Abstract
Anaplasma phagocytophilum, the causative agent of tick-borne human granulocytic anaplasmosis (HGA), is an intracellular bacterium which survives and multiplies inside polymorphonuclear neutrophil granulocytes (PMN). Increased bacterial burden in gamma interferon (IFN-γ)-deficient mice suggested a major role of IFN-γ in the control of A. phagocytophilum. Here we investigated whether infection of human PMN with A. phagocytophilum impairs IFN-γ signaling thus facilitating intracellular survival of the bacterium. The secretion of the IFN-γ-inducible chemokines IP-10/CXCL10 and MIG/CXCL9 was markedly inhibited in infected neutrophils. Molecular analyses revealed that, compared to uninfected PMN, A. phagocytophilum decreased the expression of the IFN-γ receptor α-chain CD119, diminished the IFN-γ-induced phosphorylation of STAT1, and enhanced the expression of SOCS1 and SOCS3 in PMN. Since IFN-γ activates various antibacterial effector mechanisms of PMN, the impaired IFN-γ signaling in infected cells likely contributes to the survival of A. phagocytophilum inside PMN and to HGA disease development.
Anaplasma phagocytophilum, the causative agent of the tick-borne human granulocytic anaplasmosis (HGA), is an obligate intracellular gram-negative bacterium that infects polymorphonuclear neutrophil granulocytes (PMN) (17). Inside neutrophils the bacteria replicate within an endosomal vacuole where they form a microcolony termed morula. In order to evade elimination by neutrophils, the bacteria inhibit phagosome-lysosome fusion and the production of reactive oxygen species (6, 28), delay PMN apoptosis (21), and modulate the neutrophil chemokine response (11, 17).
Anaplasma infection in immunocompetent mice is usually mild and self-limiting. However, an increased bacterial burden was observed during the early course of infection in gamma interferon (IFN-γ)-deficient mice (2, 9) or IFN-γ receptor-deficient mice (2). Similarly, mice lacking the apoptotic specklike protein with a caspase-activating recruiting domain (ASC) or caspase-1, both of which were critical for the production of interleukin-18 (IL-18) and IFN-γ in response to A. phagocytophilum, showed a strongly increased bacterial load in the blood during the first week of infection (29). In addition, deficiency of interleukin-12/23p40 or depletion of natural killer (NK) cells were associated with reduced IFN-γ production and increased bacterial burden during early infection (2, 9, 30). Together, these in vivo data suggest a protective role of IFN-γ during the innate phase of A. phagocytophilum infection.
IFN-γ is an important mediator for the activation of antimicrobial effector mechanisms in macrophages infected with intracellular pathogens such as Mycobacterium, Toxoplasma, and Leishmania spp. (15, 31, 34). To partially evade host defense these pathogens have evolved mechanisms to interfere with various steps of the IFN-γ signaling cascade (33). Macrophages infected with Mycobacterium avium, Leishmania major, or L. mexicana show a reduced expression of the IFN-γ receptor and impaired phosphorylation of Janus kinase 1 (JAK1), JAK2, and signal transducer and activator of transcription 1 (STAT1) (8, 22). M. bovis BCG and L. donovani were reported to induce suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in macrophages (7, 23). All of these findings suggest that IFN-γ signaling is a vulnerable point in host macrophages that is targeted by intracellular pathogens.
IFN-γ is also a potent modulator of neutrophil functions. Recent studies have demonstrated that treatment of PMN with IFN-γ elicits a variety of responses, including differential gene expression, increased production of reactive oxygen species, and enhanced expression of surface markers (19). IFN-γ has also been demonstrated to augment the antimicrobial activity of PMN toward several bacterial species (19). Upon exposure to IFN-γ, neutrophils produce a variety of IFN-γ-inducible chemokines, including IFN-γ-inducible 10-kDa protein (IP-10/CXCL10), monokine induced by IFN-γ (MIG/CXCL9), and IFN-inducible T-cell α-chemoattractant (I-TAC/CXCL11) (32). These inflammatory chemokines greatly influence leukocyte trafficking and activation and are important mediators of protective immune mechanisms against intracellular pathogens (25).
Similar to what has been observed in mice (2, 29), the cytokine response in human A. phagocytophilum infection is characterized by a high serum level of IFN-γ (18). However, in spite of the strong IFN-γ response, A. phagocytophilum survives in neutrophils. Therefore, we hypothesized that A. phagocytophilum is able to compromise IFN-γ-mediated activation of neutrophils and thereby prolongs at least transiently its survival within PMN. To this end, we investigated the effect of A. phagocytophilum infection on IFN-γ signaling in vitro in human neutrophils. Our results show for the first time that an intracellular pathogen partially blocks the IFN-γ response in primary human PMN.
MATERIALS AND METHODS
Bacterial strain.
