Abstract
Plasmodium falciparum merozoite surface protein 3 (MSP3), the target of antibodies that mediate parasite killing in cooperation with blood monocytes and are associated with protection in exposed populations, is a vaccine candidate under development. It belongs to a family of six structurally related genes. To optimize immunogenicity, we attempted to improve its design based on knowledge of antigenicity of various regions from the conserved C terminus of the six proteins and an analysis of the immunogenicity of “tailored” constructs. The immunogenicity studies were conducted in BALB/c and C57BL/6J mice, using MSP3 (referred to here as MSP3-1) as a model. Four constructs were designed in order to assess the effect of sequences flanking the 69-amino-acid region of MSP3-1 previously shown to be the target of biologically active antibodies. The results indicate major beneficial effects of removing (i) the subregion downstream from the 69-amino-acid sequence, since antibody titers increased by 2 orders of magnitude, and (ii) the upstream subregion which, although it defines a T-helper cell epitope, is not the target of antibodies. The construct, excluding both flanking sequences, was able to induce Th1-like responses, with a dominance of cytophilic antibodies. This led to design a multigenic construct based on these results, combining the six members of the MSP3 family. This new construction was immunogenic in mice, induced antibodies that recognized the parasite native proteins, and inhibited parasite growth in the functional antibody-dependent cellular inhibition assay, thus satisfying the preclinical criteria for a valuable vaccine candidate.
The development of a malaria vaccine holds considerable promise but has been limited by major conceptual and practical difficulties (13). We have chosen an approach based on the analysis of Plasmodium falciparum-human interactions in order to select both vaccine candidates and surrogate markers of protection able to guide vaccine development. Merozoite surface protein 3 (MSP3) is a blood-stage antigen identified by this novel approach in which the protection, which could be passively transferred by IgG from protected African adults into naive, infected individuals (20), was used to identify a mechanism of defense (2, 16). The latter, called antibody-dependent cellular inhibition (ADCI), was used to identify MSP3 as the target of protective antibodies in humans.
We continued to apply this rationale to analyze within the chosen vaccine candidate the immunological function of various regions in an attempt to “tailor” vaccine constructs based on knowledge and thereby optimize them. This strategy is applied by performing detailed antigenicity studies and analyzing the function of antibodies directed against each antigenic determinant of the molecule and by studying the immunogenicity of various constructs in preclinical models. The C-terminal part of MSP3 was chosen based on (i) identification of critical epitopes targeted by antibodies associated with protection (16, 21); (ii) full conservation of this region across different parasite strains in contrast to polymorphisms in N-terminal and repeat regions (10, 11, 16); and (iii) immunoclinical studies that demonstrated that IgG3 antibodies to the C-terminal conserved region of the molecule are associated with a strong reduction of the incidence of malaria attacks (24) and long-term protection (19).
The detailed analysis of the C-terminal part of MSP3 highlighted the importance of a 69-amino-acid (aa) domain targeted by naturally occurring cytophilic antibodies that inhibit parasite growth in functional in vitro ADCI assays (21). This helped us to design the first construction that was taken into the clinics (1, 7), the MSP3-LSP, a long synthetic peptide that included this 69-aa domain. This construct was tested in a phase I vaccine trial and induced antibodies able to inhibit the parasite multiplication in vivo. However, a subset of the volunteers' sera failed to react with the native protein in Western blots (1, 7). This occasional lack of recognition of parasite native proteins and the recent finding that MSP-3 belongs to a family of six merozoite-surface proteins (23), all of them targeted by biologically active antibodies (5), led us to hypothesize that a detailed study of the immunogenicity of MSP3 may help to design a multi-antigenic vaccine construct with higher immunogenicity than the mono-antigenic construct. Therefore, in the first part of the present work, the immunogenicity of MSP3 (referred to here as MSP3-1) was studied in the light of previous detailed antigenicity studies of its C-terminal part, which revealed important differences between subregions of the molecule (21). Thus, we investigated the immunogenicity in mice of four MSP3-1 C-terminal based constructs and their ability to induce cytophilic antibodies against the region previously identified as a target of protective antibodies (21).
The immunogenicity investigations conducted in this work, despite the obvious limitation of having been performed with mice, were considered together with the antigenicity studies of humans (5, 21). The combined antigenicity and immunogenicity data guided the design, based on knowledge, of a first multigenic vaccine, constructed from the critical antigenic regions of the six members of the MSP3 family. In the second part of this article, we present the results of an immunogenicity study in mice of this MSP3 family-based multigenic vaccine.
MATERIALS AND METHODS
Antigens.
MSP3 recombinant proteins and synthetic peptides were designed on the basis of the P. falciparum 3D7 strain sequence (see Fig. S1 in the supplemental material). The denomination of peptides as “a, b, c, d, e, and f” is described elsewhere (21). For the first part of the study concerning the immunogenicity of MSP3-1 (PlasmoDB ID PF10_0345), two recombinant hexahistidine-tagged proteins MSP3-1(a-f) (MSP3-1-CTHis 167-354) and MSP3-1(b-f) (MSP3-1-CTHis191-354) were produced and purified as described elsewhere (27) and then detoxified by using Triton X-114 (17). Seven peptides were synthesized as previously described (18): five short peptides—MSP3-1a167-191, MSP3-1b184-210, MSP3-1c 203-230, MSP3-1d 211-252, and MSP3-1e 275-307—and two long peptides, MSP3-1 LSP(a-d) (MSP3-1-LSP154-249) and MSP3-1 LSP(b-d) (MSP3-1 LSP184-250). For the second part of this study concerning the immunogenicity of the five other molecules of the MSP3 proteins family and the new multigenic protein, we expressed and purified, using the same protocol as for MSP3-1, five recombinant proteins corresponding to the C-terminal part of each: MSP3-2-CTHis161-371 (PlasmoDB ID PF10_0346), MSP3-3-CTHis228-424 (PlasmoDB ID PF10_0347), MSP3-4-CTHis508-697 (PlasmoDB ID PF10_0348), MSP3-7-CTHis214-405 (PlasmoDB ID PF10_ 0352), and MSP3-8-CTHis537-762 (Plasmo DB ID PF10_0355). We also produced the new multigenic construct as a recombinant protein expressed in Lactococcus lactis and purified as described by Theisen et al. (26).
