Abstract
Plasmodium falciparum merozoite surface protein (MSP3) is a main target of protective immunity against malaria that is currently undergoing vaccine development. It was shown recently to belong, together with MSP6, to a new multigene family whose C-terminal regions have a similar organization, contain both homologous and divergent regions, and are highly conserved across isolates. In an attempt to rationally design novel vaccine constructs, we extended the analysis of antigenicity and function of region-specific antibodies, previously performed with MSP3 and MSP6, to the remaining four proteins of the MSP3 family using four recombinant proteins and 24 synthetic peptides. Antibodies to each MSP3 family antigen were found to be highly prevalent among malaria-exposed individuals from the village of Dielmo (Senegal). Each of the 24 peptides was antigenic, defining at least one epitope mimicking that of the native proteins, with a distinct IgG isotype pattern for each, although with an overall predominance of the IgG3 subclass. Human antibodies affinity purified upon each of the 24 peptides exerted an antiparasite antibody-dependent cellular inhibition effect, which in most cases was as strong as that of IgG from protected African adults. The two regions with high homology were found to generate a broad network of cross-reactive antibodies with various avidities. A first multigenic construct was designed using these findings and those from related immunogenicity studies in mice and demonstrated valuable immunological properties. These results indicate that numerous regions from the MSP3 family play a role in protection and provide a rationale for the tailoring of new MSP3-derived malaria vaccines.
Malaria vaccine development is both urgently needed and remarkably challenging in scientific terms, particularly in the choice of concepts determining which path(s) should be followed to achieve this end. The criteria used in the decision-making process obviously affect the choice of a candidate among the 5,300 Plasmodium falciparum proteins, i.e., the vaccine discovery process, but they also markedly affect the type of construct, expression system, or formulation chosen, as well as the selection of candidates to be included in vaccine combinations (18).
Given the uncertainties related to experimental animal models, particularly their relevance to human malaria (10), we have made the choice to base our research orientations on clinical observations, since we trust they are the most reliable. According to this strategy, animal models come into play only in a second step, mainly for detailed immunogenicity studies that obviously cannot be conducted initially in humans. For vaccine discovery, immunity was passively transferred into P. falciparum-infected recipients (3, 31) by human IgG from protected African adults. This led us to compare nonprotective with protective antibodies upon each parasite isolate and to identify a novel defense mechanism that had not been foreseen by research in models, in which the effective antibodies act in a monocyte-dependent manner. This antibody-dependent cellular inhibition (ADCI) of parasite multiplication (3, 5) was in turn used to identify merozoite surface protein 3 (MSP3) as a main target of protective antibodies (25). Its C-terminal region was found to be fully conserved across P. falciparum isolates, in contrast to most other vaccine candidates (30).
Studies in exposed populations show that MSP3 induces antibody responses that are strongly associated with clinical protection against disease (28, 30, 36). A detailed antigenic analysis of the C-terminal portion of MSP3 highlighted the importance of three antigenic determinants targeted by cytophilic antibodies induced by natural infection and able to inhibit parasite growth in functional in vitro ADCI assays (32). This information was instrumental in the design of the MSP3-LSP (long synthetic peptide) construct that was taken into the clinics (1) and which induced in human volunteers antibodies that inhibited P. falciparum multiplication both in vitro and in vivo (12).
We have recently reported that msp3 belongs to a multigene family, differing in its features from other P. falciparum gene families (34). Indeed, the six msp3 genes are transcribed simultaneously, and the corresponding proteins are expressed in all isolates investigated at late schizont stage. Their C-terminal portions share a similar organization and include regions with sequence homologies flanked by regions that markedly differ from one protein to the other and yet are very well conserved among isolates. The original msp3 was renamed as msp3.1, msp6 (41) was renamed as msp3.2, and the remaining genes were renamed msp3.3, msp3.4, msp3.7, and msp3.8 (see Fig. S1 in the supplemental material). In view of the unique features of this MSP3 family, particularly the high degree of conservation of homologous and divergent sequences implying that they had a role for parasite survival, we thought it valuable to develop a polyantigenic construct combining carefully chosen regions among the most immunologically relevant from these six proteins.
A detailed immunological study combining immunogenicity and antigenicity data provides a means to design malaria vaccines based on the new knowledge resulting from this analysis, where immunogenicity and antigenicity studies are complementary. Indeed, immunogenicity studies analyze the responses induced by a given vaccine formulation in experimental models but have the great limitation of doing so in animals, whose relevance to humans is not evident (10). In contrast, antigenicity studies have the paramount advantage of identifying immune responses developed by the target population, human beings, although these responses have been induced by exposure to the whole parasite. However, our first-generation MSP3-LSP vaccine readily induced in naive European volunteers an immune response with the same characteristics (cytophilicity, titer, and fine epitope specificity) as the immune response observed in exposed populations with an acquired protection (10).
In the present detailed antigenicity study we characterize the antibody responses of humans naturally exposed to malaria to recombinant antigens and to a series of overlapping peptides spanning the C-terminal portions of MSP3.3, MSP3.4, MSP3.7, and MSP3.8 and assessed the biological antiparasite activity of the corresponding antibodies. The data obtained were compared to the results previously obtained with MSP3.1 and MSP3.2 (32, 33). The antigenicity data of all six members of the MSP3 family, combined with the detailed study of the immunogenicity of the C-terminal portion of MSP3.1 (7), led to the design of the first multi-antigenic vaccine construct based on the MSP3 family of proteins, which was shown to have valuable immunological features.
MATERIALS AND METHODS
Recombinant antigens.
The current gene identification numbers of the P. falciparum MSP3 family proteins in the PlasmoDB (www.plasmodb.org) release 5.5 database are PF10_0345, PF10_0346, PF10_0347, PF10_0348, PF10_0352, and PF10_0355 for MSP3.1, MSP3.2, MSP3.3, MSP3.4, MSP3.7, and MSP3.8, respectively. The set of six recombinant His6-tagged proteins that were designed to cover the related C-terminal regions of these MSP3 proteins are MSP3.1191-354, MSP3.2161-371, MSP3.3228-424, MSP3.4508-697, MSP3.7214-405, and MSP3.8537-762 (34). The overall percent homology of the MSP3 family proteins were analyzed by using EMBOSS pairwise sequence alignments (www.ebi.ac.uk/Tools/emboss/align/).
Peptide design.