The A. phagocytophilum Webster strain (4) was cultured in HL60 cells grown in RPMI 1640 medium (Sigma, Deisenhofen, Germany) containing 2 mM l-glutamine (Biochrom, Berlin, Germany) and 1% fetal calf serum (Sigma).
Isolation of neutrophil granulocytes.
Granulocytes were isolated from heparinized blood collected from healthy adult volunteers as described previously (1). Blood was layered on a two-layer density gradient consisting of lymphocyte separation medium 1077 (upper layer; PAA, Pasching, Austria) and Histopaque 1119 (bottom layer; Sigma) and centrifuged for 20 min at 800 × g. The granulocyte-rich layer of Histopaque 1119 was collected and further fractionated on a discontinuous Percoll (Amersham Biosciences, Uppsala, Sweden) gradient consisting of layers with densities of 1.105 g/ml (85%), 1.100 g/ml (80%), 1.093 g/ml (75%), 1.087 g/ml (70%), and 1.081 g/ml (65%). After centrifugation for 25 min at 800 × g, the interface between the 65 and 70% Percoll layers was collected and washed twice in RPMI 1640 medium. The purity of granulocytes was >99.9% (27). The viability of cells was >99% as assessed by trypan blue dye exclusion. The studies with human neutrophils have been reviewed and approved by the ethical review committee of the Medical Faculty, University of Luebeck.
Infection of neutrophils with A. phagocytophilum.
Cell-free A. phagocytophilum was obtained from 2 × 107 infected HL-60 cells (infection rate > 70%). Cell-free A. phagocytophilum were resuspended in 1 ml of complete medium and added to 5 × 106 PMN, followed by an incubation for 5 h at 37°C. Subsequently, PMN were washed three times with medium, at 256 × g for 10 min each in order to remove noningested bacteria. Immunohistochemical staining with an A. phagocytophilum polyclonal rabbit antibody confirmed the infection in at least 90% of the neutrophils (data not shown).
Washed infected PMN were cultured at 37°C at a concentration of 5 × 106 cells/ml in complete medium. For all procedures described above, culture medium without antibiotics was used. To obtain supernatants of infected PMN, neutrophils were infected with cell-free A. phagocytophilum as described above and cultured for 6 h at 37°C. Supernatants were obtained after spinning the cultures at 14,000 × g for 10 min at 4°C twice.
Flow cytometry analysis.
The cell surface expression of CD119 was assessed by staining PMN with anti-CD119-phycoerythrin (PE) monoclonal antibody (MAb; BD Biosciences, San Diego, CA) for 20 min on ice and analyzed with a FACSCalibur flow cytometer using CellQuest software (BD Biosciences). PE-conjugated mouse immunoglobulin G2b (IgG2b) isotype control antibody was purchased from BD Biosciences.
Cytokine assays.
Uninfected and A. phagocytophilum-infected PMN (5 × 106/ml) were cultured for 18 h in medium alone or in the presence of 200 ng of lipopolysaccharide (LPS)/ml and 200 U of IFN-γ/ml. CXCL10/IP-10 and CXCL9/MIG were measured in the supernatants by using enzyme-linked immunosorbent assay (ELISA; R&D Systems, Wiesbaden, Germany).
Real-time reverse transcription-PCR (RT-PCR).
To isolate total cellular RNA, 107 PMN were lysed in 1 ml of RNApure (Peqlab, Erlangen, Germany). Further isolation procedure was carried out as recommended by the manufacturer. DNA was removed by using a DNA-Free kit (Ambion, Huntingdon, United Kingdom). Total RNA (500 ng) was reverse transcribed by using a Transcriptor first-strand cDNA synthesis kit (Roche Applied Science, Mannheim, Germany). Real-time PCR was carried out by using a LightCycler FastStart DNA Master SYBR green I kit (Roche Diagnostics, Mannheim, Germany) and a LightCycler detection system (Roche Diagnostics). The high-pressure liquid chromatography-grade primers for beta actin (NCBI accession no. NM_001101; forward, CCT GGC ACC CAG CAC AAT; reverse, GGG CCG GAC TCG TCA TAC), for SOCS1 (NCBI accession no. NM_003745; forward, 5′-TTT TTC GCC CTT AGC GTG AA; reverse, 5′-GCC ATC CAG GTG AAA GCG), for SOCS2 (NCBI accession no. NM_003877; forward, 5′-CAGGGAATGGCAGAGACACT; reverse, 5′-TGGCAGAGAGAGAAGGGATG) (24), and for SOCS3 (NCBI accession no. NM_003955; forward, GAA GAT CCC CCT GGT GTT GA; reverse, TTC CGA CAG AGA TGC TGA AGA) were purchased from TIB Molbiol, Berlin, Germany. The PCR mixture had a final volume of 20 μl in each capillary tube and contained 2 μl of DNA Master Mix, 12.6 μl of sterile water, 2.4 μl of MgCl2 (final concentration, 4 mM), 0.5 μl of each primer (final concentration, 0.5 μM), and 2 μl of cDNA. An initial denaturation step at 95°C for 10 min was followed by 45 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 5 s, and extension at 72°C for 10 s.