This multigenic MSP3 construct, shown below, was designed on the basis of immunogenicity studies conducted on an animal model reported here, antigenicity (5) and immunogenicity studies carried out on human volunteers (7), and bioinformatics alignments (5, 23) to choose in each protein of the family the sequence most related to the 69-aa region of MSP3-1 that proved crucial for vaccine development (see Fig. S2 in the supplemental material). The resulting multigenic construct combines in the following order these regions from, respectively, MSP3-1, MSP3-2, MSP3-3, MSP3-4, MSP3-7, and MSP3-8: [AKEASSYDYILGWEFGGGVPEHKKEENMLSHLYVSSKDKENISKENDDVLDEKEEEAEETEEEELEEK][LNNNILGWEFGGGAPQNGAAEDKKTEYLLEQIKIPS WDRNNIPDENEQVIEDPQEDNKDEDEDEETETENLETEDDNNEE][SNE KGRPPTYSPILDDGIEFSGGLYFNEKKSTEENKQKNVLESVNLTSWDKEDI VKENEDVK][LERGLGSGALPGTNIITEEKYSLELIKLTSKDEEDIIKHNED VREEIEEQQEDIE][YNHYFAWEIGGGAPTYKPENNKNDNILLEHVKITSWDKEDIIKENEDTKREVQE][SKTIDPSKIDDRLELSSGSSSLEQHSKEDVK KGCALELVPLSLSDIEQIANESEDVLEEIEEEINTD].
Mice.
BALB/c and C57BL/6J mice, 6 to 10 weeks old, were purchased from Charles River Laboratories (L'Arbresle, France).
Immunization.
For each experiment, groups of four to five mice were immunized with one of the four constructs for MSP3-1 [MSP3-1(a-f), MSP3-1(b-f), MSP3-1 LSP(a-d), or MSP3-1 LSP(b-d)], with the C-terminal recombinant proteins of the other proteins of the family, or with the multigenic construct. A 20-μg portion of antigen supplemented with Montanide ISA720 adjuvant was administered subcutaneously to each mouse at 2-week intervals. Since the cumulative effects of repeated immunizations were of interest in preliminary experiments, we performed three immunizations, sampled the animals after the second and the third immunizations, and continued the immunizations to reach a total of six per animal.
Determination of total IgG responses.
Specific IgG responses to the various MSP3 constructs and MSP3 peptides were assessed by enzyme-linked immunosorbent assay (ELISA) in sera collected from immunized mice. ELISA plates were coated overnight at 4°C with the MSP3 antigens diluted in phosphate-buffered saline (PBS) to a final concentration of either 5 μg/ml (peptides) or 2.5 μg/ml (recombinant proteins). Wells were blocked with 300 μl of PBS supplemented with 3% nonfat milk (Regilait, France) for 1 h at room temperature, and then washed in PBS containing 0.05% Tween 20. Sera were added at serial twofold dilutions (beginning at a 1/100 dilution) for 90 min at room temperature and washed as described above, followed by the addition of 50 μl of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Caltag/Invitrogen, Cergy Pontoise, France) per well diluted 1:2,000 in PBS-3% milk-0.05% Tween 20. Plates were incubated for 1 h at room temperature, washed, and then developed with 50 μl of tetramethylbenzidine (TMB) for 15 min. Reactions were stopped by adding 50 μl of 1 M H3PO4 and were read on an ELISA plate reader at 450 nm.
Assessment of isotype distribution of antibodies.
IgG subclass ELISAs were performed essentially as described above, using class-specific second antibodies. Sera were tested in duplicate at a single dilution of 1/1,000. HRP-conjugated antibodies specific for mouse IgG subclasses (Caltag and Invitrogen, Cergy Pontoise, France) were used at dilutions of 1/4,000 for anti-IgG1, anti-IgG2a, and anti-IgG2b and 1/2,000 for anti-IgG3. The antibody concentration of each subclass was determined using murine standards for IgG1, IgG2a, IgG2b, or IgG3 (Zymed Laboratories and Invitrogen, Cergy Pontoise, France) at concentrations of 4, 8, 16, 32, 64, 128, and 256 ng/ml and assayed in triplicate in 96-well microtiter plates coated with a 2 μg of a goat anti-mouse immunoglobulin antibody (Caltag and Invitrogen)/ml.
Western blotting.
Western blotting was performed using a total parasite extract prepared from the 3D7 clone of P. falciparum; proteins were separated by gel electrophoresis under denaturing conditions (SDS-PAGE, 12% acrylamide) and then transferred onto nitrocellulose. Nitrocellulose membranes were blocked with Tris-buffered saline (TBS)-5% milk and then incubated with sera diluted 1:100 in TBS-5% milk-0.05% Tween 20. Bound immunoglobulins were detected using an alkaline-phosphatase conjugated antibody (Promega, Charbonnieres-les-Bains, France) diluted 1/7,500 in TBS-0.05% Tween 20, followed by an incubation with NBT/BCIP substrate (Promega).
Immunofluorescence assays.
Immunofluorescence assays were performed using air-dried, acetone-fixed, thin smears from red blood cell cultures containing predominantly mature schizonts of P. falciparum (3D7). Sera diluted (1/100) in PBS-1% bovine serum albumin were incubated at 37°C in a humid chamber for 1 h. After being washed with PBS, antibodies were detected by using an Alexa Fluor-conjugated goat anti-mouse IgG (Molecular Probes and Invitrogen) diluted to 1/400 in PBS with Evans blue counterstain (1/200).
T-cell assays.
Spleens were removed from mice sacrificed by cervical dislocation 7 days after the third or the sixth immunization. Splenocytes were resuspended at a concentration of 3 × 106 cells/ml in RPMI supplemented with 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, 50 μM β-mercaptoethanol, 1% nonessential amino acids, and 10% fetal calf serum. Aliquots containing 100 μl of cell suspension were distributed into round-bottom 96-well microculture plates (Costar and Sigma, Lyon, France), and 100 μl of each antigen was added at concentrations of 10, 20, or 40 μg/ml. Splenocytes stimulated with 2.5 μg of concanavalin A (Sigma)/ml or with 1 μg of lipopolysaccharide (Sigma)/ml were used as positive controls and with medium alone as negative controls. Cultures were performed in triplicate. After 48 h of culture (37°C and 5% CO2), 50 μl of supernatant/well was collected and stored at −70°C for cytokine titration. [3H]thymidine (Amersham Life Science, Buckinghamshire, United Kingdom) was added (1 μCi/well) during the last 12 h of a 72-h incubation period. The cells were harvested by using an automatic cell harvester (Skatron, Sterling, VA), and [3H]thymidine incorporation was quantified by scintillation counting. The stimulation index (SI) was calculated as follows: (mean cpm of triplicate test wells)/(mean cpm + two standard deviations of sextuplicate control wells). Proliferation was considered positive when the SI was >1.
