Abstract
Francisella tularensis is a Gram-negative bacterium that causes acute, lethal disease following inhalation. We have previously shown that viable F. tularensis fails to stimulate secretion of proinflammatory cytokines following infection of human dendritic cells (hDC) in vitro and pulmonary cells in vivo. Here we demonstrate that the presence of the CD14 receptor is critical for detection of virulent F. tularensis strain SchuS4 by dendritic cells, monocytes, and pulmonary cells. Addition of soluble CD14 (sCD14) to hDC restored cytokine production following infection with strain SchuS4. In contrast, addition of anti-CD14 to monocyte cultures inhibited the ability of these cells to respond to strain SchuS4. Addition of CD14 or blocking CD14 following SchuS4 infection in dendritic cells and monocytes, respectively, was not due to alterations in phagocytosis or replication of the bacterium in these cells. Administration of sCD14 in vivo also restored cytokine production following infection with strain SchuS4, as assessed by increased concentrations of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-12p70, and IL-6 in the lungs of mice receiving sCD14 compared to mock-treated controls. In contrast to homogenous cultures of monocytes or dendritic cells infected in vitro, mice treated with sCD14 in vivo also exhibited controlled bacterial replication and dissemination compared to mock-treated controls. Interestingly, animals that lacked CD14 were not more susceptible or resistant to pulmonary infection with SchuS4. Together, these data support the hypothesis that the absence or low abundance of CD14 on hDC and in the lung contributes to evasion of innate immunity by virulent F. tularensis. However, CD14 is not required for development of inflammation during the last 24 to 48 h of SchuS4 infection. Thus, the presence of this receptor may aid in control of virulent F. tularensis infections at early, but not late, stages of infection.
Daily insults of inhaled particulate and foreign antigens into the lungs could result in devastating inflammation. However, the lung counters these attacks by tightly regulating inflammatory responses. This regulation occurs both in the form of inhibitory molecules, such as surfactants that dampen macrophage and dendritic cell (DC) responsiveness, as well as production of immunosuppressive cytokines, such as transforming growth factor β (TGF-β) (1, 10, 15, 33, 34). Given the immunosuppressive nature of the lung environment, it is not surprising that pathogens capable of causing lethal disease following inhalation, such as Francisella tularensis, take advantage of this property for rapid replication while evading detection by the host immune response.
Francisella tularensis is a Gram-negative, facultative intracellular bacterium and is the causative agent of tularemia. Pneumonic tularemia is an acute, lethal disease mediated by F. tularensis, following inhalation of as few as 10 to 15 bacteria in mice and humans (20, 49). Surprisingly, despite the rapidity by which pulmonary F. tularensis infections progress, there is little to no evidence of inflammation in the lung until the very end stage of infection (11, 20, 54). The mechanisms by which F. tularensis replicates within the lung while evading detection by the host are not well understood and represent an important hurdle for the development of novel therapeutics and vaccines directed against tularemia.
Recently we have demonstrated that, similar to pulmonary cells, conventional human dendritic cells (hDC) derived from peripheral blood fail to secrete proinflammatory cytokines following infection with virulent F. tularensis strain SchuS4 (17). The absence of production of proinflammatory cytokines was not due to the inability of the cells to become infected or support replication of strain SchuS4, nor was it due to induction of apoptosis among infected hDC. There are multiple explanations for the lack of cytokine production in hDC and pulmonary cells following SchuS4 infection. One possibility is that these cells fail to detect F. tularensis during the initial phases of infection.
CD14 is a glycosylphosphatidylinositol receptor that exists in both a membrane-bound form and a soluble form in vivo. CD14 is present on monocytes, most macrophages, fibroblasts, and neutrophils (39). Conventional human DC lose surface expression of CD14 following their differentiation from blood monocytes, primarily due to exposure to interleukin-4 (IL-4) (40). Similar to hDC, alveolar macrophages have been reported to have little to no CD14 present on their surface (9, 45). Soluble CD14 (sCD14) is abundant in serum. However, it is present at very low levels to nearly undetectable concentrations in the airways of mammals (47). CD14 (both in its soluble and membrane-bound forms) is best known as a coreceptor for lipopolysaccharide (LPS), facilitating optimal delivery of LPS to the Toll-like receptor 4 (TLR4)/MD-2 complex on the cell surface (35, 64). In addition to delivery of LPS to TLR4, CD14 has been described as an important coreceptor for delivery of other microbial antigens, including polyuronic acids from Pseudomonas, lipoteichoic acid (LTA) from Staphylococcus aureus, outer surface protein of Borrelia burgdorferi and WI-1 antigen of Blastomyces dermatitidis to TLR2 (reviewed in reference 58). Thus, CD14 is an important coreceptor for initiating inflammatory responses via TLR2 and TLR4 against a wide variety of bacterial and fungal diseases.