Multiple sequence alignments for the MSP3 antigens were generated through CLUSTAL W2 (www.ebi.ac.uk/Tools/clustalw2) and DIALIGN2 (mobyle.pasteur.fr). The scheme of alignments and further manual refinements were used to direct the design of six overlapping peptides (see Fig. S2 in the supplemental material), from 21 to 61 amino acids in length, covering the C-terminal portion (excluding the glutamic acid-rich region) for each of the four MSP3 proteins MSP3.3, MSP3.4, MSP3.7, and MSP3.8. They were designed in order to fit those previously synthesized for MSP3.1 and MSP3.2 (32, 33) and were named “a,” “b,” “c,” “d,” “e,” and “f,” as was the case for the latter. (For the positions and sequences of the peptides, see Table S1 and Fig. S2 in the supplemental material.) Peptide synthesis was carried out through standard stepwise solid-phase peptide synthesis (GenScript Corp., Piscataway, NJ).
ELISAs.
The immunoepidemiological studies aimed at determining the prevalences and IgG subclass distributions were performed with serum samples from the inhabitants of Dielmo, a village located in an area of Senegal, West Africa, where malaria is holoendemic (40). These samples were collected during a cohort prospective follow-up study designed to identify and analyze all malaria-associated episodes of morbidity (40). The detection of total IgG and IgG subclasses was performed by enzyme-linked immunosorbent assay (ELISA) as described previously (4, 9), using secondary monoclonal antibodies originally selected as reacting with African subclass dominant allotypes. Mouse monoclonal antibodies specific for human IgG subclasses (clones NL16 [IgG1; Boehringer Mannheim, Meylan, France], HP6002 [IgG2; Sigma-Aldrich, Steinheim, Germany], and Zg4 and RJ4 [IgG3 and IgG4, respectively; Immunotech, Marseilles, France] were used at final dilutions of 1/2,000, 1/10,000, 1/10,000, and 1/1,000, respectively). For the total IgG detection, the horseradish peroxidase-conjugated goat anti-human (Caltag/Invitrogen, Carlsbad, CA) was used at a 1/3,000 dilution. For each antigen (peptides or recombinant proteins), the results were expressed as optical density (OD) ratios, or arbitrary units, calculated by dividing the OD of each serum sample over the mean OD + three standard deviations (SD) of five control serum samples (30). These control sera were randomly selected among French blood donors without exposure to malaria, and their antibody reactivities were assumed to be normally distributed. Therefore, the mean OD + 3 SD covered all of the negative control responses.
Affinity purification of specific antibodies.
The antibodies specific for the 24 overlapping peptides covering the C-terminal sequence of MSP3.3, MSP3.4, MSP3.7, and MSP3.8 were affinity purified from a pool of sera derived from 333 rural African adults from Ivory Coast, West Africa, who had reached a state of premunition, i.e., a pool similar to that used to prepare the IgG used in a passive-transfer experiment (31).
Antibodies specific to either peptides or recombinant antigens were prepared as described previously (6, 32). Briefly, plain polystyrene beads (mean diameter, 10 μm; Polysciences, Eppelheim, Germany) were coated with antigens in a 5% (wt/vol) phosphate-buffered saline (PBS) suspension and washed thoroughly in PBS. The coated beads were incubated for 2 h at room temperature with the pool of African sera mentioned above diluted 1/10 and then washed twice in PBS-0.01% Tween 20. The antibodies captured on the beads were eluted by using a 0.2 M glycine buffer (pH 2.5) and immediately neutralized with the required volume of 2 M Tris buffer (pH 11.2). The antibodies eluted were dialyzed extensively against PBS, followed by RPMI, and then concentrated by centrifugation using the molecular sieve Vivaspin2 (30,000 MWCO PES; Sartorius Stedim, Göttingen, Germany). They were stored at 4°C after addition of 1% AlbumaxI (Gibco/Invitrogen). The specificity of affinity-purified antibodies was ascertained by ELISA on each peptide, using bovine serum albumin and the peptide SALSA-1 from the sporozoite and liver stage antigen 1 (2), as negative controls. The purity of antibodies preparation was ascertained in SDS-PAGE using a 10% polyacrylamide gel.
Indirect immunofluorescence assays (IFA) were performed according to previously described techniques (3) in order to assess recognition of the native parasite proteins by affinity-purified antibodies. Air-dried acetone-fixed thin smears from the P. falciparum clone 3D7 at the mature schizont stage were incubated with serial dilutions of affinity-purified antibodies and using a 1/200 dilution of fluorescein-labeled goat anti-human IgG (Alexa Fluor 488; Invitrogen, Carlsbad, CA) as a second antibody.
Functional ADCI assays.
The P. falciparum 3D7 clone (MRA-102, MR4; ATCC, Manassas, VA) was cultivated in complete medium, i.e., RPMI 1640 supplemented with 0.5% AlbumaxI (both from Gibco/Invitrogen), as described earlier (21). Schizont enrichment by flotation on 1% (wt/vol) gelatin (Sigma-Aldrich, Steinheim, Germany) (21) was performed 48 h before initiating the ADCI-based functional assays.
IgG was extracted from the same pool of sera derived from Ivory Coast adults used for affinity purification by ion-exchange chromatography on DEAE Ceramic HyperD F (Pall BioSepra, Cergy-Saint-Christophe, France) and named PIAG (for pool of immune African globulins). In the ADCI assays performed to evaluate the biological activity of antibodies specific to each antigen, two positive controls were used: PIAG and antibodies affinity purified on MSP3.1-Cterm. The negative control was IgG from a pool of French donors with no malaria history (N-IgG). Peripheral blood mononuclear cells (PBMC) from healthy French donors were prepared and cryopreserved as previously described (20). For the ADCI assay, PBMC were rapidly thawed at 37°C and assessed for viability by trypan blue exclusion. Cell suspensions were adjusted to a concentration of 107 cells per ml with RPMI 1640, and 200 μl/well was distributed in flat-bottom 96-well plates (TPP, Trasadingen, Switzerland), followed by incubation for 1 h at 37°C and 5% CO2. The nonadherent cells were removed by two washing steps with RPMI 1640, and then the cell appearance and the relative homogeneity of the distribution of the adherent cells in the different wells were checked by observation under an inverted microscope. Antibodies affinity purified upon the different peptides or recombinant antigens were tested at a final concentration, yielding a 1/20 IFA titer, and the N-IgG and PIAG were used at 2 mg/ml (ca. 10% the IgG concentration in the sera from African adults). To each well were added 50 μl of the test antibody and 50 μl of a schizont-infected red blood cell suspension at final hematocrits of 2.5 and 0.5% parasitemia, both in complete medium. The control wells consisted of either monocytes alone, monocytes with N-IgG or parasites with antibodies but without monocytes, or parasites alone (8, 25). At 48 and 72 h, 50 μl of complete medium was added to each well. After cultivation for 96 h, the level of parasitemia was determined on Giemsa-stained thin smears from each well by microscopic examination of at least 50,000 erythrocytes. The specific growth inhibitory index (SGI), which takes into account the possible inhibition induced by monocytes or antibodies alone, was calculated as follows: SGI = 100 × [1 − (parasitemia with monocytes and test IgG/parasitemia with test IgG)/(parasitemia with monocytes and N-IgG/parasitemia with N-IgG)], where parasitemias are expressed as the percentage of infected red blood cells. The ADCI assays were performed in duplicate for each affinity-purified antibody, using monocytes showing <10% direct nonspecific inhibition of parasite growth. Based on previous experience, the threshold of positivity of the ADCI assay was considered to be ≥30% of the positive control (12).