Cytokine concentrations were assayed in culture supernatants by using the CBA flex set kit for mouse gamma interferon (IFN-γ), tumor necrosis factor (TNF), interleukin-12p70 (IL-12p70), monocyte chemoattractant protein 1 (MCP-1), IL-10, IL-6, and IL-4 (BD Biosciences, Le Pont de Claix, France). The results were acquired on a FACSCalibur according to BD Biosciences instructions and analyzed by using FCAP array software (Soft Flow, Kedves, Hungary).
ADCI assay.
The ADCI assay was carried out essentially as described previously (2, 6).
RESULTS
Immunogenicity of MSP3-1. (i) Immunogen components influence the B-cell epitope dominance and the induction of appropriate antibody responses against the 69-aa target region.
MSP3-1 is the molecule that was first described among the MSP3 family of proteins. Immunoepidemiological studies and functional assays have led us to define a 69-aa region (aa 184 to 252), covering peptides MSP3-1b, MSP3-1c, and MSP3-1d, as a target of protective antibodies (21). Subregions with identical sequences or a very high degree of homology were identified in other members of the MSP3 family. We thus considered MSP3-1 as a model to provide indications for the design of a multigenic construct based on the MSP3 family. We investigated in two mouse strains, BALB/c and C57BL/6J, the immunogenicity of four MSP3-1 constructs in order to assess the effect of amino acid sequences upstream and downstream from the region from aa 184 to 252 upon the immune responses to the three peptides MSP3-1b, MSP3-1c, and MSP3-1d.
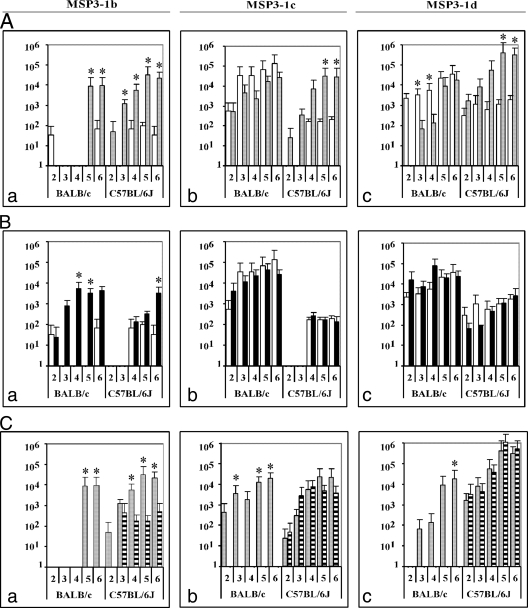
In a first instance, we questioned whether the downstream region (aa 253 to 354) of this sequence of interest, containing peptides “e” and “f,” had an effect on the induction of appropriate antibody responses. We therefore compared the MSP3-1(a-f) and the MSP3-1 LSP(a-d) immunogens (Fig. 1A). The results show that the inclusion of this region clearly impaired the anti-b responses (Fig.1Aa) in both mouse strains and the anti-c (Fig.1Ab) and anti-d (Fig.1Ac) responses in C57BL/6J mice.
FIG. 1.
Antibody responses in BALB/c or C57BL/6J mice immunized with each of the four constructs of MSP3-1. Mice were immunized with MSP3-1(a-f) (□), MSP3-1(b-f) (▪), MSP3-1 LSP(a-d) (░⃞), and MSP3-1 LSP(b-d) (▤). The immunogens were administered at regular intervals of 2 weeks. Sera were collected after two to six immunizations and tested by ELISA on the MSP3-1b (left column), MSP3-1c (center column), and MSP3-1d (right column) peptides. (A) Comparison of IgG responses elicited in MSP3-1(a-f)- and MSP3-1 LSP(a-d)-immunized mice to peptides MSP3-1b (a), MSP3-1c (b), and MSP3-1d (c). (B) Comparison of IgG responses elicited in MSP3-1(a-f)- and MS3-1(b-f)-immunized mice to peptides MSP3-1b (a), MSP3-1c (b), and MSP3-1d (c). (C) Comparison of IgG responses elicited in MSP3-1 LSP(a-d)- and MSP3-1 LSP(b-d)-immunized mice to peptides MSP3-1b (a), MSP3-1c (b), and MSP3-1d (c). The results are expressed as mean ELISA titers ± the standard deviation (SD) in groups of five mice. Titers lower than 50 are considered negative. A nonparametric statistical analysis was performed; P values of <0.05 are indicated by an asterisk.
In a second instance, we assessed the effect of the upstream region (aa 167 to 184) of the 69-aa sequence, which corresponds to peptide MSP3-1a. Comparison of the immunogenicity of MSP3-1(a-f) and MSP3-1(b-f) constructs (Fig. 1B) indicates that, in both mouse strains, the “a” sequence also has a deleterious influence upon the induction of anti-b responses (Fig.1Ba); however, it has no effect upon the anti-c (Fig.1Bb) and anti-d (Fig.1Bc) responses. This deleterious effect of the “MSP3-1a” region upon the anti-b response was no longer detected when the MSP3-1 LSP(a-d) and the MSP3-1 LSP(b-d) were compared (Fig. 1C), most likely because MSP3-1 LSP(b-d) proved to be poorly immunogenic. It did not induce antibody responses against any of the three peptides in BALB/c mice and induced a low anti-b response in C57BL/6J mice (Fig.1Ca). Antibodies induced by the four MSP3-1 constructs recognized native parasite proteins in immunofluorescence and Western blot tests; however, antibodies induced by MSP3-1(a-f) showed very weak reactivity compared to those raised by the three other immunogens (data not shown). Taken together, these results suggest that the simultaneous presence, and to a lesser extent the simultaneous absence, of both upstream and downstream regions of the 69-aa sequence profoundly impairs the immunogenicity toward this sequence targeted by biologically active antibodies, particularly toward peptide b. Considering both mouse strains, LSP(a-d) is the only construct that induced antibody responses against the three overlapping peptides covering the 69-aa target region.
(ii) Immunogen components influence the Th1/Th2 balance.