In this report, we demonstrate that CD14 serves as a critical coreceptor for detection of F. tularensis SchuS4 during the initial stage of in vitro infection in hDC and primary human monocytes. Further, CD14 was also found to have an important role in the early in vivo detection and control of pulmonary infections with strain SchuS4. However, development of inflammatory responses associated with bacterial sepsis observed at late stages of SchuS4 infection were not dependent on the presence of CD14. Thus, in contrast to infections with other microbial pathogens where the presence of CD14 in the lungs is detrimental to control of infection, CD14 represents an important sensor to initiate protective host immune responses during pulmonary infections with strain SchuS4, but it is not required to elicit responses at the end of the disease process.
MATERIALS AND METHODS
Bacteria.
Francisella tularensis strain SchuS4 was kindly provided by Jeannine Peterson (Centers for Disease Control and Prevention, Fort Collins, CO). F. tularensis SchuS4 was cultured in modified Mueller-Hinton broth (Mueller-Hinton broth supplemented with CaCl2, MgCl2, 0.1% glucose, 0.025% ferric pyrophosphate, and 2% IsoVitaleX [BD Biosciences]) (MMH) at 37°C with constant shaking overnight, aliquoted into 1-ml samples, frozen at −80°C, and thawed just prior to use as previously described (11). The titers of frozen stocks were determined by enumerating viable bacteria from serial dilutions plated on modified Mueller-Hinton agar as previously described (13, 24). The number of viable bacteria in frozen stock vials varied less than 5% over a 10-month period. DsRed-expressing Brucella abortus strain 2038 was kindly supplied by Jean Celli (Rocky Mountain Laboratories, Hamilton, MT). B. abortus was cultured on tryptic soy agar (TSA) plates for 72 h at 37°C. Individual colonies were then transferred to tryptic soy broth (TSB), and bacteria were cultured overnight at 37°C with constant shaking. The number of bacteria present in broth cultures was determined by the optical density at 600 nm. The actual numbers of viable bacteria were confirmed by plating an inoculum on TSA plates as previously described (50).
Mice.
Specific-pathogen-free, 6- to 8-week-old, male C57BL/6J (wild-type) mice and CD14-deficient (CD14−/−) mice (four or five mice per group) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in sterile microisolater cages in the biosafety level 3 (BSL-3) facility at the NIAID/Rocky Mountain Laboratories (RML). All mice were provided sterile water and food ad libitum, and all research involving animals was conducted in accordance with Animal Care and Use guidelines and animal protocols were approved by the Animal Care and Use Committee at RML.
Isolation of human monocytes and differentiation of dendritic cells.
Human monocytes were obtained from peripheral blood samples provided by the Department for Transfusion Medicine and the NIH Clinical Center at the National Institutes of Health (Bethesda, MD) and enriched by apheresis. Apheresed, CD14+ monocytes were further enriched using Ficoll-Paque (GE Healthcare) and negative selection using Dynabeads MyPure Monocytes kit for untouched human cells per the manufacturer's instructions (Invitrogen). Monocytes were used immediately following negative selection or were differentiated into conventional, myeloid dendritic cells as previously described (14, 48). For differentiation of monocytes into hDC, CD14+ monocytes were resuspended at 5 × 105/ml in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 0.2 mM l-glutamine, 1 mM HEPES buffer, and 0.1 mM nonessential amino acids (all from Invitrogen) (complete RPMI medium [cRPMI]) and 100 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) and 20 ng/ml IL-4 (both from Peprotech) plated at 2 ml/well in six-well plates and incubated at 37°C and 5% CO2. On day 2 of culture, 100% of each cytokine per well in 1 ml of cRPMI was added, and cells were used on day 4 of culture. The resulting differentiated DC were >97% CD1a+/DC-SIGN+ and <1% CD14+. Human blood cells were collected from anonymous volunteers under a protocol reviewed and approved by the NIH Institutional Biosafety Committee.
Infection of cells.