The mRNA expression of SOCS proteins and beta actin was analyzed by relative quantification. Using the 2−ΔΔCT method, the data are presented as relative expression of the mRNA expression normalized to the reference gene beta actin and relative to uninfected control.
Western blot analysis.
Infected and noninfected PMN were incubated for 15 min (for STAT1) or 6 h (for SOCS3) at 37°C in the absence or presence of 200 U of IFN-γ/ml. For the Western blot analysis of SOCS3 the cells were incubated in the presence of a 10 μM concentration of the proteasome inhibitor MG132 (Sigma). Neutrophils (3 × 106) were resuspended in ice-cold phosphate-buffered saline (PBS) containing 25 mM NaF and 0.5 mM Na3VO4. Cells were centrifuged twice at 500 × g for 5 min at 4°C. Cells were resuspended in lysis buffer (20 mM HEPES [pH 7.9], 420 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% [vol/vol] Nonidet P-40, 20% [vol/vol] glycerol, 1 mM dithiothreitol) containing inhibitors of proteases (5 μg of leupeptin/ml, 5 μg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride, 5 mg of α1-antitrypsin [Sigma]/ml), and phosphatases (1 mM Na3VO4, 50 mM NaF, 10 μM phenylarsine oxide). After a 15-min incubation on ice, cell debris was spun down at 12,000 × g for 20 min at 4°C. Supernatants were then boiled with 4× sample buffer for 10 min at 95°C.
Lysates from 106 PMN were electrophoresed on 7.5% (for STAT1) or 10% (for SOCS3) sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. After blocking, the membranes were incubated overnight at 4°C in the presence of the Tyr701-phospho-specific anti-STAT1 rabbit polyclonal antibody (New England Biolabs, Beverly, MA) or with anti-SOCS3 rabbit polyclonal antibody (ImmunoBiological Laboratories, Tokyo, Japan) and probed with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (Santa Cruz, Heidelberg, Germany). The signal was detected by using the chemiluminescence system (Immobilon Western Chemiluminescence HRP Substrate; Millipore, Billerica, MA). To assure equal sample loading, membranes were reprobed with rabbit polyclonal anti-beta actin antibody (New England Biolabs) and HRP-conjugated goat anti-rabbit IgG antibody.
Statistical analysis.
Data from at least three independent experiments are presented as means ± the standard errors of the mean (SEM). Statistical differences were determined with the Student t test. The results were considered statistically significant where P < 0.05.
RESULTS
A. phagocytophilum infection results in decreased secretion of IFN-γ-inducible chemokines IP-10 and MIG.
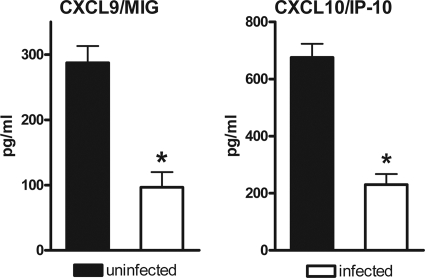
The IFN-γ-inducible chemokines CXCL10/IP-10 and CXCL9/MIG favor the recruitment of NK cells and Th1 cells to the site of infection, thus promoting the defense against intracellular pathogens (25). As a read-out for intact IFN-γ-signaling, we measured the release of CXCL10/IP-10 and CXCL9/MIG by uninfected PMN versus PMN infected with A. phagocytophilum. Since LPS potentiates the IFN-γ-induced release of CXCL10/IP-10 by PMN (37), neutrophils were treated with IFN-γ and LPS to obtain optimal IFN-γ-induced chemokine levels. Although uninfected PMN released high levels of CXCL10/IP-10 and CXCL9/MIG in response to IFN-γ plus LPS, the secretion of both chemokines was markedly diminished after infection with A. phagocytophilum (Fig. 1). The observed decrease in chemokine release was not due to an enhanced rate of cell death in the infected cultures. The viability of the cells was 82.0% ± 7.5% in the noninfected IFN-γ/LPS-treated cultures and 86.0% ± 5.6% of the cells in the Anaplasma-infected and IFN-γ/LPS-treated cultures after 18 h of incubation. These findings suggest that A. phagocytophilum interferes with the IFN-γ response of primary human neutrophils.
FIG. 1.
Infection with A. phagocytophilum results in reduced secretion of CXCL10/IP-10 and CXCL9/MIG by neutrophil granulocytes. Uninfected and A. phagocytophilum-infected neutrophil granulocytes (5 × 106/ml) were cultured for 18 h in medium alone or in the presence of 200 ng of LPS/ml and 200 U of IFN-γ/ml. The CXCL9/MIG and CXCL10/IP-10 content in the supernatants was measured by ELISA. The results are the means ± the SEM of three independent experiments. *, Significant (P < 0.05) difference between infected and uninfected PMN.