To determine the T-cell responses induced by the MSP3-1 construct immunization, we assessed the lymphoproliferation of splenocytes taken from immunized mice, in response to in vitro stimulation with the MSP3-1 short peptides or long constructs, at two distinct points during the follow-up period (after the third and sixth injections of the vaccine). Different epitopes were inducers of lymphoproliferation depending on the MSP3-1 construct used as immunogen.
Spleen cells taken from C57BL/6J mice immunized with MSP3-1(a-f) or MSP3-1(b-f) showed no proliferation in response to stimulation with MSP3-1 peptides (a, b, c, d, or e) after the third injection. Low lymphoproliferation was recorded in response to the stimulation with “e” peptide after the sixth immunization (SI6 injections = 1.80 ± 0.48) with splenocytes isolated from the MSP3-1(b-f)-immunized mice. MSP3-1 LSP(a-d)-vaccinated mice splenocytes responded at the two time points against the “a” peptide only (SI3 injections = 1.72 ± 0.27; SI6 injections = 2.58 ± 0.46), whereas splenocytes taken from C57BL/6J mice immunized with MSP3-1 LSP(b-d) responded to the “c” peptide only (SI3 injections = 2.61 ± 0.48; SI6 injections = 3.79 ± 0.22). Similar lymphoproliferation patterns were observed when using immunized BALB/c splenocytes. Higher lymphoproliferative responses were obtained when stimulating cells with the MSP3-1 recombinants or long peptides compared to the short peptides.
In order to determine the Th1/Th2 balance of T-cell responses, the levels of TNF, IFN-γ, IL-10, IL-6, IL-12p70, and IL-4 were determined in 48-h supernatants after in vitro stimulation by the four immunogens and by the five short peptides “a” to “e.” Similar cytokine profiles were observed in supernatants of stimulated spleen cells from both mouse strains, and these profiles did not differ substantially after the third or sixth immunizations. Therefore, we present here representative examples of this large series of experiments: those obtained in C57BL/6 after the third immunization (Fig. 2 and 3). Values for IL-12p70 and IL-4 are not reported on the figures, since IL-12p70 secretion was at the background levels and IL-4 was detected only in the supernatants of spleen cells of MSP3-1 LSP(a-d)-immunized groups.
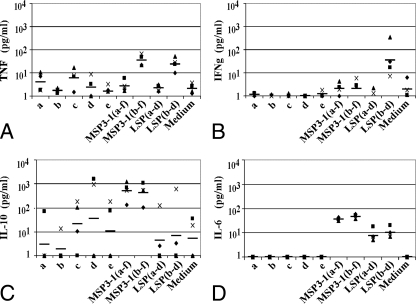
FIG. 2.
Cytokine levels in 48-h culture supernatants of splenocytes from MSP3-1(b-f)-immunized C57BL/6J mice stimulated in vitro with MSP3-1 peptides or long constructs. (A) TNF levels; (B) IFN-γ levels; (C) IL-10 levels; (D) IL-6 levels. Shown are individual results for each mouse tested in duplicate with samples taken after the third immunization, and the black horizontal bars represent the geometric means of five different mice.
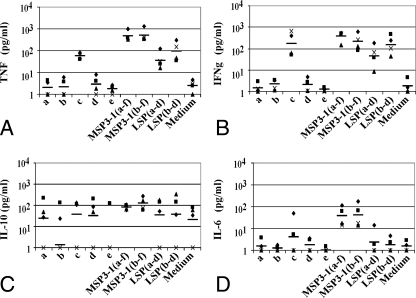
FIG. 3.
Cytokine levels in 48-h culture supernatants of splenocytes from MSP3-1 LSP(b-d)-immunized C57BL/6J mice stimulated in vitro with MSP3-1 peptides or long constructs. (A) TNF levels; (B) IFN-γ levels; (C) IL-10 levels; (D) IL-6 levels. Shown are individual results for each mouse tested in duplicate with samples taken after the third immunization, and the black horizontal bars represent the geometric means of five different mice tested in duplicate in samples taken after the third immunization.
The results obtained in MSP3-1(a-f)-immunized mice splenocytes did not indicate either a Th1 profile (with predominant TNF and IFN-γ secretion) or a Th2 profile (with predominant IL-10 and IL-6 secretion). High levels of TNF secretion were detected upon boosting by the immunogen itself, varying from 315 to 836 pg/ml, but IFN-γ levels were low and varied between 43 and 72 pg/ml. IL-10 secretion was heterogeneous, varying between 1 and 53 pg/ml, and IL-6 levels varied between 48 and 74 pg/ml (data not shown).
As shown in Fig. 2, in mice immunized with MSP3-1(b-f) the responses were skewed toward a Th2 profile of cytokines; after stimulation with the immunogen itself, high concentrations of IL-10 were detected (range, 101 to 1,051 pg/ml), and IL-6 levels ranged from 37 to 57 pg/ml; on the contrary, IFN-γ levels were borderline negative, and TNF secretion was low (range, 21 to 66 pg/ml).
The cytokine pattern observed upon stimulation of spleen cells taken from MSP3-1 LSP(a-d)-immunized mice, corresponded to a Th2 response. IL-10 and IL-6 levels were high, varying from 31 to 225 pg/ml for IL-10 and from 32 to 145 pg/ml for IL-6. In addition, IL-4, a Th2-type cytokine, was detected in stimulated spleen cells supernatants of this group, and its concentration ranged from 90 to 138 pg/ml after the in vitro boost with the immunogen itself. The very low levels of IFN-γ (5 to 26 pg/ml) observed are also consistent with a Th2 response despite a high production of TNF (200 to 1,066 pg/ml) (data not shown).
It is only in MSP3-1 LSP(b-d)-immunized animals that a clear dominance of a Th1 profile of cytokines was observed (Fig. 3), since high concentrations of both of the Th1-type cytokines, TNF and IFN-γ, were detected after stimulation by each of the constructs or by MSP3-1c peptide, whereas the Th2 type cytokine secretion remained at the background level for IL-10 and low to moderate for IL-6.
Taken together, these results confirm that immune responses toward the 69-aa target region are profoundly affected by the flanking sequences, since immunogens that include both the upstream and the downstream sequences of the 69-aa domain proved unable to induce T-cell responses toward the different peptides; deletion of both flanking regions was beneficial since the MSP3-1 LSP(b-d) construct was the only construct able to elicit a dominant IFN-γ and TNF secretion and thus a Th1 response.
(iii) The isotype distribution of the antibody response confirms the Th1/Th2 balance observed.