Monocytes and hDC were infected at a multiplicity of infection (MOI) of 50 with F. tularensis strain SchuS4 as previously described (17). In additional experiments, monocytes and hDC were infected with a MOI of 50 of B. abortus. Briefly, monocytes were infected immediately following their enrichment described above. hDC were removed from their original cultures and pelleted. The resulting hDC conditioned medium was reserved for replating of cells. Monocytes and hDC were adjusted to 2 × 107/ml to 3 × 107/ml in either fresh cRPMI (monocytes) or reserved hDC medium (hDC). As indicated, DC and monocytes were incubated with soluble CD14 (sCD14) (R&D Systems) (4 μg/3 × 106 cells) or anti-CD14 antibody (R&D Systems) (5 μg/3 × 106 cells), respectively, at room temperature 30 min prior to infection. Cells treated with medium alone or with mouse IgG1 (isotype control) (R&D Systems) served as negative controls. F. tularensis SchuS4 or DsRed-expressing B. abortus strain 2308 was added, the cells were incubated at 37°C in 7% CO2 for 1 h, then 50 μg/ml (for SchuS4) or 100 μg/ml (for B. abortus) gentamicin (Invitrogen) was added, and the cells were incubated for an additional 45 min to kill extracellular bacteria. Then the cells were washed extensively and adjusted to 5 × 105 cells/ml in fresh cRPMI (monocytes) or reserved DC medium (hDC). Cells were then plated at 1 ml/well in 24-well tissue culture plates. After plating, sCD14 was added to DC at 2 μg/5 × 105 cells. Alternatively, anti-CD14 or mouse IgG1 was added to monocyte cultures at 10 μg/5 × 105 cells after plating. sCD14, anti-CD14, and isotype control antibodies remained in the culture medium throughout the course of the experiment. At the indicated time points, intracellular bacteria were enumerated as previously described (17, 52). Additionally, intracellular infection was monitored by microscopy as described below.
Fluorescence microscopy.
Monocytes and hDC were fixed and stained for F. tularensis and tubulin as previously described with the following modifications (18). Briefly, monocytes and hDC were infected with F. tularensis SchuS4 as described above and then allowed to adhere to a glass slide using a Shandon Cytospin 4. The cells were fixed in ice-cold 90% methanol for 2 min at −20°C. Tubulin and bacteria were visualized following incubation with rabbit anti-tubulin (Cell Signal Technologies) followed by either Alexa Fluor 488-conjugated anti-rabbit antibodies (Invitrogen) or Alexa Fluor 546-conjugated anti-rabbit antibodies (Invitrogen) and Alexa Fluor 488-conjugated goat anti-F. tularensis (U.S. Biological, Swampscot, MA), respectively. Samples were visualized using a Carl Zeiss LSM 510 confocal scanning laser microscope (Thornwood, NY) for quantitative analysis and image acquisition. Approximately 75 to 100 cells/field and a minimum of three fields per slide were analyzed for the presence of intracellular bacteria. The percentage of infected cells was calculated as follows: (number of infected cells/total number of cells) × 100. Confocal images of 1,024 × 1,024 pixels were acquired and assembled using Adobe Photoshop CS2 software (Adobe Systems, San Jose, CA).
Collection of airway and lung cells.
Airway cells were obtained by bronchoalveolar lavage (BAL) as previously described (11). Briefly, mice were euthanized by administration of overdoses of inhaled isofluorane, and an 18-gauge (18G) catheter was immediately inserted into the trachea. Approximately 1.5 ml of ice-cold phosphate-buffered saline (PBS) was injected and then aspirated from the lungs. This was repeated three times. Bronchoalveolar lavage cells were centrifuged at 1,200 rpm for 5 min at 4°C. BAL cells were then resuspended in fluorescence-activated cell sorting (FACS) buffer (PBS with 2% fetal bovine serum and 0.05% sodium azide) prior to staining. Lung cells were isolated as previously described with minor modifications (11). Briefly, the lungs were excised, minced, and incubated in PBS supplemented with 0.35 mg/ml Blendzyme 3 (Roche, Nutley, NJ) for 45 min at 37°C and 5% CO2. Tissues were triturated using a 5-ml syringe and 18G needle. The cells were then pelleted by centrifugation at 1,200 rpm for 5 min, and red blood cells were lysed with NHCl4 and passed through a 70-mm nylon screen. The cells were then washed twice in PBS and resuspended in FACS buffer prior to analysis. Total live cells from the airways and lungs were enumerated using trypan blue and a hemacytometer.
Flow cytometry and analysis of human cells and mouse lung and airway cells.
