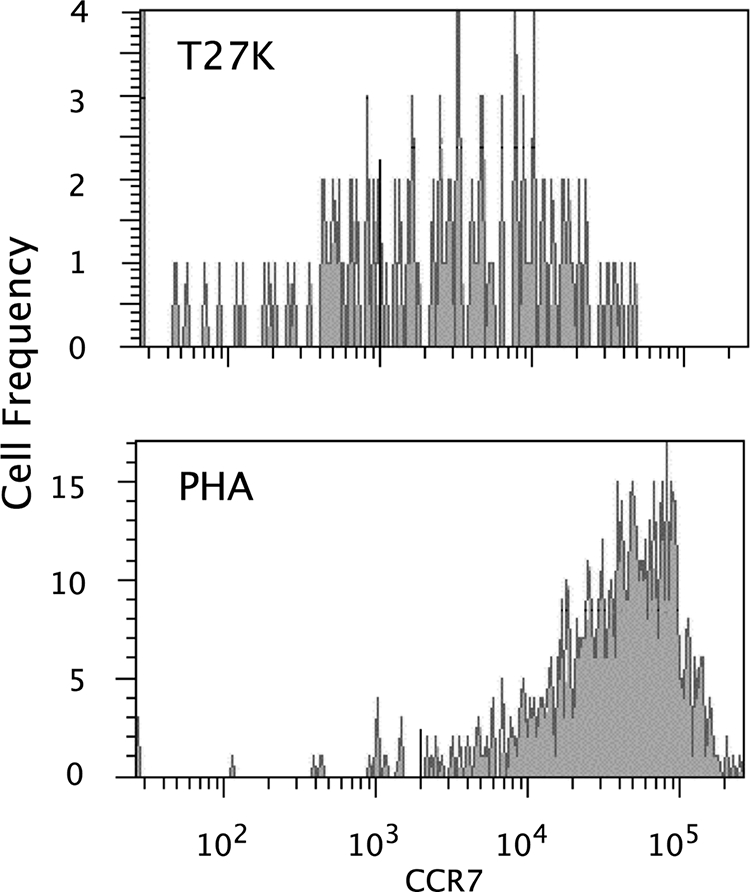
Representative histograms for CD4+/CD45RO+ polyfunctional lymphocytes expressing CCR7 from PBMC incubated with either T27K or phytohemagglutinin (PHA) from an immune donor.
CCR7 expression was also determined after incubation with autologous mDC loaded with T27K. For samples from nonimmune donors, no analysis was done because the number of polyfunctional memory cells was too small (data not shown). For samples from immune donors, the median (minimum to maximum) number of CCR7− polyfunctional CD4+/CDRO45+ lymphocytes was 23 (6 to 24) and the number of CCR7+ cells was 49 (34 to 69). The ratio of CCR7− cells to CCR7+ cells was not significantly different from that for PBMC incubated with T27K only (P = 0.14). For CD8/CDRO45+ polyfunctional lymphocytes, the median number of CCR7− cells was 4 (2 to 4) and the median number of CCR7+ cells was 11 (10 to 12), again not significantly different from those for cells incubated with T27K only (P = 0.76).
DISCUSSION
As pointed out by Seder and colleagues (28), the quality of the T-lymphocyte response, measured using multiple parameters, is a crucial factor in defining a protective cellular immune response in a variety of infections. In the current study, we have demonstrated that donors with naturally acquired coccidioidomycosis who have controlled their illness have T lymphocytes circulating in their peripheral blood in response to in vitro incubation with the coccidioidal antigen preparation T27K that simultaneously express IL-2, IFN-γ, and TNF-α. These cells occurred in both CD4 and CD8 lymphocyte populations and were activated, based on CD69 expression. Such results supply needed additional information on the complex elements of immune protection for this important medical mycosis.
Polyfunctional T lymphocytes were not found to significantly increase after incubation with mDCT27K. Dendritic cells are powerful antigen-presenting cells, capable of stimulating lymphocytes to respond to a variety of antigens and influencing cytokine production (21). Richards and colleagues (24) showed that mature dendritic cells activate lymphocytes in response to T27K using PBMC obtained from nonimmune donors and from anergic donors. Cytokine assays performed in that study demonstrated secretion of large amounts of IFN-γ after the initial stimulation. However, the net lymphocyte activation response among PBMC from nonimmune donors in those experiments was only half that of cells from immune donors, similar to the cytokine release assay results in our current experiments. Hence, mature dendritic cells pulsed with coccidioidal antigen do not appear to induce the same type of immune response in vitro that naturally immune healthy donors express.