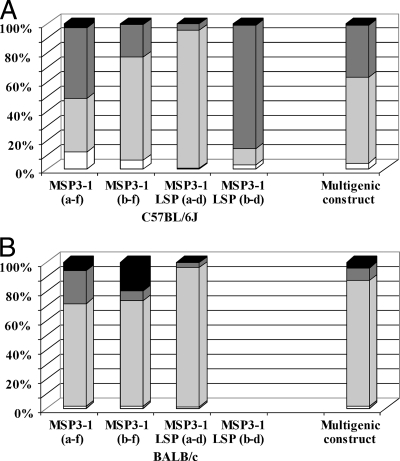
Since protective antibodies against P. falciparum blood stages have been shown to belong to cytophilic classes (3, 19), we analyzed the isotype distribution of MSP3-1 antibodies induced in both mouse strains by each of the four MSP3-1 constructs, assuming that the noncytophilic IgG1 and IgG3 (8) mouse isotypes would correspond to a Th2 response, whereas the cytophilic IgG2a and IgG2b (8) would correspond to a Th1 response.
As shown in Fig. 4, noncytophilic antibodies (IgG1 and IgG3) are major components of the antibody response in sera of MSP3-1(b-f)- or MSP3-1 LSP(a-d)-immunized mice. The same imbalance was observed in the BALB/c group immunized with MSP3-1(a-f) since the sum of specific IgG1 and IgG3 calculated represented 64% of the total IgG response, whereas in the C57BL/6J mice there was rather an equilibrium between cytophilic (which represented 55% of the total IgG) and noncytophilic isotypes (which represented 45% of the total IgG response). This observation is consistent with the Th1/Th2 balance described above for the T-cell response in these mice.
FIG. 4.
Isotypic distribution of the IgG responses against each of the four MSP3-1 immunizing antigens and the multigenic construct. Specific IgG2a (black), IgG2b (dark gray), IgG1 (light gray), and IgG3 (white) against the immunogen itself were determined by ELISA. Geometric means were calculated for each of the four isotypes in each group of five mice and are represented as percentages of the total IgG responses in C57BL/6J (A) and BALB/c (B) mice. The geometric means (95% confidence interval) of the total IgG responses in C57BL/6J mice immunized with MSP3-1(a-f), MSP3-1(b-f), MSP3-1 LSP(a-d), MSP3-1 LSP(b-d), and the multigenic construct were 42.1 μg/ml (41.1 to 42.9), 262.0 μg/ml (258.5 to 265.5), 743.7 μg/ml (712.1 to 776.7), 179.4 μg/ml (172.3 to 186.8), and 30.2 μg/ml (29.2 to 32.1), respectively. The geometric means (95% confidence interval) of the total IgG responses in BALB/c mice immunized with MSP3-1(a-f), MSP3-1(b-f), MSP3-1 LSP(a-d), and the multigenic construct were 556.4 μg/ml (536.1 to 577.5), 313.4 μg/ml (306.3 to 320.6), 277.7 μg/ml (268.1 to 287.6), and 652.6 μg/ml (635.1 to 670.6), respectively. No antibody responses were detectable in BALB/c mice that were immunized with the MSP3-1 LSP(b-d) construct. The results for the multigenic antigen are represented on this figure for easier comparison.
Interestingly, MSP3-1 LSP(b-d) is the only construct that induced a cytophilic antibody response, since >80% of the total specific IgGs consisted of cytophilic isotypes mainly belonging to the IgG2b subclass in C57BL/6J mice (Fig. 4), although no antibody response was induced in BALB/c mice (Fig. 1). This cytophilic response is in keeping with the Th1 cytokine profile described above.
Immunogenicity of the five other C-terminal recombinant proteins of the MSP3 family.
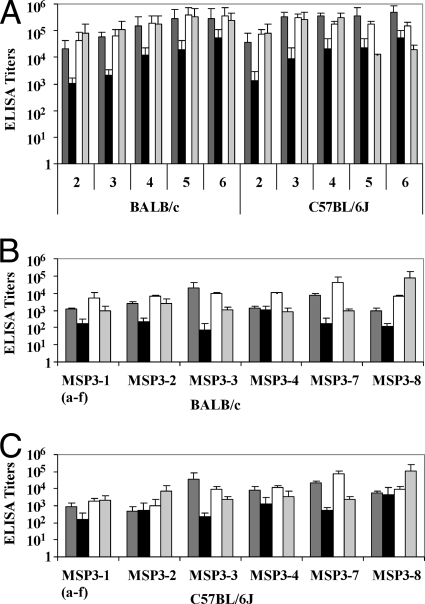
Prior to the construction of a new multigenic protein, we investigated the immunogenicity of each of the five other members of the MSP3 protein family. We immunized both mouse strains with recombinant proteins corresponding to the five C-terminal regions (a to f) of MSP3-2, MSP3-3, MSP3-4, MSP3-7, and MSP3-8. As shown in Fig. 5A, four of them induced antibodies in both mouse strains, whose titers increased over repeated immunizations and ranged from one thousand to one million. Only MSP3-4 C-term was somewhat less immunogenic than the remaining proteins in both mouse strains.
FIG. 5.
IgG responses in BALB/c and C57BL/6J mice immunized with the C-terminal recombinant proteins MSP3-3, MSP3-4, MSP3-7, and MSP3-8. (A) IgG titers using the immunogen as a coating antigen in mice immunized two to six times with MSP3-3 (dark gray bars), MSP3-4 (black bars), MSP3-7 (white bars), or MSP3-8 (light gray bars). (B) Cross-reactivity on the six members of the MSP3 protein family of sera from BALB/c mice after two injections of MSP3-3 (dark gray bars), MSP3-4 (black bars), MSP3-7 (white bars), or MSP3-8 (light gray bars). (C) Cross-reactivity on the six members of the MSP3 protein family of sera from C57BL/6J mice after two injections of MSP3-3 (dark gray bars), MSP3-4 (black bars), MSP3-7 (white bars), or MSP3-8 (light gray bars). The results are expressed as mean ELISA titers ± the SD.
Given the structural homologies among the MSP3 protein family, particularly within peptides “b” and “d,” antibodies elicited by one member cross-reacted with the remaining ones. This has been documented for antibodies elicited by MSP3-1 and MSP3-2 (22). In order to further document this phenomenon, we tested the antibodies induced by the immunization with the C-terminal regions of MSP3-3, MSP3-4, MSP3-7, and MSP3-8 for their capacity to recognize all six MSP3 family members.