Macrophage and dendritic cell populations obtained from human blood samples and mouse lungs and airways were assessed for CD14 by flow cytometry. Directly conjugated antibodies for these analyses were purchased from BD Biosciences (San Jose, CA). The following antibodies in various combinations were used for flow cytometric analysis: allophycocyanin (APC)-conjugated anti-mouse CD11c (clone N418), PeCy7-conjugated anti-mouse CD11b (clone M1/70), fluorescein isothiocyanate (FITC)-conjugated anti-mouse I-A/I-E (major histocompatibility complex class II [MHC-I]; clone M5/114.15.2), phycoerythrin (PE)-conjugated anti-mouse CD14 (clone rmC5-3), PE-conjugated anti-human CD14 (clone M5E2), and APC-conjugated anti-human CD1a (clone HI149). Staining with directly conjugated antibodies was done in FACS buffer at 4°C. Then, the cells were washed and fixed in 1% paraformaldehyde for 30 min at 4°C. The cells were washed a final time, resuspended in FACS buffer, and stored at 4°C until analyzed. Flow cytometry was done using a Partec ML flow cytometer (Partec, Swedesboro, NJ). Analysis gates were set on viable unstained cells and were designed to include all viable cell populations. Of note, the side scatter (SSC) and forward scatter (FSC) settings were reduced from those typically used for analysis of lymphocytes in order to include macrophages and dendritic cells, which have high forward and side scatter properties. Approximately 25,000 (BAL fluid samples, human monocytes, and dendritic cells) and 100,000 (lung) events were analyzed for each sample. Isotype control antibodies were included when the analyses and panels were first being performed to ensure specificity of staining but were not routinely included with each experiment. Data were analyzed using FlowJo software (Treestar, Ashland, OR).
Infection of mice.
Mice were infected intranasally (i.n.) with approximately 50 CFU/25 μl F. tularensis SchuS4. Briefly, bacteria were thawed and diluted in PBS. As indicated, sCD14 (Cell Sciences, Canton, MA) was added to diluted bacterial suspensions so that the final concentration was 1 μg sCD14/25 μl. Mice were anesthetized by injection of 100 μl of 12.5 mg/ml ketamine plus 3.8 mg/ml xylazine intraperitoneally. Approximately 50 CFU with or without 1 μg sCD14 was administered into the nares of each mouse in a total volume of 25 μl. The actual inoculum concentration was confirmed by plating a portion of the inoculum onto MMH agar plates, incubating plates at 37°C with 5% CO2, and enumerating colonies. This dose routinely resulted in a mean time to death of 5 days in naïve C57BL/6J mice. As indicated, mice were also treated with either 1 μg sCD14/25 μl or 25 μl PBS (control) i.n. 1 day after infection.
Collection of tissue homogenate and enumeration of bacteria.
Bacteria were enumerated from the lungs and spleens as previously described with minor modifications (11). Briefly, the organs were aseptically collected and placed in ice-cold homogenization buffer (150 mM Tris-HCl, 5 mM EDTA, 10 mM Trizma base) supplemented with a 1:100 dilution of phosphatase inhibitor cocktail I, phosphatase inhibitor cocktail II, and proteinase inhibitor cocktail III (all from AG Scientific, San Diego, CA). The organs were immediately homogenized by grinding tissues through a sterile S/S type 304 no. 60 mesh wire mesh screen (Billeville Wire Cloth Co., Cedar Grove, NJ) using a 3-ml syringe plunger. A portion of the resulting homogenate was immediately serially diluted in PBS and plated on MMH agar for enumeration of bacterial loads. The remaining homogenate was centrifuged at 14,000 × g for 30 min at 4°C. The resulting supernatants were sterile filtered through 0.2-μm syringe filters (Schleicher and Schuell, Keene, NH) and stored at −80°C before assessment for cytokines.
Measurement of cytokines.
Culture supernatants and lung homogenates were assayed for the presence of tumor necrosis factor alpha (TNF-α), IL-6, IL-10, IL-1β, IL-12p70, monocyte chemotactic peptide 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), and MIP-1β by cytometric bead array using a LSRII multiparameter flow cytometer and FCAP array software (all from BD Biosciences, San Jose, CA) according to the manufacturer's instructions.
Statistical analysis.
Statistical differences between two groups were determined using an unpaired t test with the significance set at P < 0.05. For comparison between three or more groups, analysis was done by one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test with significance determined at P < 0.05.
RESULTS
Differential induction of proinflammatory cytokines by F. tularensis SchuS4 in monocytes and hDC.