A. phagocytophilum infection impairs IFN-γ-signaling in neutrophils.
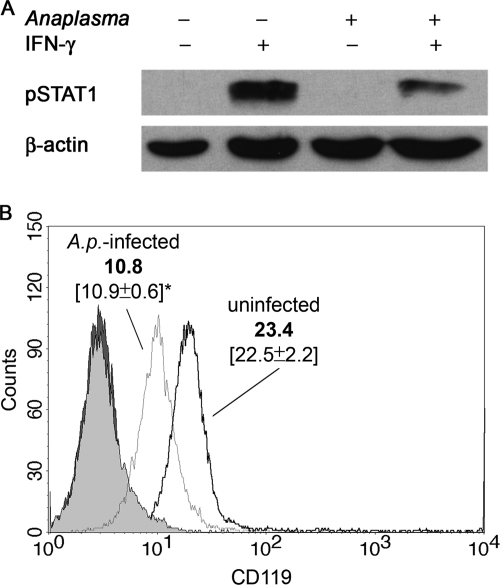
Having observed a decreased production of the IFN-γ-inducible chemokines CXCL10/IP-10 and CXCL9/MIG, we investigated the underlying mechanism. Since IFN-γ-induced effects in neutrophils are mediated through the JAK-STAT1 signaling pathway, Western blot analysis was carried out to assess IFN-γ-induced STAT1 phosphorylation in A. phagocytophilum-infected neutrophils. Compared to uninfected PMN, a marked decrease in STAT1 phosphorylation in infected neutrophils after exposure to IFN-γ was observed (Fig. 2A).
FIG. 2.
Infection with A. phagocytophilum leads to decreased STAT1 phosphorylation and CD119 expression in neutrophil granulocytes. PMN were infected with A. phagocytophilum as described in Materials and Methods. (A) Western blot analysis of phosphorylated STAT1. A. phagocytophilum-infected and noninfected PMN were incubated for 15 min at 37°C in the absence or presence of 200 U of IFN-γ/ml. pSTAT1 was detected using an antibody specific for pSTAT1. To assure equal sample loading, membranes were stripped and reprobed with anti-β-actin MAb. The results shown are from one of three experiments that yielded similar results. (B) Flow cytometry analysis of cell surface expression of CD119. Infected and noninfected PMN were cultured for 5 h and stained with PE-conjugated anti-CD119 MAb. Filled histograms indicate the population stained with isotype control antibody for infected (dark gray) and uninfected (light gray) PMN. The fluorescence intensity of the cells was analyzed by flow cytometry. The histograms are from one representative experiment. In brackets, mean fluorescence intensities ± the SEM of three experiments are given. *, Significant (P < 0.05) difference between CD119 expression of infected and uninfected PMN.
Next, we addressed the question whether this decline in STAT1 phosphorylation resulted from an altered signaling event upstream of STAT1. Based on the previous findings that infection with Mycobacterium or Leishmania spp. can reduce the expression of the IFN-γ receptor on macrophages (8, 22), the cell surface expression of the IFN-γ receptor alpha chain (CD119) was assessed on A. phagocytophilum-infected versus uninfected neutrophils. As shown in Fig. 2B, A. phagocytophilum infection resulted in a markedly decreased cell surface expression of CD119. Thus, A. phagocytophilum hampers the expression of the IFN-γ receptor in neutrophils.
Infection with A. phagocytophilum induces mRNA and protein expression of SOCS molecules in neutrophil granulocytes.
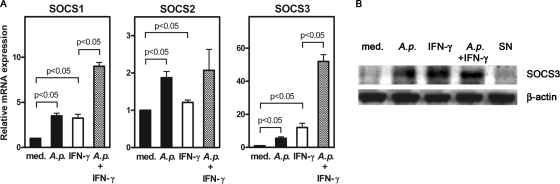
SOCS proteins are induced by IFN-γ as part of a negative-feedback mechanism to limit IFN-γ-mediated inflammatory responses. Pathogens are known to take advantage of this inhibitory mechanism. Accordingly, induction of SOCS1 and SOCS3 has been described for several microorganisms that reside inside macrophages (7, 36, 40). Using quantitative RT-PCR, we assessed the gene expression of SOCS1, SOCS2, and SOCS3 in A. phagocytophilum-infected PMN to investigate whether the upregulation of SOCS proteins is involved in the impairment of IFN-γ signaling by A. phagocytophilum. As expected, exposure of PMN to IFN-γ alone enhanced the mRNA expression of SOCS1 and SOCS3 (Fig. 3A). An upregulation of SOCS1 and SOCS3 mRNA expression was also seen after infection of PMN with A. phagocytophilum, which was most prominent in the presence of IFN-γ (Fig. 3A). Consistent with its minor role in IFN-γ signaling, SOCS2 gene expression was only weakly induced (Fig. 3A).