Cross-reactivity studies.
In a first set of experiments, the cross-reactivity of antibodies was assessed toward the sequence of the peptides “b,” which are quasi-identical for MSP3.1, MSP3.2, and MSP3.7. The competition ELISA was performed as described elsewhere (14), with competing antigen at concentrations ranging from 0 to 800 μg/ml. The results, expressed as the percent reactivity, were calculated as follows: [(mean OD values with the competing antigen at a given concentration)/(mean OD values without the competing antigen)] × 100. To control the specificity of the competition between antigen and antibody, the nonrelevant antigen SALSA-1 was added at the same concentration as the competing antigen.
The second set of experiments, performed to characterize the overall cross-reactivity network among the MSP3 family, were based on the reactivity of antibodies specific to the peptide “d.” Indeed, the sequences of the “d” peptides are highly homologous among the MSP3 family. The ELISA reactivity of antibodies specific to each peptide “d” upon all of the six recombinant MSP3-Cterm antigens was determined.
Avidity studies.
The binding strength of antibodies induced in mice by a first MSP3 polyantigenic construct (7) was evaluated by ELISA toward each MSP3 recombinant C terminus protein as described elsewhere (29). Briefly, antibodies affinity purified upon the polyantigenic construct from a pool of sera from immunized mice were tested on microplates coated with each of the six MSP3 recombinant proteins. After allowing the formation of antigen-antibody complexes, the latter were dissociated with increasing concentrations of the chaotropic ion ammonium thiocyanate (NH4SCN) from 0.25 to 4 M. After a wash step, the remaining antibodies were quantified. The percent reactivity was calculated as follows: [(mean OD values with a given concentration of NH4SCN)/(mean OD values without NH4SCN)] × 100. The avidity index was estimated as the molar concentration of NH4SCN required to reduce the initial OD by 50%.
RESULTS
Antibodies specific for the six MSP3 antigens are highly prevalent in subjects living in areas where malaria is endemic.
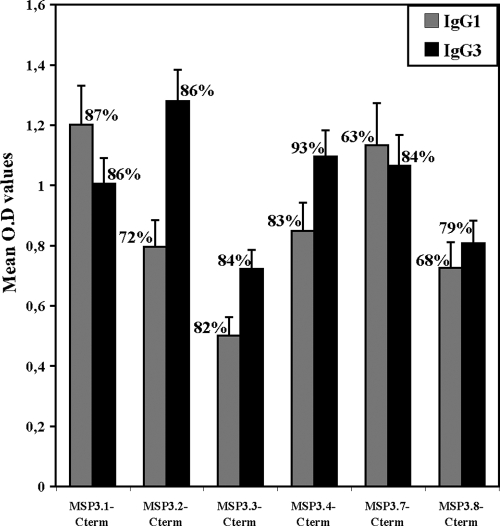
IgG antibodies specific for all of the six MSP3 antigens were detected in serum samples from adults living in the village of Dielmo, Senegal. Prevalences, i.e., percentages of individuals with a positive response, were very high, ranging from 89 to 95%, even though IgG levels varied from one antigen to the other (Table 1). The high antibody reactivity obtained with MSP3.2 was similar to that obtained with MSP3.4 or with MSP3.1. The lowest reactivity was with MSP3.7, which was in the same range as those of MSP3.8 and MSP3.3. Given the role of cytophilic antibodies in parasite killing and their association with protection against malaria, we performed IgG cytophilic subclass analysis (IgG1 and IgG3) of responses to the six MSP3 antigens using sera from a cohort of 76 malaria-exposed individuals of all ages (0.9 to 80.4 years) from the same village (Fig. 1). The results confirm the high prevalence of responses, since for each antigen except MSP3.8 the prevalences were ≥80% for IgG1 or IgG3. Although IgG3 antibodies were dominant in the case of MSP3.2, MSP3.4, and MSP3.7, the responses to the three remaining antigens were balanced between IgG1 and IgG3.
TABLE 1.
IgG responses of the C-terminal portion of the six MSP3 family proteins in hyperimmune serum samples from 45 adults from Dielmo, Senegala
| Protein | OD |
Prevalence (%) | |
|---|---|---|---|
| Mean ± SEM | Range | ||
| MSP3.1-Cterm | 2.2 ± 1.2 | 0.2-3.8 | 91.1 |
| MSP3.2-Cterm | 2.5 ± 1.0 | 0.2-3.8 | 88.9 |
| MSP3.3-Cterm | 1.9 ± 1.0 | 0.1-3.6 | 93.3 |
| MSP3.4-Cterm | 2.3 ± 1.1 | 0.2-4.1 | 95.6 |
| MSP3.7-Cterm | 1.3 ± 0.8 | 0.1-3.4 | 91.1 |
| MSP3.8-Cterm | 1.8 ± 0.9 | 0.2-3.6 | 91.1 |
To calculate the prevalence values, antibody responses were considered positive when the net OD value of each serum sample was greater than the mean OD + 3 SD of control serum samples. The prevalence values were not statistically different (Pearson's χ2 test). The mean net OD values represent the mean responses of the positive serum samples.
FIG. 1.