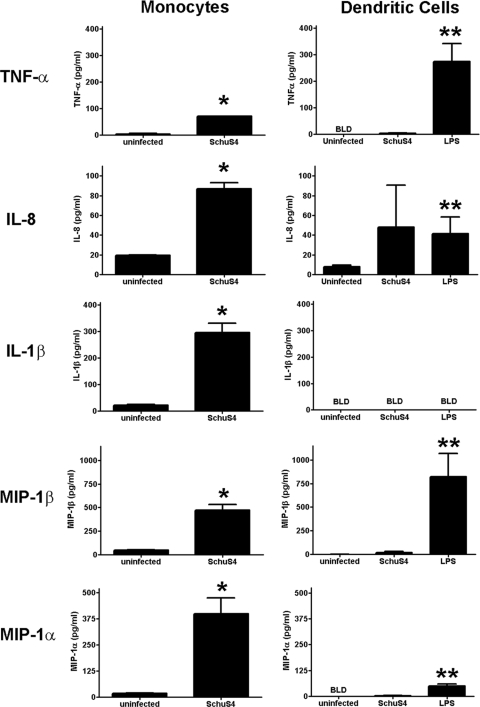
We have previously shown that hDC fail to secrete detectable levels of proinflammatory cytokines following infection with F. tularensis SchuS4 (17). In contrast, it has been reported that primary human monocytes secrete IL-6, IL-8, and IL-1β in response to SchuS4 infection (16). To confirm and extend these initial observations, we directly compared production of cytokines in hDC and monocyte cultures infected with strain SchuS4. As expected, hDC infected with SchuS4 did not produce TNF-α, IL-8, IL-1β, MIP-1β, or MIP-1α at concentrations that were significantly different from those of uninfected controls (Fig. 1). The absence of cytokine production from SchuS4-infected hDC was not due to an inability of hDC to respond to microbial stimuli, since hDC treated with E. coli LPS secreted significantly higher concentrations of all cytokines tested than untreated/uninfected controls (P < 0.05) (Fig. 1). In contrast, monocytes responded to SchuS4 infection by secreting significantly more TNF-α, IL-8, IL-1β, MIP-1β, and MIP-1α than uninfected monocytes (P < 0.01) (Fig. 1). Thus, unlike hDC, primary human monocytes vigorously respond to SchuS4 infection by secreting both proinflammatory cytokines and chemokines.
FIG. 1.
Monocytes, but not human dendritic cells, secrete cytokines in response to F. tularensis SchuS4 infection. Primary human monocytes and donor-matched hDC were infected with F. tularensis SchuS4 at a MOI of 50. Twenty-four hours after infection, culture supernatants were analyzed for cytokines. Monocytes infected with F. tularensis SchuS4 secreted significantly more TNF-α, IL-6, IL-1β, MIP1β, and MIP-1α compared to monocytes from uninfected controls (P < 0.01 [*]). In contrast, DC failed to secrete cytokines in response to SchuS4 infection, and these values were significantly different from those of uninfected controls. hDC readily secreted significantly greater concentrations of TNF-α, IL-6, IL-1β, MIP-1β, and MIP-1α in response to LPS compared to DC from uninfected controls (P < 0.01 [**]). Thus, the absence of cytokine in SchuS4-infected hDC cultures was not due to a general defect in hDC recognition of microbial antigens. Values that are below the level of detection (BLD) are indicated. Data are representative of six experiments of similar design, using different donor cells of similar design. Values are means plus standard errors of the means (SEM) (error bars).
CD14 expression on monocytes and hDC.
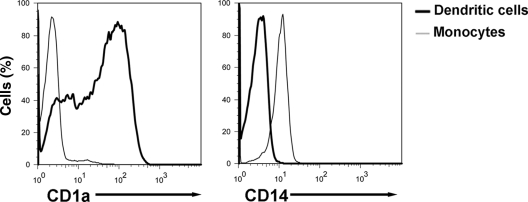
CD14 is a receptor that is critical for optimal induction of responses to multiple microbial antigens including LPS, glucuronoxylomannan of Cryptococcus neoformans, lipoteichoic acid (LTA), and lipoarabinomannan lacking terminal mannosyl units (AraLAM) from Mycobacterium tuberculosis (6, 38, 64, 66). Monocytes circulating in the peripheral blood express CD14 on their surfaces, whereas hDC lose surface expression of CD14 and gain CD1a following their differentiation from monocytes (48). Given the important role CD14 plays in the initial recognition of microbes and the marked disparity in expression of this receptor on monocytes and hDC, we reasoned that CD14 may play a role in induction of cytokine responses following SchuS4 infection of monocytes and hDC. We first confirmed transition of these receptors on hDC by assessing CD1a and CD14 expression on primary human monocytes and donor-matched hDC. As expected, monocytes had uniform levels of CD14 on their surface, whereas CD1a was undetectable on these cells (Fig. 2). In contrast, hDC differentiated from monocytes had undetectable levels of CD14 and had increased expression of CD1a (Fig. 2).