The antibody responses induced in BALB/c (Fig. 5B) and C57BL/6J (Fig. 5C) mice immunized with each of the four MSP3 proteins cited above were cross-reactive with the other six proteins, although the degree of cross-reactivity varied. In general, the highest titers were observed against the immunogen and were lower but very substantial against the remaining five members of the family.
The first MSP3 family-based multigenic construct elicited IgG reacting with the six MSP3 family proteins.
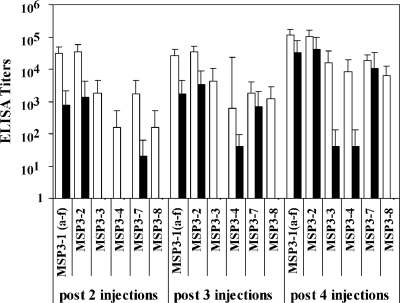
The first multigenic construct was designed on the basis of bioinformatic alignments determining in each MSP3 protein the cognate sequence to the 69-aa region defined on MSP3-1. The new protein is the combination of these six sequences, without the addition of the upstream or the downstream sequences of the 69-aa domain, since the immunogenicity studies reported above suggest that such addition might be deleterious. This molecule was used to immunize C567BL/6J and BALB/c mice. Specific IgG responses to the immunogen itself were induced in both mouse strains; titers ranged between 3,200 and 51,200 in C57BL/6J mice and between 51,200 and 409,600 in BALB/c mice after the fourth immunization.
Sera from BALB/c mice immunized with the multigenic protein reacted in ELISAs with all six MSP3 proteins (Fig. 6), whereas sera from C57BL/6J mice reacted more strongly with three of the six molecules: MSP3-1(a-f), MSP3-2, and MSP3-7; moderately with MSP3-3 and MSP3-4; and not at all with MSP3-8.
FIG. 6.
IgG responses against the C-terminal recombinant proteins of the MSP3 family in mice immunized with the new multigenic construct. Groups of five BALB/c (□) and C57BL/6J (▪) mice were immunized with the multigenic construct, and IgG responses were tested by ELISA after two, three, and four injections against MSP3-1(a-f), MSP3-2, MSP3-3, MSP3-4, MSP3-7, or MSP3-8. The results are expressed as mean titers ± the SD.
Antibodies induced by the multigenic construct react with native parasite proteins.
Since a vaccine should induce immune effectors that react with native proteins presented by the parasite, antibodies elicited in mice were assessed for reactivity in Western blot assays with P. falciparum blood-stage extract and in an immunofluorescence assay upon mature blood forms.
Western blots with the sera of C57BL/6J and BALB/c mice immunized with the multigenic protein demonstrate the recognition of parasitic proteins, notably of MSP3-1 (∼50 kDa) and other molecular masses corresponding to other members of the family. Sera from mice immunized with single proteins of the MSP-3 family were assessed as well. The reactivity profile to native proteins of sera from MSP3-2 immunized mice was very similar to that observed with the sera of the MSP3-1 construct-immunized mice or of mice immunized with the multigenic protein, stressing the high cross-reactivity between the MSP3-1 and the MSP3-2 proteins, possibly due to the identity among the two “b” peptide antigenic determinants (22). Sera collected from MSP3-3-, MSP3-4-, MSP3-7-, or MSP3-8-immunized mice did not cross-react with MSP3-1 or MSP3-2, and the molecular masses they displayed corresponded to the respective native proteins.
The immunofluorescence assays also confirmed the recognition of native antigens on parasitized red blood cells or free merozoites by the sera collected from mice immunized with the new multigenic vaccine with titers varying, after the fourth injection, from 100 to 800 with sera from C57BL/6J mice (40% positive mice) and from 800 to 6,400 with sera from BALB/c mice (100% positive mice).
Isotype distribution and functional activity of antibodies induced by the multigenic construct.
The isotype pattern of IgG elicited by the multigenic construction was determined (Fig. 4). The results show that the isotype distribution was well balanced between cytophilic (IgG2a and IgG2b) and noncytophilic (IgG1 and IgG3) subclasses in C57BL/6J mice, whereas an imbalance in favor of the IgG1 subclass was observed in BALB/c mice.
In order to assess their functional activity, we tested the induced antibodies for their ability to inhibit parasite growth in the ADCI assay, carried out with additional controls using immunoglobulins from naive mice to check for the specificity of the parasite growth inhibition obtained with antibodies prepared from the sera of immunized mice.
Since the concentrations of antigen specific antibodies were higher in this mouse strain, we analyzed the antiparasitic activity of IgG prepared from a pool of sera collected from five BALB/c mice immunized four times with the multigenic construct. We found that this IgG preparation had kept its ability to cross-react with the six MSP3 recombinant proteins (optical density [OD] values ranging from 2.5 to 4.3, similar to the OD of 4.2 recorded using the multigenic protein itself). In the ADCI assay, this IgG preparation was able to very efficiently inhibit the in vitro parasite growth, in cooperation with human monocytes. Indeed, the specific inhibition index (59.85% ± 1.62%) was as high as that obtained with a pool of hyperimmune African sera IgG protective upon passive transfer in humans (63.36% ± 4.45%) or with previous experiments performed with anti-MSP3-1b mouse antibodies (16).
DISCUSSION
In the search for an effective malaria vaccine, we focused our studies on antigens targeted by the most potent immunity that can be achieved in humans, that is, the immunity acquired over the years by individuals living in areas of hyperendemicity. This led us to characterize MSP3-1, and especially the highly conserved C-terminal region, as a most promising vaccine candidate. In a phase I vaccine trial of a prototype construct, only 60% of the volunteers developed antibodies able to react with the native protein (1, 7). For this reason we undertook a detailed immunogenicity study of this antigen in two strains of mice. The present study was intended to guide the design, based on knowledge, of a multigenic vaccine constructed from the recently described six members of the MSP3 multigene family (23). Indeed, antigenicity studies (5) indicate that the members of this new family of genes are the target of biologically active antibodies.