Dendritic cells control the induction of T-lymphocyte immunity in hosts by stimulating naïve T cells. After processing antigen and presenting the antigen in the context of MHC to lymphocytes, costimulatory molecules, such as CD40/CD80/CD86, bind to receptors on the lymphocyte and cause a signaling cascade, leading to cytokine production by the lymphocyte. Expression of IL-12p40 from dendritic cells aids in increasing inflammatory cytokines, such as IL-1 and IL-6, which aids in increasing Th1 responses (8, 31). In this study, we observed an increase in both IL-2 and IFN-γ when PBMC were incubated with autologous mDCT27K from nonimmune donors. These cytokines were released in smaller quantities than in PBMC obtained from naturally immune donors. In the case of TNF-α, the amount released by nonimmune cells increased with stimulation with mDCT27K but actually decreased in PBMC from immune donors. The reason for this is unclear. However, in all cases, PBMC from immune donors produced more Th1 cytokine than cells from nonimmune donors. Moreover, no increase in the number of polyfunctional T lymphocytes was observed when mDCT27K were incubated with PBMC from either nonimmune or immune donors. These results may indicate that natural immunization against Coccidioides might allow for a small responsive memory population of cells which could more rapidly release cytokine in response to coccidioidal antigens. In other models, memory CD4+ and CD8+ T lymphocytes are maintained and remain responsive to antigen (27).
Immunization strategies using dendritic cells have been sought as immunotherapy to circumvent host tolerance to antigens and overcome other potential host deficiencies that may limit this response (8). Similarly, dendritic cells could be used to overcome host tolerance that may be present in donors with disseminated coccidioidomycosis and anergy to stimulate cellular responses to Coccidioides. It has previously been shown that this can be done to some degree (24). However, our current data suggest that the type of immune response induced by dendritic cells is different in both the quantity of cytokine release and the induction of polyfunctional T lymphocytes.
An anomalous result of this study is that mDC induced release of Th1 cytokines into the culture supernatant, but we could not detect an increase either in the frequency or the intensity of intracellular cytokine expression among CD3+ lymphocytes. It is possible that other cells are the source of these cytokines. Given our restriction to six-color flow, we were not able to assess this. It is also possible that CD3 lymphocytes with low-intensity expression of cytokine are the source and were not detected in our assay.
To establish long-term immunity, an individual must establish a population of memory cells. Within our system, there were equivalent numbers of CD45RO-positive lymphocytes from naturally immune donors that simultaneously expressed IL-2 and IFN-γ that contained on their surface either low or high levels of CCR7 after stimulation with T27K. In contrast, when the mitogen PHA was employed as the stimulus, the vast majority of cells showed high CCR7 expression. This bimodal population of cells containing both an effector (low CCR7) and a central (high CCR7) memory phenotype among cells responding to coccidioidal antigen indicates a heterogeneous immune response. Effector memory cells are posited to produce cytokines which influence the response of migrating lymphocytes to the site of infection. Central memory cells establish a long-lived population of lymphocytes primed to react to subsequent infection by the pathogen (25). Based on our observation, T27K elicits in vitro memory cells capable of both reacting to Coccidioides and establishing long-term memory.
In the context of the experiments performed, there are several caveats that must be addressed regarding the analysis of the flow cytometry. First, we analyzed only 400,000 events. While this is a large number, it may not be enough to capture rare responding lymphocytes for fungal antigens (28). On the other hand, we were able to identify a significant increase in polyfunctional T-lymphocyte frequency among PBMC from immune donors on the order of 10-fold above that for nonimmune donors. Second, we were limited to six-color analysis due to the limitations of our flow cytometer (28). A more thorough analysis could have been achieved if more colors for analysis were available. For example, this might have allowed specific exclusion of nonviable cells or the identification of activation via degranulation through expression of CD107 or by measurement of the release of perforin or granzymes (28). Finally, we screened only a small population of healthy donors (7 nonimmune and 6 immune). While we were able to identify statistically significant differences within our samples, a larger donor population might have allowed for subdivisions of immune response based on donor characteristics.