FIG. 2.
Differential expression of CD14 on human dendritic cells and monocytes. Primary human monocytes and donor-matched hDC were analyzed for surface expression of CD1a and CD14 by flow cytometry. Monocytes were uniformly CD14+ and CD1a−, whereas the majority of hDC were CD1a+ and CD14−. Data are representative of four experiments of similar design, using different donor cells.
CD14 aids in induction of cytokines following infection of human cells with F. tularensis SchuS4.
We next determined whether addition of soluble CD14 to hDC cultures enhanced the ability of these cells to secrete cytokine in response to SchuS4 infection. Surprisingly, addition of sCD14 to hDC infected with F. tularensis SchuS4 induced secretion of significantly higher concentrations of TNF-α, MIP-1β, and MIP-1α compared to those of untreated, SchuS4-infected and uninfected controls (Fig. 3). Interestingly, addition of sCD14 did not elicit production of IL-8 (Fig. 3) or IL-1β (data not shown) in SchuS4-infected hDC. sCD14 treatment of uninfected hDC induced modest, to barely detectable, amounts of TNF-α and MIP-1β. However, the concentrations of these cytokines were significantly lower than those observed in sCD14-treated, SchuS4-infected hDC (P < 0.01). Thus, sCD14 enabled hDC to recognize and respond to SchuS4 infection.
FIG. 3.
CD14 is critical for induction of cytokines during F. tularensis SchuS4 infection of human cells. Human dendritic cells (A) or primary human monocytes (B) were infected with F. tularensis SchuS4 at a MOI of 50 in the presence of sCD14 (DC), anti-CD14 (monocytes), or isotype control (monocytes). (A) sCD14 elicited secretion of significantly greater concentrations of TNF-α and MIP-1α from SchuS4-infected cells compared to the cells from all other groups (P < 0.01 [*]). sCD14 also elicited secretion of significantly more MIP-1β from SchuS4-infected cells compared to untreated, SchuS4-infected DC (Un) (P < 0.05 [**]). (B) Presence of CD14 blocking antibodies resulted in secretion of significantly less TNF-α, MIP1-α, MIP-1β, and IL-1β by SchuS4-infected monocytes compared to monocytes from other groups (P < 0.01 [*]). Data are pooled from six separate experiments, using different donors. Values are means plus SEM (error bars).
We next examined the role of CD14 in the recognition of F. tularensis SchuS4 in monocytes. Addition of anti-CD14 antibodies to SchuS4-infected monocytes resulted in a significant decrease in secretion of TNF-α, MIP-1β, MIP-1α, and IL-1β compared to isotype-treated controls (P < 0.01) (Fig. 3). Similar to hDC, secretion of IL-8 by SchuS4-infected monocytes was not impacted by blocking CD14. Together, these data demonstrate that the presence of CD14 in either a membrane-bound or soluble form is essential for initiation of cytokine and chemokine responses following SchuS4 infection in human monocytes and DC.
To determine whether the role of CD14 in the elicitation of cytokines and chemokines was specific for F. tularensis SchuS4, we examined the contribution of CD14 in the detection of B. abortus by human monocytes and dendritic cells. We selected B. abortus for several reasons. First, B. abortus is also a Gram-negative, facultative intracellular pathogen. Further, similar to F. tularensis SchuS4, Brucella species have been previously demonstrated both to fail to induce inflammatory responses in hDC and to actively suppress production of cytokines following infection of these mammalian cells (7, 8). Surprisingly, addition of sCD14 failed to induce inflammatory responses in hDC following infection with B. abortus. In contrast, blocking CD14 resulted in a significant decrease in secretion of both TNF-α and IL-1β by human monocytes infected with B. abortus. Thus, unlike infections with SchuS4, CD14 was not sufficient to provoke a proinflammatory response from hDC. This suggests that the dependence of human cells on CD14 for detection of SchuS4 may be unique to this bacterium.
CD14 does not play a role in phagocytosis of F. tularensis SchuS4.