Our previous antigenicity studies, and functional assays of antibodies directed against the C-terminal region of MSP3, identified a 69-aa conserved domain, covered by the overlapping peptides MSP3-1b, MSP3-1c, and MSP3-1d, as a target of biologically active antibodies to be included in MSP3-1-based vaccine constructs. Indeed, the MSP3-1 LSP tested in a phase I vaccine trial (7) corresponded to the region covered by peptides MSP3-1a to −1d. The present immunogenicity study in mice assessed the effect of the addition of the N-flanking sequence (“a”) and of the C-flanking sequence (“e-f”) upon the response against the crucial region from MSP3-1b to -1d. The addition of the sequence downstream from the 69-aa domain proved to be clearly deleterious because the MSP3-1 LSP(a-d) construct, compared to the MSP3-1(a-f) construct, induced markedly higher antibody and T-cell responses. Surprisingly, the deleterious effect of the C-flanking region was less intense in the absence of the MSP3-1a region, since the antibody response induced by the MSP3-1(b-f) construct was better than that induced by the MSP3-1(a-f). These observations raise the question of the spatial conformation of these different constructs and of the possible interaction between the two sequences surrounding the sequence of interest, both of which have a coiled structure potentially allowing a coiled-coil superstructure (4, 14). The effect of the addition of MSP3-1a was more complex. Whereas comparison of the MSP3-1(a-f) and MSP3-1(b-f) constructs suggests a deleterious effect related to the “a” region, comparison of the MSP3-1 LSP(a-d) and MSP3-1 LSP(b-d) constructs shows that the antibody response is somewhat better with the former. Indeed, the MSP3-1 LSP(a-d) construct, which has been tested in clinics, was validated as the only one able to induce a high and stable antibody response, in both C57BL/6J and BALB/c mouse strains, against the three antigenic determinants (MSP3-1b, MSP3-1c, and MSP3-1d) targeted by biologically active antibodies. The MSP3-1a peptide present in this construct defines a T-cell epitope eliciting responses in mice, which is in accordance with the results of the human clinical trial (1). However, the MSP3-1 LSP(b-d) construct, in which the MSP3-1a sequence was eliminated, had the remarkable feature to induce a Th1-like cellular response in both mouse strains and to induce mainly cytophilic antibodies in C57BL/6J. Thus, the deletion of the MSP3-1a antigenic determinant unveiled a new T-helper epitope located on the MSP3-1c sequence which became immunodominant in both BALB/c and C57BL/6J mice but improved the humoral response only in the latter.
Taken together, these results indicated a deleterious effect of the subregion downstream from the 69-aa sequence of interest and shed light on the peculiarity of the MSP3-1a subregion. The sequence of MSP3-1a includes heptad repeats with a corresponding coiled structure that is unique to MSP3-1 (i.e., not found in the other members of the MSP3 family) and contains an immunodominant T-helper cell epitope that is not the target of antibody responses in either mice or in humans immunized with the MSP3-1 LSP(a-d) construct (1). This is in accordance with the observation that in naturally exposed individuals no substantial antibody response was detected against the MSP3-1a peptide (21). Thus, the flanking sequences profoundly affected the humoral and cellular immune responses toward the target “b-d” sequence. The direct recognition of the antigen in its native form by the B-cell antigen receptor is necessary for the activation of a B cell. As a consequence, the humoral response is directly dependent on the structural context in which an antigenic determinant is presented, which could explain the differences observed between the constructs in terms of antibody response directed against the various subregions. This may be the case also for T-cell activation. Indeed, several groups have reported the influence of the antigen structure, i.e., of the three-dimensional presentation of the epitopes, on the determination of the T-helper cell epitope immunodominance (12, 15, 25). They showed that the extent of antigen uptake by the antigen-presenting cells, the rate of its processing, and the affinity of the resulting peptides for major histocompatibility complex molecules were affected by its structure, which consequently should also affect the presentation to T-cell receptor and the activation of T cells.
The study of immunogenicity in mice of an antigen from a human specific parasite, i.e., a protein that has evolved under pressure from the human immune system, has obvious limitations. It is also obvious that an immunogenicity study, as detailed as the one presented here, cannot be conducted in humans.
In this context, it is nevertheless worth stressing that the negative immunological effect of the MSP3[e-f] region indicated by our study in models was recently supported by results from human trials. Whereas MSP3-1 LSP(a-d) (1) and Glurp R0 (9) both generated strong IFN-γ responses in human volunteers, a fusion of both molecules that included the “e-f” region failed to do so when used with the same adjuvant in volunteers (M. Theisen, unpublished data).
To attempt to circumvent the qualitative limitations of models and the quantitative limitations of clinical trials, our strategy has been to interpret immunogenicity studies in experimental animal models in the context of detailed antigenicity analysis in humans of MSP3-1 (21) and MSP3-2 (22) and of the four remaining members of the MSP3 family (5).
These detailed antigenicity studies, conducted using similar peptides covering the six proteins of the MSP3 family, have shown that each peptide was antigenic and that a large proportion was the target of cytophilic antibody responses which also mediated the ADCI mechanism. The “b” peptide contains an 11-aa stretch that is shared among three of the MSP3 proteins and generates full cross-reactivity. The “d” peptide contains a 20-aa sequence that is also present with limited sequence variation among the six members inducing cross-reactivity of antibody responses. Thus, the 69-aa region of interest is, overall, antigenically conserved across the MSP3 family. In contrast, the flanking regions are divergent from one gene to the other but are also fully conserved as MSP3-1 among diverse P. falciparum isolates. These results, together with the present analysis of the immunogenicity of various MSP3-1-based constructs, were used to design a MSP3 multigenic vaccine. In contrast to other combined malaria vaccine candidates already in development, our approach was to group similar antigenic determinants from six proteins of the same family in order to increase immunogenicity toward cross-reactive sequences that are targets of protective immunity. However, the immunogenicity study of MSP3-1 considered as a model clearly demonstrated that the addition of various antigenic regions may profoundly affect the humoral and cellular responses to these targets. These findings finally led to a construct limited to the subregions homologous to MSP3-1(b-d), that is, excluding both the N-flanking regions (which markedly differed among the six proteins) and the C-flanking regions [with marked homologies, but suspected to be deleterious, as was established for the MSP3-1(e-f) subregion]. This new construction was immunogenic in mice, induced antibodies that recognized the native proteins, and inhibited parasite growth in in vitro functional ADCI assays in cooperation with human monocytes, i.e., it satisfied the preclinical criteria. In addition, the human antigenic and epidemiological studies conducted on the other members of the MSP3 family of proteins (5) and general considerations about the relationship between protein structure and immunogenicity are now available and can be exploited to design other MSP3 multigenic vaccine constructs.
Supplementary Material
Acknowledgments
L.-J.D. was supported by a fellowship from the French Ministry of Research.