Cytophilic IgG subclasses responses to the C-terminal portion of the six MSP3 antigens in serum samples of individuals of all age groups (n = 76; range, 0.9 to 80.4 years; mean age, 14.4 years) from Dielmo, Senegal. To calculate the prevalence values, antibody responses were considered positive when the net OD value of each serum sample was greater than the mean OD + 3 SD of control serum samples. The mean of net OD values represents the mean responses of the positive serum samples. Bars indicate the standard errors of the mean.
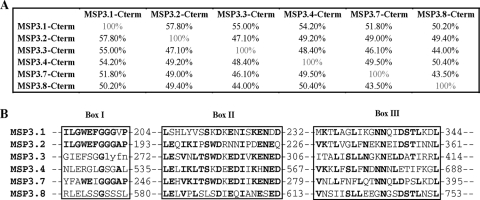
The C-terminal regions of the six MSP3 antigens contain highly homologous sequences flanked by nonhomologous ones.
In addition to their overall structural homology, the proteins of the MSP3 family share sequence similarities in their C-terminal portions. The overall percent homology, which is between 32 and 49% for the full-length protein, increases to 43 to 57% for the C-terminal portions (Fig. 2A). The first conserved motif, made of 11 amino acid residues centered on three glycine residues (Fig. 2B, box I), is included in the peptides called “b.” The sequences upstream and downstream are unique for each antigen, which directed the design of peptides “a” and “c,” respectively (see Table S1 in the supplemental material). Noticeably, the MSP3.1a sequence has the unique feature of heptad repeats. For each MSP3 antigen the peptide “d” located upstream of the glutamic acid rich region is characterized by a stretch of 20 amino acids with high position-specific scoring, i.e., identical or homologous residues being conserved for a given position (Fig. 2B, box II). The “e” peptides are located downstream of the Glu-rich region and, finally, the homologous leucine zipper-like pattern is displayed in the “f” peptides (Fig. 2B, box III).
FIG. 2.
(A) Matrix of overall percent homology (identity and similarity) of the C-terminal sequences of the six MSP3 proteins obtained with EMBOSS pairwise alignments. (B) Truncated multiple sequence alignment of the MSP3 proteins family showing the three regions with common linear motifs present in the C-terminal portion. Conserved residues are highlighted in boldface.
The 24 peptides from MSP3.3, MSP3.4, MSP3.7, and MSP3.8 are all antigenic and predominantly targeted by cytophilic antibodies.
The detailed analysis of the IgG subclass distribution among a cohort of malaria-exposed individuals of all age groups, versus the 24 peptides covering the C-terminal regions of these four antigens, showed that each of them was recognized by antibodies from sera from areas of endemicity, i.e., all of them were antigenic. The prevalence of specific antibodies was high; indeed, >60% of the individuals had antibodies reacting with two-thirds of the peptides (Fig. 3). For all of them, the antibodies belonged predominantly to the IgG1 and IgG3 subclasses, and only one (MSP3.8b) was targeted by a noncytophilic IgG2 response. In most cases, the IgG1 and IgG3 levels were of similar magnitude, but a trend, although not significant, toward a predominance of IgG3 was observed against some peptides (MSP3.3a, MSP3.4b, and MSP3.7e) or of IgG1 for others (e.g., MSP3.3b and MSP3.7d) (Fig. 3).
FIG. 3.
Prevalences (A) and titers (B) of IgG subclasses distribution in 24 peptides derived from MSP3.3, MSP3.4, MSP3.7, and MSP3.8 in serum samples of malaria-exposed individuals (n = 25; age range, 2 to 54 years; mean age, 16.86 years) from Dielmo village. Bars indicate the standard errors of the mean.
The present results, together with those previously reported for MSP3.1 and MSP3.2 (32, 33), show that the subclass patterns of responses to the homologous peptides “b,” “d,” or “f” from the six antigens of the MSP3 family differ markedly. This suggests that the flanking regions, defining T-helper cell epitopes (31), may play an important role in the modulation of the IgG1-to-IgG3 balance. Additional differences in antigenicity emerge for the various “a” peptides: in contrast to the low prevalence and low levels of antibodies previously observed to peptide MSP3-1a, high prevalences and levels are observed for MSP3-3a and MSP3-8a. Conversely, responses to the various “b” peptides, which is a major target of ADCI for MSP3-1, are high in five of the six proteins (i.e., all except MSP3-7).
Multiple targets of antibodies triggering ADCI are found throughout the MSP3 family.
Human antibodies affinity purified upon each of the 24 peptides of MSP3.3, MSP3.4, MSP3.7, and MSP3.8 recognized both the corresponding peptide and the recombinant C-terminus region (data not shown). More importantly, these affinity-purified antibodies were all able to recognize the parasite native proteins as determined by IFA, indicating that each synthetic peptide mimicked at least one natural parasite epitope.
In order to allow comparisons in ADCI assays, these antibodies were adjusted according to their IFA titers. The ADCI assay measures the intraerythrocytic killing effect of mediators released by monocytes and the resulting reduction of parasite density. As shown in Fig. 4, each of the 24 peptides from the four antigens was found to be a target of human antibodies with potent monocyte-dependent antiplasmodial activity in the ADCI assay. For a few of them (anti-3.3c, anti-3.3f, anti-3.4c, and anti-3.7c) the ADCI effect was only marginal and yet significant, whereas for the majority it was comparable to that of the PIAG, i.e., the IgG of protected African adults. The antiplasmodial effect mediated by some of them, such as anti-3.3e, anti-3.4b, and anti-3.8d antibodies, was even higher than that observed using the PIAG. This strong effect is remarkable since each peptide specific antibody corresponds to a very minor subset of total malarial antibodies and a small subset of antibodies directed to the six MSP3 family proteins. Although antibodies specific to the recombinant antigens were all efficient in ADCI (Fig. 4), the antibodies specific to several peptides (MSP3.3a, MSP3.3e, MSP3.4a, MSP3.4b, MSP3.7a, MSP3.8a, MSP3.8b, MSP3.8d, MSP3.8e, and MSP3.3f) mediated an effect stronger than that observed with the corresponding recombinant antigen. These results differ from those obtained previously with MSP3.1, where only three of the six peptides were the target of biologically active antibodies (32). They are in keeping with results obtained using MSP3.2 peptide-specific antibodies, which all had a strong inhibitory effect (33). Thus, the present data complement those obtained in our two previous studies to characterize a very large series of new targets for antibodies mediating the killing of P. falciparum parasite through the ADCI mechanism.
FIG. 4.