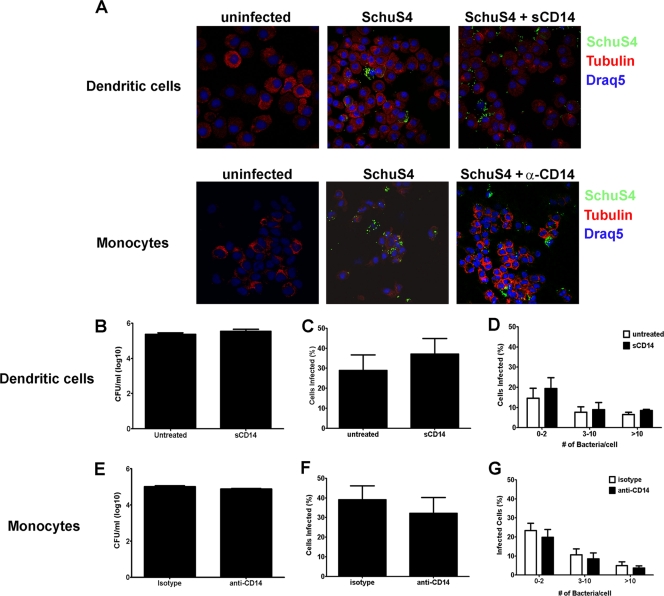
CD14 has been implicated in mediating phagocytosis of other Gram-negative bacteria (50). Thus, it is possible that the enhancement of cytokine secretion observed in sCD14-treated hDC, or the inhibition of this response in monocytes treated with anti-CD14, was due to alternations in the phagocytosis of strain SchuS4 by the host cell. To determine the role of CD14 in phagocytosis of SchuS4 in monocytes and hDC, we measured the uptake of SchuS4 in sCD14-treated hDC or anti-CD14-treated monocytes 3 h after infection. As depicted in Fig. 4, addition of sCD14 to hDC had no impact on the number of viable intracellular bacteria, the overall number of cells infected with SchuS4, or the quantity of bacteria entering each cell (Fig. 4A to D). Similarly, blocking CD14 on human monocytes did not impact the uptake of viable bacteria, the overall number of monocytes infected with SchuS4, or the number of bacteria taken up by each cell (Fig. 4E to H).
FIG. 4.
CD14 does not play a role in phagocytosis of F. tularensis SchuS4 by human cells. Human dendritic cells and monocytes were infected with F. tularensis SchuS4 at a MOI of 50 in the presence of sCD14 and anti-CD14 or isotype-matched antibodies, respectively. Three hours after infection, the cells were lysed or stained for SchuS4 (green) and tubulin (red) and assessed for intracellular bacteria by enumerating bacteria in cell lysates or by confocal microscopy. (A to C) Addition of sCD14 had no effect on the uptake of viable SchuS4 by hDC (A). Furthermore, there were no significant differences in either the number of infected DC or the number of bacteria per cell among any group (B to D). (E to H) Blocking CD14 had no effect on the phagocytosis of viable F. tularensis SchuS4 by human monocytes (E). Further, there were no significant differences in either the number of monocytes infected or the number of bacteria per cell in any group (F to H). Data are representative of six experiments of similar design, using different donors. Values are means plus SEM (error bars).
We have previously shown that live, replicating SchuS4 fails to stimulate cytokine production in hDC. Further, viable SchuS4 actively suppresses the ability of hDC to respond to secondary microbial stimuli. Thus, an additional explanation for increased cytokine production in sCD14-treated hDC is that F. tularensis SchuS4 replicates poorly in these treated cells and, therefore, is unable to inhibit production of proinflammatory cytokines. To assess the effects CD14 had on the replication of SchuS4 in hDC and monocytes, we evaluated the bacterial load, the number of infected cells, and the number of bacteria per cell 24 h after infection in hDC and monocytes treated with sCD14 or anti-CD14 antibodies, respectively. There were no differences in bacterial loads among DC treated with sCD14 and untreated cells (Fig. 5A). Furthermore, addition of sCD14 did not impact the number of cells infected or the number of bacteria per cell compared to untreated controls (Fig. 5B to D). Similar to hDC, CD14 did not appear to have a role in SchuS4 replication in monocytes. Blocking CD14 on monocytes failed to significantly alter the intracellular replication, the number of cells infected, or the number of bacteria per cell (Fig. 5A and E to G). Thus, although CD14 was essential for stimulation of cytokine responses in monocytes and hDC following SchuS4 infection, this receptor does not play a direct role in phagocytosis or replication of SchuS4 in these two independent cell types.
FIG. 5.