FIG. 3.
Infection with A. phagocytophilum leads to the upregulation of SOCS1, SOCS2, and SOCS3 gene expression and to the enhanced expression of SOCS3 protein in neutrophil granulocytes. (A) Real-time RT-PCR analysis of SOCS1, SOCS2, and SOCS3 gene expression. Freshly isolated human PMN were either left untreated (med) or infected with cell-free A. phagocytophilum (A.p.) at 37°C for 5 h. Subsequently, infected and noninfected PMN were incubated for 18 h in the presence or absence of 200 U of IFN-γ/ml. Total RNA was isolated, and the gene expression of SOCS1, SOCS2, and SOCS3 was assessed by real-time RT-PCR as described in Materials and Methods. (B) Western blot analysis of SOCS3 in neutrophil granulocytes. Noninfected (med) or infected (A.p.) PMN were incubated in the absence or presence of 200 U of IFN-γ/ml and noninfected PMN in the presence of supernatants of A. phagocytophilum-infected neutrophils (SN) for 6 h at 37°C. To assure equal sample loading, membranes were stripped and reprobed with anti-β-actin MAb. The results shown are from one of three experiments that all yielded similar results.
Western blot analysis was carried out to assess whether A. phagocytophilum induces the expression of SOCS proteins. Infection with A. phagocytophilum resulted in a strong upregulation of SOCS3 protein in PMN (Fig. 3B).
Since SOCS proteins are induced by several cytokines other than IFN-γ, we investigated whether the observed upregulation of SOCS3 protein is a direct effect of A. phagocytophilum or whether it is mediated by soluble factors secreted by infected cells. Therefore, freshly isolated PMN were exposed to bacterium-free supernatants derived from A. phagocytophilum-infected PMN cultures. Cultures of PMN with such supernatants did not enhance SOCS3 protein expression (Fig. 3B). This finding argues for a direct pathogen-induced upregulation of SOCS proteins in A. phagocytophilum-infected PMN.
DISCUSSION
A. phagocytophilum displays a unique tropism for PMN. We previously demonstrated that the susceptibility of mice to A. phagocytophilum is dependent on the presence of PMN (9). At the same time, PMN are thought to function as antibacterial effector cells, suggesting that A. phagocytophilum has developed mechanisms to facilitate its survival within PMN. In the present study we investigated whether A. phagocytophilum is able to counteract the response of PMN to IFN-γ, one of the key cytokines for the induction of both oxidative and nonoxidative antimicrobial effector mechanisms in mouse and human phagocytes. We showed that infection of human PMN with A. phagocytophilum caused a decrease in the cell surface expression of the IFN-γ receptor α-chain (CD119), a diminished STAT1 phosphorylation, an enhanced mRNA expression of SOCS1 and SOCS3, and increased protein levels of SOCS3. As a downstream consequence of the impaired IFN-γ signaling cascade, the IFN-γ-inducible chemokines IP-10/CXCL10 and MIG/CXCL9 were secreted at a markedly reduced level.
The cytokine response in human A. phagocytophilum infection is characterized by a high serum level of IFN-γ (18). In spite of this strong IFN-γ response, A. phagocytophilum survives inside neutrophils. Compromised IFN-γ signaling is likely to favor survival of A. phagocytophilum in neutrophils, which in turn contributes to HGE disease development. Thus far, such an evasion strategy has been described only in the context of macrophage infection with intracellular pathogens (8, 22). Our results clearly extend these findings to primary neutrophils infected with an intracellular pathogen. Moreover, our results are the first to demonstrate that a bacterium belonging to the order of Rickettsiales can induce SOCS proteins.
The mechanism for how A. phagocytophilum downregulates IFN-γ signaling is not yet clear. However, our results indicate that the observed effect is not mediated by soluble mediators. Previous studies indicated that downregulation of IFN-γ-induced macrophage response by mycobacteria was mediated by the mycobacterial 19-kDa lipoprotein involving host cell TLR2 and MyD88 (20). M. avium was demonstrated to decrease expression of the IFN-γ receptor in RAW264.7 macrophages also in a TLR2-dependent manner (14). In this latter study, in addition to M. avium, downregulation of IFN-γ receptor expression could be achieved by exposure of RAW264.7 cells to the TLR2 agonist Pam3CSK4 (14). Furthermore, the enhanced protein levels of the dominant-negative STAT1β in M. avium-infected murine macrophages were shown to be TLR2 dependent (3). TLR2 was reported to be involved in the enhanced SOCS1 and SOCS3 expression in murine macrophages exposed to M. avium or to mycobacterial lipoarabinomannan (39). All of these observations suggest that impairment of macrophage IFN-γ signaling in response to mycobacterial infection is mediated by TLR2. Recent data show that A. phagocytophilum can also affect host cell functions in a TLR2-dependent manner. Activation of NF-κB in primary murine macrophages by A. phagocytophilum was shown to be mediated by TLR2 but not by TLR4 (12). This finding suggests a possible role of TLR2 in the A. phagocytophilum-mediated impairment of IFN-γ signaling.