Antibody-dependent cellular inhibitory effect of human antibodies affinity purified upon the 24 peptides and the four recombinant antigens covering the C-terminal regions of MSP3 proteins. ▪, Positive controls; , anti-MSP3.3 peptides; □, anti-MSP3.4 peptides; ▒, anti-MSP3.7 peptides; ░⃞, anti-MSP3.8 peptides. Each of the affinity-purified antibodies was tested in duplicate at a concentration corresponding to a 1/20 final IFA titer. The PIAG and antibodies affinity purified upon recombinant MSP3.1-Cterm were used as positive controls in each experiment. The SGI values are expressed as a percentage of the effect of PIAG (100%); values of >30% are considered significant.
Cross-reactions among MSP3 family members.
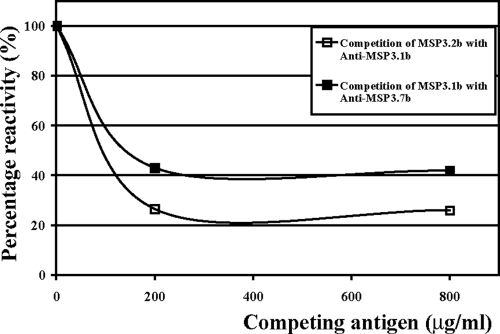
As mentioned above, a stretch of 11 amino acids is quasi-identical in the “b” peptides derived from MSP3.1 and MSP3.2 and highly homologous in MSP3.7b, which suggests that antibodies specific to these peptides may be cross-reactive. Indeed, in competition ELISAs, the MSP3.2b peptide at a concentration of 200 μg/ml was able to decrease by 80% the binding of anti-MSP3.1b antibodies to MSP3.1-Cterm. Similarly, the binding of anti-MSP3.7b was decreased by 60% by the competing MSP3.1b peptide (Fig. 5). These findings extend the cross-reactivity observed previously between MSP3.1b and MSP3.2b (33). The competition was less efficient between MSP3.1b and MSP3.7b than between MSP3.1b and MSP3.2b (Fig. 5), most probably due to the fact that, within the highly conserved stretch of 11 amino acids of MSP3.7b, there are substitutions in the first three positions—YFA versus ILG—and in the sixth position F/I, whereas MSP3.1b and MSP3.2b only differ by a V/A substitution in the tenth position (Fig. 2B, box I).
FIG. 5.
Antibody cross-reactivities between “b” peptides of the MSP3 proteins family. □, Binding of anti-MSP3.1b specific antibodies upon the recombinant antigen MSP3.1-Cterm in the presence of increasing concentrations of the MSP3.2b peptide; ▪, binding of anti-MSP3.7b specific antibodies upon the recombinant antigen MSP3.7-Cterm in the presence of increasing concentrations of the MSP3.1b peptide. Affinity-purified antibodies were preincubated with different amounts of peptides before being tested by ELISA.
The cross-reactivity was also assessed among the second group of homologous peptides, i.e., the “d” peptides (Fig. 2B, box II), using antibodies immunopurified on each peptide. The cross-reactivity network summarized in Fig. 6 indicates that naturally occurring human antibodies specific to peptide “d” of each MSP3 protein cross-reacted with at least one other antigen of the MSP3 family. Anti-MSP3-8d presented cross-reactivity with only one other member of the gene family, whereas anti-MSP3.1d and anti-MSP3.2d presented cross-reactivities with each of the remaining five proteins, i.e., they reacted with all six MSP3 proteins. On the other hand, MSP3.4-Cterm and MSP3.8-Cterm appeared as the “most antigenic” since they were recognized by antibodies immunopurified on each of the six “d” peptides. Conversely, these results indicate that the limited point mutations occurring in the highly conserved motif of the peptides “d” can also induce true antigenic divergence despite a high level of sequence homology. In this manner, the various homologous sequences generate a large number of distinct antibody species with a wide pattern of reactivity to the six proteins.
FIG. 6.
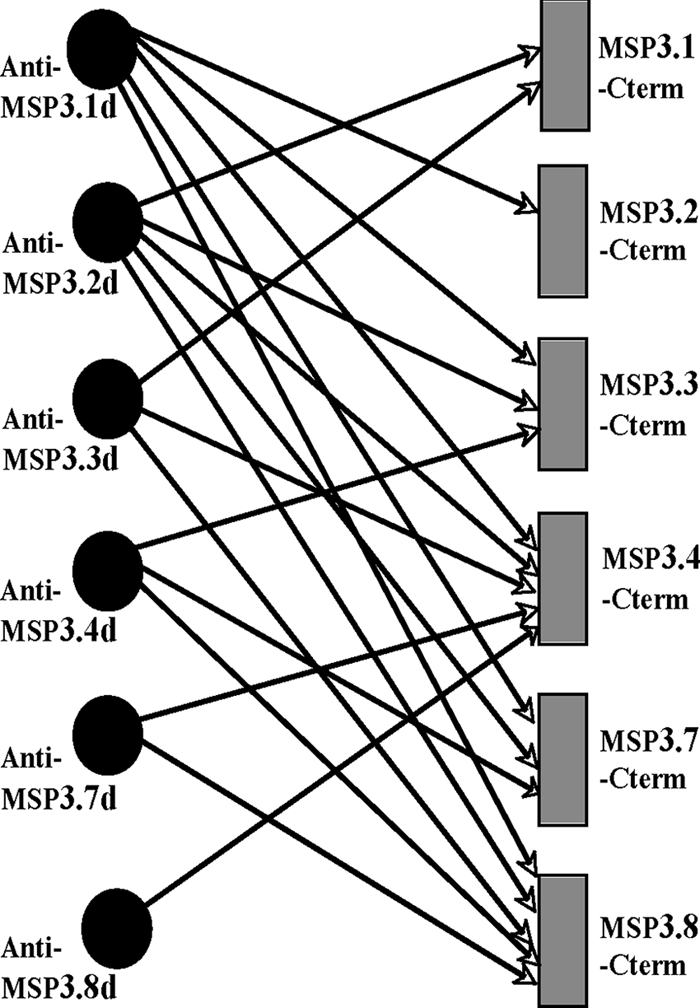
Pattern of cross-reactivity of antibodies to “d” peptides from the MSP3 proteins family. Binding patterns of antibodies specific to the “d” peptides of the six MSP3 proteins were deduced from their ELISA reactivities against the different recombinant antigens. Note that the reactivity to the homologous protein (e.g., anti-MSP3-8d peptide with MSP3-8 Cterm protein) was present for each of them; however, it is not shown for the sake of clarity. A significant cross-reactivity corresponds to OD values of ≥0.2.