There is considerable interest in developing a human vaccine for coccidioidomycosis (12, 16, 23). However, neither the antigens nor the mode of delivery is established (19, 29, 32). The results of this study suggest a model to test such vaccine candidates in vitro prior to larger-scale studies based on the presumption that an antigen capable of eliciting an increase in the frequency of polyfunctional T lymphocytes among PBMC from naturally immune donors cultured in vitro is likely to be a good vaccine candidate. Such a model has already found utility in human tuberculosis (9). Moreover, this model could be employed to ascertain the precise elements of anergy among those with disseminated extrathoracic coccidioidomycosis and assist in devising novel immunological strategies to treat this difficult entity.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NIAID grant IPO1AI061310-01) and by the Southern Arizona Veterans Affairs Health Care System (SAVAHCS).
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 9 November 2009.
REFERENCES
- 1.Ampel, N. M., G. C. Bejarano, S. D. Salas, and J. N. Galgiani. 1992. In vitro assessment of cellular immunity in human coccidioidomycosis: relationship between dermal hypersensitivity, lymphocyte transformation, and lymphokine production by peripheral blood mononuclear cells from healthy adults. J. Infect. Dis. 165:710-715. [DOI] [PubMed] [Google Scholar]
- 2.Ampel, N. M., and L. Christian. 1997. In vitro modulation of proliferation and cytokine production by human peripheral blood mononuclear cells from subjects with various forms of coccidioidomycosis. Infect. Immun. 65:4483-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ampel, N. M., L. A. Kramer, K. M. Kerekes, S. M. Johnson, and D. Pappagianis. 2001. Assessment of the human cellular immune response to T27K, a coccidioidal antigen preparation, by flow cytometry of whole blood. Med. Mycol. 39:315-320. [DOI] [PubMed] [Google Scholar]
- 4.Ampel, N. M., and L. A. Kramer. 2003. In vitro modulation of cytokine production by lymphocytes in human coccidioidomycosis. Cell Immunol. 221:115-121. [DOI] [PubMed] [Google Scholar]
- 5.Ampel, N. M., D. K. Nelson, S. Chavez, K. A. Naus, A. B. Herman, L. Li, et al. 2005. Preliminary evaluation of whole-blood gamma interferon release for clinical assessment of cellular immunity in patients with active coccidioidomycosis. Clin. Diagn. Lab. Immunol. 12:700-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ampel, N. M., R. F. Hector, C. P. Lindan, and G. W. Rutherford. 2006. An archived lot of coccidioidin induces specific coccidioidal delayed-type hypersensitivity and correlates with in vitro assays of coccidioidal cellular immune response. Mycopathologia 161:67-72. [DOI] [PubMed] [Google Scholar]
- 7.Arizona Department of Health Services. 2008. Valley fever annual report 2007. Arizona Department of Health Services, Phoenix, AZ.