In macrophages, infection with a number of intracellular pathogens was reported to induce SOCS1 and/or SOCS3 gene expression (7, 36, 40). Since SOCS proteins were shown to partially reduce STAT1 phosphorylation and to interfere with IFN-γ signaling (35), these studies suggested a role for SOCS proteins in pathogen-mediated interference with IFN-γ signaling in macrophages. We demonstrate here enhanced expression of SOCS proteins and reduced IFN-γ signaling in A. phagocytophilum-infected human PMN. The induction of SOCS proteins, therefore, appears to be a potent inhibitory mechanism by which intracellular microorganisms suppress cellular activation in PMN. Since the exposure of neutrophils to bacterium-free supernatants of infected PMN did not lead to enhanced SOCS expression, the upregulation of SOCS appears to be a direct effect of bacteria and not mediated by soluble factors. In previous studies with macrophages, transwell experiments demonstrated that TLR ligands directly induce SOCS proteins (5). Furthermore, SOCS1 and SOCS3 could be induced in DC from IFNAR−/− mice by TLR stimuli, indicating that SOCS expression does not require type I IFN. Moreover, the expression of SOCS1 and SOCS3 in response to Borrelia burgdorferi did not require de novo synthesis of soluble mediators (16). Taken together, the upregulation of SOCS proteins is presumably mediated by direct contact with pathogens both in neutrophils and in macrophages.
In contrast to the strong upregulation of SOCS1 and SOCS3 gene expression, only a marginal upregulation of SOCS2 was observed in A. phagocytophilum-infected neutrophils. This is in line with the earlier finding that TLR ligands induce SOCS1 and SOCS3 but not SOCS2 in macrophages (5). Moreover, the progression of Leishmania infection in SOCS2−/− mice did not differ from that in resistant wild-type C57BL/6 mice, suggesting that SOCS2 is not a major target for evasion mechanisms of intracellular pathogens (10).
A previous study showed that the expression of gp91 phox in response to IFN-γ is inhibited in A. phagocytophilum-infected HL-60 cells (38). However, the impaired gp91 phox gene expression was not associated with reduced levels of phosphorylated STAT1 but was rather attributed to an impairment of signaling events downstream of STAT1 (38). In the present study we clearly show diminished STAT1 phosphorylation and demonstrate that at least two steps upstream of STAT1 phosphorylation, i.e., the expression of CD119 and SOCS proteins, are modulated by A. phagocytophilum. Although HL-60 cells are often used as a surrogate model for primary human PMN, the apparently conflicting results regarding STAT1 phosphorylation illustrate the importance of using primary cells in studies on host cell-pathogen interactions.
Since NK cell-derived IFN-γ is crucial for the control of intracellular pathogens such as Leishmania and Toxoplasma within the first days of infection (26), NK cells are a likely a source of IFN-γ that controls early A. phagocytophilum infection. Two independent studies recently demonstrated that NK1.1+ cells are involved in the early IFN-γ-mediated control of A. phagocytophilum infection in a murine model (9, 13). Since CXCL10/IP-10 and CXCL9/MIG target among other cells of the cellular immune response also NK cells, decreased release of CXCL10/IP-10 and CXCL9/MIG by infected PMN can disturb the reciprocal interaction between IFN-γ-secreting cells and neutrophils releasing CXCL10/IP-10 and CXCL9/MIG. Decreased release of these chemokines by infected PMN may result in a reduced recruitment of NK cells and Th1 cells leading in turn to diminished antibacterial response.
In summary, our data demonstrate that A. phagocytophilum impairs IFN-γ signaling in neutrophils, a mechanism that has, thus far, neither been shown for neutrophils nor in the context of an infection with members of the order of Rickettsiales.
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft (La 1267/1-3, GRK 288/B1, and Cluster of Excellence “Inflammation at Interfaces”).