An MSP3 multigenic construct induces in mice antibodies that cross-react with each antigen of the MSP3 family.
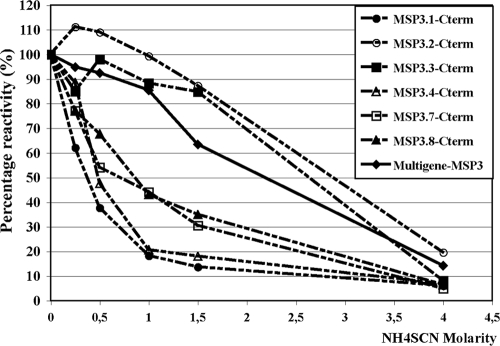
The studies described above show that sequence homologies among MSP3 proteins generate a large network of cross-reactions, despite sequence divergences, particularly among the flanking regions. Based on the combination of data from immunogenicity studies (7) and the present antigenicity data, a first MSP3 polyantigenic construct covering the “b-c-d” peptide sequences was designed and used to immunize mice. The mouse antibodies affinity purified upon the MSP3 multigenic construct reacted with each of the six antigens of the MSP3 family. However, they differently recognized each MSP3 antigen since the avidity indexes were estimated to be 0.4, 0.5, 0.7, 0.9, 2.6, and 2.8 M for MSP3.1, MSP3.4, MSP3.7, MSP3.8, MSP3.3, and MSP3.2, respectively. Therefore, the avidities of these antibodies were high for MSP3.3 and MSP3.2, medium for MSP3.8 and MSP3.7, and low for MSP3.4 and MSP3.1 (Fig. 7).
FIG. 7.
Avidity of antibodies induced in mice after immunization with an MSP3 family multigenic construct. The curves represent the reactivity on the MSP3 multigenic construct itself and on the different MSP3 antigens in the presence of increasing concentrations of chaotropic ion, i.e., ammonium thiocyanate (NH4SCN).
Our results indicate that the antigens of the MSP3 family are the targets of a family of cross-reactive antibodies, with wide differences in terms of avidity and specificity.
DISCUSSION
In order to design a multisubunit MSP3 vaccine based on a sound immunological rationale, we performed a detailed immunological study of the remaining four members of the MSP3 family of proteins to complement the data previously obtained on the other two, MSP3.1 and MSP3.2 (32, 33). As a complement to the immunogenicity study reported elsewhere (7), we performed here a detailed antigenicity analysis of the homologous C-terminal portions of these proteins and evaluated the biological activity of the corresponding antibodies. As in our previous studies with MSP3.1 and MSP3.2, we used both recombinant proteins and peptides corresponding to homologous or divergent regions, in order to select, based on the immune responses from exposed individuals, those to include in vaccine constructs.
Using sera from subjects spanning all ages living in a holoendemic region of Senegal, we found that the conserved C-terminal regions of the six MSP3 proteins were all antigenic and that a large majority of subjects harbored antibodies to each of them. A more detailed analysis of MSP3.3, MSP3.4, MSP3.7, and MSP3.8 was performed using for each of them six overlapping peptides covering their C-terminal portion. Each of these 24 peptides was antigenic, in accordance with what had previously been observed for the 12 peptides from MSP3.1 and MSP3.2. This shows that each synthetic peptide defines at least one B-cell epitope in the correct conformation. The recognition of the parasite native protein by anti-peptide antibodies, demonstrated by IFA assays and indirectly confirmed by ADCI assays, is an additional proof of the similarity of the peptides’ epitopes to those of the native proteins.
Besides their overall structural homology, the six C-terminal MSP3 proteins share highly homologous sequences displayed on the “b” and “d” (and “f”) peptides. The sequence ILGWEFGGGAP from the “b” peptide is well conserved among MSP3.1, MSP3.2, and MSP3.7 and was therefore found to be targeted by antibodies that are fully cross-reactive to these three antigens. The antibodies directed to the various “d” peptides presented a broad range of cross-reactivities on each of the six MSP3 proteins, a finding consistent with the occurrence of single-point amino acid changes among the homologous sequences. A malaria vaccine combining these antigens would thus not only have the advantage of inducing antibodies to an increased number of targets of ADCI but would presumably also broaden the types of antibody species raised against these proteins.
Twenty of the twenty-four peptides analyzed reacted with antibodies that displayed an IgG subclass pattern with dominant cytophilic responses. This was also found to be the case for four of the six MSP3.1 peptides and all of the six MSP3.2 peptides tested previously (32, 33). The IgG3 type of response was more prevalent than the IgG1 response, whereas the levels of both were equivalent. The importance of this finding is related to previous studies showing that IgG antibodies either mediating parasite killing in vitro or associated with protection in vivo are restricted to the IgG1 and IgG3 subclasses (4, 16, 20, 30). In agreement with this, all 24 peptides were indeed found to define antigenic determinants able to trigger the antiparasitic ADCI mechanism. The ADCI antiparasite activity of peptide-specific antibodies was, for more than half of them, as effective as or more effective than the total IgG from protected African adults. Therefore, these antibodies constitute a large series of novel valuable targets able to trigger the ADCI mechanism in addition to the nine targets previously described in the other two members of the MSP3 family (32, 33). They also add to epitopes identified in unrelated proteins such as GLURP, SERP (35, 39) and, more recently, MSP1-Block2 (15).
The multiple targets of in vitro inhibitory antibodies, found in all six members of the MSP3 family of proteins, suggest that they may play, in addition to MSP3.1, a role in protection. The additional, and most unusual, full sequence conservation among several field isolates of the C-terminal portion of each MSP3 gene (34) suggests that the conservation of differences in both homologous and nonhomologous regions has a critical function. Within the regions of highest homology, the cross-reactive B-cell epitopes generate a wider diversity in the affinity and fine specificity of the antibody repertoire than would a single antigen. Conversely, the conservation of these regions suggests a minimal selective advantage to random mutations. Antibodies elicited by any member could still cross-react with other nonmutated members and mediate parasite inhibition. Within nonhomologous regions, sequence differences from one gene to the other may be related to the T-helper function, which was documented in MSP3.1 (7, 32), each sequence being better fitted to a given major histocompatibility complex (MHC) class II subset. The conservation of the diversity would provide improved T-cell help in individuals with diverse MHC class II genetic backgrounds so as to generate the same essential antibodies directed to the conserved cross-reactive B-cell epitopes. These features open new perspectives on the role of MSP3 antigens in the natural host-parasite interaction. Their function might be to maintain the homeostasis between P. falciparum and human beings by contributing to control parasite densities through an indirect, nonsterilizing, defense mechanism (11).