- 8.Benko, S., Z. Magyarics, A. Szabo, and E. Rajnavolgyi. 2008. Dendritic cell subtypes as primary targets of vaccines: the emerging role and cross-talk of pattern recognition receptors. Biol. Chem. 389:469-485. [DOI] [PubMed] [Google Scholar]
- 9.Beveridge, N. E., D. A. Price, J. P. Casazza, A. A. Pathan, C. R. Sander, T. E. Asher, et al. 2007. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur. J. Immunol. 37:3089-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. 2009. Increase in coccidioidomycosis—California, 2000-2007. MMWR Morb. Mortal. Wkly. Rep. 58:105-109. [PubMed] [Google Scholar]
- 11.Ciuffreda, D., D. Comte, M. Cavassini, E. Giostra, L. Buhler, M. Perruchoud, et al. 2008. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur. J. Immunol. 38:2665-2677. [DOI] [PubMed] [Google Scholar]
- 12.Cole, G. T., J. M. Xue, C. N. Okeke, E. J. Tarcha, V. Basrur, R. A. Schaller, et al. 2004. A vaccine against coccidioidomycosis is justified and attainable. Med. Mycol. 42:189-216. [DOI] [PubMed] [Google Scholar]
- 13.Corry, D. B., N. M. Ampel, L. Christian, R. M. Locksley, and J. N. Galgiani. 1996. Cytokine production by peripheral blood mononuclear cells in human coccidioidomycosis. J. Infect. Dis. 174:440-443. [DOI] [PubMed] [Google Scholar]
- 14.Darrah, P. A., D. T. Patel, P. M. De Luca, R. W. Lindsay, D. F. Davey, B. J. Flynn, et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843-850. [DOI] [PubMed] [Google Scholar]
- 15.Drutz, D. J., and A. Catanzaro. 1978. Coccidioidomycosis. Part I. Am. Rev. Respir. Dis. 117:559-585. [DOI] [PubMed] [Google Scholar]
- 16.Galgiani, J. N. 1999. Coccidioidomycosis: a regional disease of national importance. Rethinking approaches for control. Ann. Intern. Med. 130:293-300. [DOI] [PubMed] [Google Scholar]
- 17.Gaucher, D., R. Therrien, N. Kettaf, B. R. Angermann, G. Boucher, A. Filali-Mouhim, et al. 2008. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 205:3119-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harari, A., F. Vallelian, P. R. Meylan, and G. Pantaleo. 2005. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 174:1037-1045. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, S. M., N. W. Lerche, D. Pappagianis, J. L. Yee, J. N. Galgiani, and R. F. Hector. 2007. Safety, antigenicity, and efficacy of a recombinant coccidioidomycosis vaccine in cynomolgus macaques (Macaca fascicularis). Ann. N. Y. Acad. Sci. 1111:290-300. [DOI] [PubMed] [Google Scholar]
- 20.Kirkland, T. N., and J. Fierer. 1996. Coccidioidomycosis: a reemerging infectious disease. Emerg. Infect. Dis. 2:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell 106:263-266. [DOI] [PubMed] [Google Scholar]
- 22.Nebbia, G., F. M. Mattes, C. Smith, E. Hainsworth, J. Kopycinski, A. Burroughs, et al. 2008. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am. J. Transplant. 8:2590-2599. [DOI] [PubMed] [Google Scholar]
- 23.Pappagianis, D. 2001. Seeking a vaccine against Coccidioides immitis and serologic studies: expectations and realities. Fungal Genet. Biol. 32:1-9. [DOI] [PubMed] [Google Scholar]
- 24.Richards, J. O., N. M. Ampel, and D. F. Lake. 2002. Reversal of coccidioidal anergy in vitro by dendritic cells from patients with disseminated coccidioidomycosis. J. Immunol. 169:2020-2025. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto, F., and A. Lanzavecchia. 2001. Exploring pathways for memory T cell generation. J. Clin. Invest. 108:805-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sancho, D., M. Gomez, and F. Sanchez-Madrid. 2005. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 26:136-140. [DOI] [PubMed] [Google Scholar]
- 27.Seder, R. A., and R. Ahmed. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4:835-842. [DOI] [PubMed] [Google Scholar]
- 28.Seder, R. A., P. A. Darrah, and M. Roederer. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247-258. [DOI] [PubMed] [Google Scholar]
- 29.Shubitz, L., T. Peng, R. Perrill, J. Simons, K. Orsborn, and J. N. Galgiani. 2002. Protection of mice against Coccidioides immitis intranasal infection by vaccination with recombinant antigen 2/PRA. Infect. Immun. 70:3287-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, C. E., R. R. Beard, E. G. Whiting, and H. G. Rosenberger. 1946. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am. J. Public Health 36:1394-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueno, H., E. Klechevsky, R. Morita, C. Aspord, T. Cao, T. Matsui, et al. 2007. Dendritic cell subsets in health and disease. Immunol. Rev. 219:118-142. [DOI] [PubMed] [Google Scholar]
- 32.Xue, J., X. Chen, D. Selby, C. Y. Hung, J. J. Yu, and G. T. Cole. 2009. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect. Immun. 77:3196-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann, C. R., S. M. Johnson, G. W. Martens, A. G. White, B. L. Zimmer, and D. Pappagianis. 1998. Protection against lethal murine coccidioidomycosis by a soluble vaccine from spherules. Infect. Immun. 66:2342-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]