We thank F. Bazzoni and M. Rossato for their assistance with the Western blot lysis protocol.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Aga, E., D. M. Katschinski, G. van Zandbergen, H. Laufs, B. Hansen, K. Muller, W. Solbach, and T. Laskay. 2002. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169:898-905. [DOI] [PubMed] [Google Scholar]
- 2.Akkoyunlu, M., and E. Fikrig. 2000. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect. Immun. 68:1827-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez, G. R., B. S. Zwilling, and W. P. Lafuse. 2003. Mycobacterium avium inhibition of IFN-γ signaling in mouse macrophages: Toll-like receptor 2 stimulation increases expression of dominant-negative STAT1β by mRNA stabilization. J. Immunol. 171:6766-6773. [DOI] [PubMed] [Google Scholar]
- 4.Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. [DOI] [PubMed] [Google Scholar]
- 5.Baetz, A., M. Frey, K. Heeg, and A. H. Dalpke. 2004. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate Toll-like receptor signaling in innate immune cells. J. Biol. Chem. 279:54708-54715. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by downregulating gp91phox. J. Immunol. 164:3946-3949. [DOI] [PubMed] [Google Scholar]
- 7.Bertholet, S., H. L. Dickensheets, F. Sheikh, A. A. Gam, R. P. Donnelly, and R. T. I. Kenney. 2003. Leishmania donovani-induced expression of suppressor of cytokine signaling 3 in human macrophages: a novel mechanism for intracellular parasite suppression of activation. Infect. Immun. 71:2095-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhardwaj, N., L. E. Rosas, W. P. Lafuse, and A. R. Satoskar. 2005. Leishmania inhibits STAT1-mediated IFN-γ signaling in macrophages: increased tyrosine phosphorylation of dominant-negative STAT1β by Leishmania mexicana. Int. J. Parasitol. 35:75-82. [DOI] [PubMed] [Google Scholar]
- 9.Birkner, K., B. Steiner, C. Rinkler, Y. Kern, P. Aichele, C. Bogdan, and F. D. von Loewenich. 2008. The elimination of Anaplasma phagocytophilum requires CD4+ T cells, but is independent of Th1 cytokines and a wide spectrum of effector mechanisms. Eur. J. Immunol. 38:3395-3410. [DOI] [PubMed] [Google Scholar]
- 10.Bullen, D. V., T. M. Baldwin, J. M. Curtis, W. S. Alexander, and E. Handman. 2003. Persistence of lesions in suppressor of cytokine signaling-1-deficient mice infected with Leishmania major. J. Immunol. 170:4267-4272. [DOI] [PubMed] [Google Scholar]
- 11.Carlyon, J. A., and E. Fikrig. 2006. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr. Opin. Hematol. 13:28-33. [DOI] [PubMed] [Google Scholar]
- 12.Choi, K. S., D. G. Scorpio, and J. S. I. Dumler. 2004. Anaplasma phagocytophilum ligation to Toll-like receptor (TLR) 2, but not to TLR4, activates macrophages for nuclear factor-κB nuclear translocation. J. Infect. Dis. 189:1921-1925. [DOI] [PubMed] [Google Scholar]
- 13.Choi, K. S., T. Webb, M. Oelke, D. G. Scorpio, and J. S. Dumler. 2007. Differential innate immune cell activation and proinflammatory response in Anaplasma phagocytophilum infection. Infect. Immun. 75:3124-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curry, H., G. R. Alvarez, B. S. Zwilling, and W. P. Lafuse. 2004. Toll-like receptor 2 stimulation decreases IFN-gamma receptor expression in mouse RAW264.7 macrophages. J. Interferon Cytokine Res. 24:699-710. [DOI] [PubMed] [Google Scholar]
- 15.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dennis, V. A., A. Jefferson, S. R. Singh, F. Ganapamo, and M. T. Philipp. 2006. Interleukin-10 anti-inflammatory response to Borrelia burgdorferi, the agent of Lyme disease: a possible role for suppressors of cytokine signaling 1 and 3. Infect. Immun. 74:5780-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumler, J. S., K. S. Choi, J. C. Garcia-Garcia, N. S. Barat, D. G. Scorpio, J. W. Garyu, D. J. Grab, and J. S. Bakken. 2005. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 11:1828-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumler, J. S., E. R. Trigiani, J. S. Bakken, M. E. Aguero-Rosenfeld, and G. P. Wormser. 2000. Serum cytokine responses during acute human granulocytic ehrlichiosis. Clin. Diagn. Lab. Immunol. 7:6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis, T. N., and B. L. Beaman. 2004. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology 112:2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortune, S. M., A. Solache, A. Jaeger, P. J. Hill, J. T. Belisle, B. R. Bloom, E. J. Rubin, and J. D. Ernst. 2004. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J. Immunol. 172:6272-6280. [DOI] [PubMed] [Google Scholar]