We thank G. P. Corradin for kindly providing the MSP3-1 LSP(b-d) synthetic peptide. We are grateful to Catherine Blanc and Catherine Nakhlé-Ghanem for their valuable technical assistance.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 6 July 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Audran, R., M. Cachat, F. Lurati, S. Soe, O. Leroy, G. Corradin, P. Druilhe, and F. Spertini. 2005. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect. Immun. 73:8017-8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun, H., and P. Druilhe. 1992. Antibodies in falciparum malaria: what matters most, quantity or quality? Mem. Inst. Oswaldo Cruz 87(Suppl. 3):229-234. [DOI] [PubMed] [Google Scholar]
- 4.Burgess, B. R., P. Schuck, and D. N. Garboczi. 2005. Dissection of merozoite surface protein 3, a representative of a family of Plasmodium falciparum surface proteins, reveals an oligomeric and highly elongated molecule. J. Biol. Chem. 280:37236-37245. [DOI] [PubMed] [Google Scholar]
- 5.Demanga, C. G., L.-J. Daher, E. Prieur, C. Blanc, J.-L. Pérignon, H. Bouharoun-Tayoun, and P. Druilhe. 2010. Toward the rational design of a malaria vaccine construct using the MSP3 family as an example: contribution of antigenicity studies in humans. Infect. Immun. 78:486-494. [DOI] [PMC free article] [PubMed]
- 6.Druilhe, P., and H. Bouharoun-Tayoun. 2002. Antibody-dependent cellular inhibition assay. Methods Mol. Med. 72:529-534. [DOI] [PubMed] [Google Scholar]
- 7.Druilhe, P., F. Spertini, D. Soesoe, G. Corradin, P. Mejia, S. Singh, R. Audran, A. Bouzidi, C. Oeuvray, and C. Roussilhon. 2005. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLoS Med. 2:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grey, H. M., J. W. Hirst, and M. Cohn. 1971. A new mouse immunoglobulin: IgG3. J. Exp. Med. 133:289-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermsen, C. C., D. F. Verhage, D. S. Telgt, K. Teelen, J. T. Bousema, M. Roestenberg, A. Bolad, K. Berzins, G. Corradin, O. Leroy, M. Theisen, and R. W. Sauerwein. 2007. Glutamate-rich protein (GLURP) induces antibodies that inhibit in vitro growth of Plasmodium falciparum in a phase 1 malaria vaccine trial. Vaccine 25:2930-2940. [DOI] [PubMed] [Google Scholar]
- 10.Huber, W., I. Felger, H. Matile, H. J. Lipps, S. Steiger, and H. P. Beck. 1997. Limited sequence polymorphism in the Plasmodium falciparum merozoite surface protein 3. Mol. Biochem. Parasitol. 87:231-234. [DOI] [PubMed] [Google Scholar]
- 11.McColl, D. J., and R. F. Anders. 1997. Conservation of structural motifs and antigenic diversity in the Plasmodium falciparum merozoite surface protein-3 (MSP-3). Mol. Biochem. Parasitol. 90:21-31. [DOI] [PubMed] [Google Scholar]
- 12.Mirano-Bascos, D., M. Tary-Lehmann, and S. J. Landry. 2008. Antigen structure influences helper T-cell epitope dominance in the human immune response to HIV envelope glycoprotein gp120. Eur. J. Immunol. 38:1231-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moorthy, V. S., M. F. Good, and A. V. Hill. 2004. Malaria vaccine developments. Lancet 363:150-156. [DOI] [PubMed] [Google Scholar]
- 14.Mulhern, T. D., G. J. Howlett, G. E. Reid, R. J. Simpson, D. J. McColl, R. F. Anders, and R. S. Norton. 1995. Solution structure of a polypeptide containing four heptad repeat units from a merozoite surface antigen of Plasmodium falciparum. Biochemistry 34:3479-3491. [DOI] [PubMed] [Google Scholar]
- 15.Nayak, B. P. 1999. Differential sensitivities of primary and secondary T-cell responses to antigen structure. FEBS Lett. 443:159-162. [DOI] [PubMed] [Google Scholar]
- 16.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 17.Reichelt, P., C. Schwarz, and M. Donzeau. 2006. Single step protocol to purify recombinant proteins with low endotoxin contents. Protein Expr. Purif. 46:483-488. [DOI] [PubMed] [Google Scholar]
- 18.Roggero, M. A., C. Servis, and G. Corradin. 1997. A simple and rapid procedure for the purification of synthetic polypeptides by a combination of affinity chromatography and methionine chemistry. FEBS Lett. 408:285-288. [DOI] [PubMed] [Google Scholar]
- 19.Roussilhon, C., C. Oeuvray, C. Muller-Graf, A. Tall, C. Rogier, J. F. Trape, M. Theisen, A. Balde, J. L. Perignon, and P. Druilhe. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 4:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 21.Singh, S., S. Soe, J. P. Mejia, C. Roussilhon, M. Theisen, G. Corradin, and P. Druilhe. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190:1010-1018. [DOI] [PubMed] [Google Scholar]
- 22.Singh, S., S. Soe, C. Roussilhon, G. Corradin, and P. Druilhe. 2005. Plasmodium falciparum merozoite surface protein 6 displays multiple targets for naturally occurring antibodies that mediate monocyte-dependent parasite killing. Infect. Immun. 73:1235-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh, S., S. Soe, S. Weisman, J. W. Barnwell, J. L. Pérignon, and P. Druilhe. 2009. A conserved multi-gene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS ONE 4:e5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soe, S., M. Theisen, C. Roussilhon, K. S. Aye, and P. Druilhe. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surman, S., T. D. Lockey, K. S. Slobod, B. Jones, J. M. Riberdy, S. W. White, P. C. Doherty, and J. L. Hurwitz. 2001. Localization of CD4+ T-cell epitope hotspots to exposed strands of HIV envelope glycoprotein suggests structural influences on antigen processing. Proc. Natl. Acad. Sci. USA 98:4587-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theisen, M., S. Soe, K. Brunstedt, F. Follmann, L. Bredmose, H. Israelsen, S. M. Madsen, and P. Druilhe. 2004. A Plasmodium falciparum GLURP-MSP3 chimeric protein; expression in Lactococcus lactis, immunogenicity and induction of biologically active antibodies. Vaccine 22:1188-1198. [DOI] [PubMed] [Google Scholar]
- 27.Theisen, M., J. Vuust, A. Gottschau, S. Jepsen, and B. Hogh. 1995. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin. Diagn. Lab. Immunol. 2:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.