Our findings also have practical consequences for vaccine development. Indeed, they pave the way toward the rational design of a multisubunit vaccine construct based on a family of proteins with both homologous and divergent sequences that are highly conserved. This combination strategy differs from constructs combining different polymorphic antigens (13, 17, 19, 23, 24, 27, 38). The C-terminal sequences being conserved, a polyantigenic MSP3 vaccine avoids antigen polymorphism, a recognized bottleneck for vaccine development (18, 22, 26, 37).
Finally, the present antigenicity data has to be combined with results from immunogenicity studies (7) to design improved constructs. Indeed, the latter suggest that certain regions are involved in the regulation of the intensity of immune responses. This was expected, since one of the remarkable characteristics of malaria infection in African children is the overwhelming number of parasites harbored over childhood and adolescence. The corresponding massive amounts of malarial antigens repeatedly stimulate their immune system for years. That the antibody titers to MSP3 and other antigens remain very moderate in children as in adults implies that malarial antigens must contain regulatory regions to prevent the induction of high immune responses that could otherwise eradicate the parasite. Such regions were identified in the immunogenicity study (7), where it was found that removing the “e-f” region from MSP3.1 resulted in an increase by 2 orders of magnitude of antibodies to the ADCI-relevant “b-d” region. In this manner the immunity induced by a carefully designed immunogen should be more effective than natural exposure to the parasite.
Our first polyantigenic construct indeed capitalizes on both sets of available data to include only the “b-d” regions of the six MSP3 antigens. The resulting multicomponent fusion protein was strongly immunogenic in mice, inducing biologically active antibodies and cross-reacting with the six MSP3 family proteins with various avidities (7; the present study). However, the combination of data emerging from detailed antigenicity and immunogenicity studies can be exploited in diverse ways, i.e., to design various constructs, so as to best fit the general strategy of a multicomponent vaccine based on the conserved MSP3 family antigens aimed at triggering more efficiently the ADCI mechanism on diverse human genetic backgrounds.
Supplementary Material
Acknowledgments
We thank Christian Roussilhon for helpful discussions and Nicolas Puchot for help with the illustrations.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 2 November 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Audran, R., M. Cachat, F. Lurati, S. Soe, O. Leroy, G. Corradin, P. Druilhe, and F. Spertini. 2005. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect. Immun. 73:8017-8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottius, E., L. BenMohamed, K. Brahimi, H. Gras, J. P. Lepers, L. Raharimalala, M. Aikawa, J. Meis, B. Slierendregt, A. Tartar, A. Thomas, and P. Druilhe. 1996. A novel Plasmodium falciparum sporozoite and liver stage antigen (SALSA) defines major B, T helper, and CTL epitopes. J. Immunol. 156:2874-2884. [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun, H., and P. Druilhe. 1992. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 60:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahimi, K., J. L. Perignon, M. Bossus, H. Gras, A. Tartar, and P. Druilhe. 1993. Fast immunopurification of small amounts of specific antibodies on peptides bound to ELISA plates. J. Immunol. Methods 162:69-75. [DOI] [PubMed] [Google Scholar]
- 7.Daher, L.-J., C. G. Demanga, E. Prieur, J.-L. Pérignon, H. Bouharoun-Tayoun, and P. Druilhe. 2010. Toward the rational design of a malaria vaccine construct using the MSP3 family as an example: contribution of immunogenicity studies in models. Infect. Immun. 78:477-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druilhe, P., and H. Bouharoun-Tayoun. 2002. Antibody-dependent cellular inhibition assay. Methods Mol. Med. 72:529-534. [DOI] [PubMed] [Google Scholar]
- 9.Druilhe, P., and H. Bouharoun-Tayoun. 2002. Human antibody subclass ELISA. Methods Mol. Med. 72:457-459. [DOI] [PubMed] [Google Scholar]
- 10.Druilhe, P., P. Hagan, and G. A. Rook. 2002. The importance of models of infection in the study of disease resistance. Trends Microbiol. 10:S38-S46. [DOI] [PubMed] [Google Scholar]
- 11.Druilhe, P., and J. L. Perignon. 1997. A hypothesis about the chronicity of malaria infection. Parasitol. Today 13:353-357. [DOI] [PubMed] [Google Scholar]
- 12.Druilhe, P., F. Spertini, D. Soesoe, G. Corradin, P. Mejia, S. Singh, R. Audran, A. Bouzidi, C. Oeuvray, and C. Roussilhon. 2005. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLoS Med. 2:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fluck, C., S. Schopflin, T. Smith, B. Genton, M. P. Alpers, H. P. Beck, and I. Felger. 2007. Effect of the malaria vaccine combination B on merozoite surface antigen 2 diversity. Infect. Genet. Evol. 7:44-51. [DOI] [PubMed] [Google Scholar]
- 14.Friguet, B., A. F. Chaffotte, L. Djavadi-Ohaniance, and M. E. Goldberg. 1995. Under proper experimental conditions the solid-phase antigen does not disrupt the liquid phase equilibrium when measuring dissociation constants by competition ELISA. J. Immunol. Methods 182:145-150. [DOI] [PubMed] [Google Scholar]
- 15.Galamo, C. D., A. Jafarshad, C. Blanc, and P. Druilhe. 2009. Anti-MSP1 block 2 antibodies are effective at parasite killing in an allele-specific manner by monocyte-mediated antibody-dependent cellular inhibition. J. Infect. Dis. 199:1151-1154. [DOI] [PubMed] [Google Scholar]
- 16.Garraud, O., S. Mahanty, and R. Perraut. 2003. Malaria-specific antibody subclasses in immune individuals: a key source of information for vaccine design. Trends Immunol. 24:30-35. [DOI] [PubMed] [Google Scholar]