- 21.Ge, Y., and Y. Rikihisa. 2006. Anaplasma phagocytophilum delays spontaneous human neutrophil apoptosis by modulation of multiple apoptotic pathways. Cell. Microbiol. 8:1406-1416. [DOI] [PubMed] [Google Scholar]
- 22.Hussain, S., B. S. Zwilling, and W. P. Lafuse. 1999. Mycobacterium avium infection of mouse macrophages inhibits IFN-gamma Janus kinase-STAT signaling and gene induction by downregulation of the IFN-gamma receptor. J. Immunol. 163:2041-2048. [PubMed] [Google Scholar]
- 23.Imai, K., T. Kurita-Ochiai, and K. Ochiai. 2003. Mycobacterium bovis bacillus Calmette-Guerin infection promotes SOCS induction and inhibits IFN-γ-stimulated JAK/STAT signaling in J774 macrophages. FEMS Immunol. Med. Microbiol. 39:173-180. [DOI] [PubMed] [Google Scholar]
- 24.Isomaki, P., T. Alanara, P. Isohanni, A. Lagerstedt, M. Korpela, T. Moilanen, T. Visakorpi, and O. Silvennoinen. 2007. The expression of SOCS is altered in rheumatoid arthritis. Rheumatology 46:1538-1546. [DOI] [PubMed] [Google Scholar]
- 25.Khan, I. A., J. A. MacLean, F. S. Lee, L. Casciotti, E. DeHaan, J. D. Schwartzman, and A. D. Luster. 2000. IP-10 is critical for effector T-cell trafficking and host survival in Toxoplasma gondii infection. Immunity 12:483-494. [DOI] [PubMed] [Google Scholar]
- 26.Laskay, T., M. Rollinghoff, and W. Solbach. 1993. Natural killer cells participate in the early defense against Leishmania major infection in mice. Eur. J. Immunol. 23:2237-2241. [DOI] [PubMed] [Google Scholar]
- 27.Lotz, S., E. Aga, I. Wilde, G. van Zandbergen, T. Hartung, W. Solbach, and T. Laskay. 2004. Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. J. Leukoc. Biol. 75:467-477. [DOI] [PubMed] [Google Scholar]
- 28.Mott, J., and Y. Rikihisa. 2000. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect. Immun. 68:6697-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedra, J. H., F. S. Sutterwala, B. Sukumaran, Y. Ogura, F. Qian, R. R. Montgomery, R. A. Flavell, and E. Fikrig. 2007. ASC/PYCARD and caspase-1 regulate the IL-18/IFN-γ axis during Anaplasma phagocytophilum infection. J. Immunol. 179:4783-4791. [DOI] [PubMed] [Google Scholar]
- 30.Pedra, J. H., J. Tao, F. S. Sutterwala, B. Sukumaran, N. Berliner, L. K. Bockenstedt, R. A. Flavell, Z. Yin, and E. Fikrig. 2007. IL-12/23p40-dependent clearance of Anaplasma phagocytophilum in the murine model of human anaplasmosis. FEMS Immunol. Med. Microbiol. 50:401-410. [DOI] [PubMed] [Google Scholar]
- 31.Salgame, P. 2005. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr. Opin. Immunol. 17:374-380. [DOI] [PubMed] [Google Scholar]
- 32.Scapini, P., J. A. Lapinet-Vera, S. Gasperini, F. Calzetti, F. Bazzoni, and M. A. Cassatella. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177:195-203. [DOI] [PubMed] [Google Scholar]
- 33.Singhal, A., A. Jaiswal, V. K. Arora, and H. K. Prasad. 2007. Modulation of gamma interferon receptor 1 by Mycobacterium tuberculosis: a potential immune response evasive mechanism. Infect. Immun. 75:2500-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solbach, W., and T. Laskay. 2000. The host response to Leishmania infection. Adv. Immunol. 74:275-317. [DOI] [PubMed] [Google Scholar]
- 35.Stoiber, D., P. Kovarik, S. Cohney, J. A. Johnston, P. Steinlein, and T. Decker. 1999. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-γ. J. Immunol. 163:2640-2647. [PubMed] [Google Scholar]
- 36.Stoiber, D., S. Stockinger, P. Steinlein, J. Kovarik, and T. Decker. 2001. Listeria monocytogenes modulates macrophage cytokine responses through STAT serine phosphorylation and the induction of suppressor of cytokine signaling 3. J. Immunol. 166:466-472. [DOI] [PubMed] [Google Scholar]
- 37.Tamassia, N., F. Calzetti, T. Ear, A. Cloutier, S. Gasperini, F. Bazzoni, P. P. McDonald, and M. A. Cassatella. 2007. Molecular mechanisms underlying the synergistic induction of CXCL10 by LPS and IFN-γ in human neutrophils. Eur. J. Immunol. 37:2627-2634. [DOI] [PubMed] [Google Scholar]
- 38.Thomas, V., S. Samanta, C. Wu, N. Berliner, and E. Fikrig. 2005. Anaplasma phagocytophilum modulates gp91phox gene expression through altered interferon regulatory factor 1 and PU.1 levels and binding of CCAAT displacement protein. Infect. Immun. 73:208-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazquez, N., T. Greenwell-Wild, S. Rekka, J. M. Orenstein, and S. M. Wahl. 2006. Mycobacterium avium-induced SOCS contributes to resistance to IFN-γ-mediated mycobactericidal activity in human macrophages. J. Leukoc. Biol. 80:1136-1144. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann, S., P. J. Murray, K. Heeg, and A. H. Dalpke. 2006. Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-gamma signaling. J. Immunol. 176:1840-1847. [DOI] [PubMed] [Google Scholar]