- 17.Genton, B., I. Betuela, I. Felger, F. Al-Yaman, R. F. Anders, A. Saul, L. Rare, M. Baisor, K. Lorry, G. V. Brown, D. Pye, D. O. Irving, T. A. Smith, H. P. Beck, and M. P. Alpers. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 185:820-827. [DOI] [PubMed] [Google Scholar]
- 18.Genton, B., and Z. H. Reed. 2007. Asexual blood-stage malaria vaccine development: facing the challenges. Curr. Opin. Infect. Dis. 20:467-475. [DOI] [PubMed] [Google Scholar]
- 19.Hu, J., Z. Chen, J. Gu, M. Wan, Q. Shen, M. P. Kieny, J. He, Z. Li, Q. Zhang, Z. H. Reed, Y. Zhu, W. Li, Y. Cao, L. Qu, Z. Cao, Q. Wang, H. Liu, X. Pan, X. Huang, D. Zhang, X. Xue, and W. Pan. 2008. Safety and immunogenicity of a malaria vaccine, Plasmodium falciparum AMA-1/MSP-1 chimeric protein formulated in montanide ISA 720 in healthy adults. PLoS ONE 3:e1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafarshad, A., M. H. Dziegiel, R. Lundquist, L. K. Nielsen, S. Singh, and P. L. Druilhe. 2007. A novel antibody-dependent cellular cytotoxicity mechanism involved in defense against malaria requires costimulation of monocytes FcγRII and FcγRIII. J. Immunol. 178:3099-3106. [DOI] [PubMed] [Google Scholar]
- 21.Jensen, J. B. 2002. In vitro culture of Plasmodium parasites. Methods Mol. Med. 72:477-488. [DOI] [PubMed] [Google Scholar]
- 22.Lalitha, P. V., P. Malhotra, R. Chattopadhyay, and V. S. Chauhan. 1999. Plasmodium falciparum: variations in the C-terminal cysteine-rich region of the merozoite surface protein-1 in field samples among Indian isolates. Exp. Parasitol. 92:12-18. [DOI] [PubMed] [Google Scholar]
- 23.Malkin, E., J. Hu, Z. Li, Z. Chen, X. Bi, Z. Reed, F. Dubovsky, J. Liu, Q. Wang, X. Pan, T. Chen, B. Giersing, Y. Xu, X. Kang, J. Gu, Q. Shen, K. Tucker, E. Tierney, W. Pan, C. Long, and Z. Cao. 2008. A phase 1 trial of PfCP2.9: an AMA1/MSP1 chimeric recombinant protein vaccine for Plasmodium falciparum malaria. Vaccine 26:6864-6873. [DOI] [PubMed] [Google Scholar]
- 24.Miller, L. H., T. Roberts, M. Shahabuddin, and T. F. McCutchan. 1993. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol. Biochem. Parasitol. 59:1-14. [DOI] [PubMed] [Google Scholar]
- 25.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 26.Pan, W., D. Huang, Q. Zhang, L. Qu, D. Zhang, X. Zhang, X. Xue, and F. Qian. 2004. Fusion of two malaria vaccine candidate antigens enhances product yield, immunogenicity, and antibody-mediated inhibition of parasite growth in vitro. J. Immunol. 172:6167-6174. [DOI] [PubMed] [Google Scholar]
- 27.Polley, S. D., W. Chokejindachai, and D. J. Conway. 2003. Allele frequency-based analyses robustly map sequence sites under balancing selection in a malaria vaccine candidate antigen. Genetics 165:555-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polley, S. D., K. K. Tetteh, J. M. Lloyd, O. J. Akpogheneta, B. M. Greenwood, K. A. Bojang, and D. J. Conway. 2007. Plasmodium falciparum merozoite surface protein 3 is a target of allele-specific immunity and alleles are maintained by natural selection. J. Infect. Dis. 195:279-287. [DOI] [PubMed] [Google Scholar]
- 29.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83-87. [DOI] [PubMed] [Google Scholar]
- 30.Roussilhon, C., C. Oeuvray, C. Muller-Graf, A. Tall, C. Rogier, J. F. Trape, M. Theisen, A. Balde, J. L. Perignon, and P. Druilhe. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 4:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 32.Singh, S., S. Soe, J. P. Mejia, C. Roussilhon, M. Theisen, G. Corradin, and P. Druilhe. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190:1010-1018. [DOI] [PubMed] [Google Scholar]
- 33.Singh, S., S. Soe, C. Roussilhon, G. Corradin, and P. Druilhe. 2005. Plasmodium falciparum merozoite surface protein 6 displays multiple targets for naturally occurring antibodies that mediate monocyte-dependent parasite killing. Infect. Immun. 73:1235-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh, S., S. J. Barnwell, and P. Druilhe. 2009. A conserved multi-gene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS One 4:e5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soe, S., S. Singh, D. Camus, T. Horii, and P. Druilhe. 2002. Plasmodium falciparum serine repeat protein, a new target of monocyte-dependent antibody-mediated parasite killing. Infect. Immun. 70:7182-7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soe, S., M. Theisen, C. Roussilhon, K. S. Aye, and P. Druilhe. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stowers, A. W., V. Cioce, R. L. Shimp, M. Lawson, G. Hui, O. Muratova, D. C. Kaslow, R. Robinson, C. A. Long, and L. H. Miller. 2001. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect. Immun. 69:1536-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takala, S. L., D. Coulibaly, M. A. Thera, A. Dicko, D. L. Smith, A. B. Guindo, A. K. Kone, K. Traore, A. Ouattara, A. A. Djimde, P. S. Sehdev, K. E. Lyke, D. A. Diallo, O. K. Doumbo, and C. V. Plowe. 2007. Dynamics of polymorphism in a malaria vaccine antigen at a vaccine-testing site in Mali. PLoS Med. 4:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theisen, M., S. Soe, S. G. Jessing, L. M. Okkels, S. Danielsen, C. Oeuvray, P. Druilhe, and S. Jepsen. 2000. Identification of a major B-cell epitope of the Plasmodium falciparum glutamate-rich protein (GLURP), targeted by human antibodies mediating parasite killing. Vaccine 19:204-212. [DOI] [PubMed] [Google Scholar]
- 40.Trape, J. F., C. Rogier, L. Konate, N. Diagne, H. Bouganali, B. Canque, F. Legros, A. Badji, G. Ndiaye, P. Ndiaye, et al. 1994. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am. J. Trop. Med. Hyg. 51:123-137. [DOI] [PubMed] [Google Scholar]
- 41.Trucco, C., D. Fernandez-Reyes, S. Howell, W. H. Stafford, T. J. Scott-Finnigan, M. Grainger, S. A. Ogun, W. R. Taylor, and A. A. Holder. 2001. The merozoite surface protein 6 gene codes for a 36-kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 112:91-101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.