CD14 does not affect replication of F. tularensis SchuS4 in human monocytes and human dendritic cells. hDC and monocytes were infected with F. tularensis SchuS4 at a MOI of 50 in the presence of sCD14 and anti-CD14 or isotype-matched antibodies, respectively. Twenty-four hours after infection, the cells were lysed or stained for SchuS4 (green) or tubulin (red), and the nucleus was stained using Draq5 (blue). Cells were assessed for intracellular bacteria by enumerating bacteria in cell lysates or by microscopy. (A) The presence of sCD14 in hDC cultures or blocking CD14 in monocyte cultures had no qualitative effect on the number of cells infected with SchuS4. (B to D) Addition of sCD14 to SchuS4-infected hDC had no significant effect on the number of viable intracellular bacteria (B), the number of DC infected (C), or the number of bacteria per cell (D). (E to G) Blocking CD14 on the surface of monocytes had no significant effect on the ability of SchuS4 to replicate in these cells as measured by the number of bacteria in cell lysates (D), the number of cells infected (E), and the number of bacteria per cell (F). Data are representative of three experiments of similar design, using different donor cells. Values are means plus SEM (error bars).
CD14 contributes to the control of pulmonary SchuS4 infection.
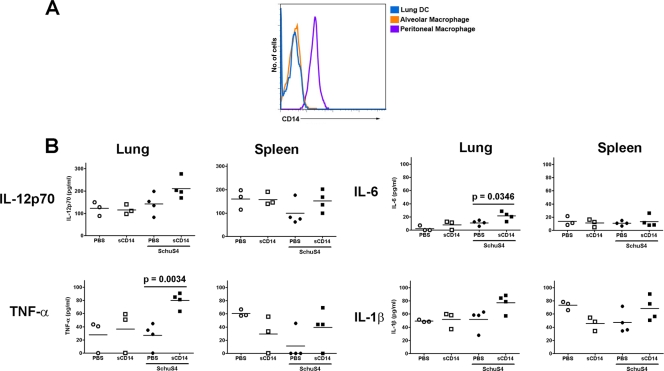
One enigma of pulmonary infection with F. tularensis SchuS4 is the absence of strong inflammatory responses in the lungs during the first few days of infection, despite rapid replication of the bacterium (3, 11, 21). The failure of F. tularensis SchuS4 to elicit inflammation in the lung is believed to be a key virulence factor for this pathogen. Thus, elicitation of a controlled inflammatory response early after infection would likely aid in the resolution of pulmonary SchuS4 infection. As discussed above, the absence of CD14 on hDC appears to aid in SchuS4 evasion of induction of proinflammatory responses in these cells. Similar to hDC, pulmonary cells, including alveolar macrophages (AvMØ), have been noted to express very low to undetectable levels of CD14 (2, 9). Furthermore, sCD14 is not abundant in the airways of healthy mammals (22, 51). Alveolar macrophages and airway DC are the primary targets of Francisella infection following inhalation of the bacterium during the first few days of infection, and these cells have both functional and phenotypic properties that are similar to those of human dendritic cells derived from peripheral blood (5, 11, 12, 28, 29, 36, 56). Therefore, as observed above in human dendritic cells (Fig. 1, 3, and 4), lack of CD14 on these important airway cells may aid in the evasion of induction of early cytokine responses by virulent F. tularensis. Thus, we next hypothesized that administration of sCD14 to the airways of SchuS4-infected mice might aid in control of pulmonary infection. We first confirmed that cells collected from mouse airways and lungs poorly expressed CD14. In comparison to peritoneal cells, airway cells and lung dendritic cells had nearly undetectable levels of CD14 on their surfaces (Fig. 6A). Next we examined the ability of sCD14 to facilitate production of cytokines in the lungs following SchuS4 infection. As previously observed, SchuS4 infection in the absence of sCD14 failed to elicit production of IL-12p70, TNF-α, IL-6, and IL-1β in the lungs in concentrations that were significantly different from those of mock-treated animals. In contrast, lungs from mice infected with SchuS4 that also received sCD14 had significantly more TNF-α and IL-6 (P = 0.0034 and 0.0346, respectively) than all other groups of animals (Fig. 6B). sCD14-treated, SchuS4-infected mice also had greater concentrations of IL-12p70 and IL-1β in their lungs, but these values were not significantly different from the values of the other groups of mice. Uninfected mice treated with sCD14 did not have significantly different concentrations of cytokine in their lungs compared to mock-treated controls (Fig. 6B). Thus, addition of exogenous sCD14 alone could not account for the enhanced production of TNF-α, IL-6, IL-12p70, and IL-1β observed in SchuS4-infected animals treated with sCD14.
FIG. 6.
CD14 aids in elicitation of proinflammatory cytokines in vivo. (A) Alveolar macrophages, lung DC, and peritoneal cells from naïve mice were assessed for cell surface expression of CD14 by flow cytometry. Peritoneal cells expressed uniform levels of CD14 on their cell surface. In contrast, alveolar macrophages and lung DC were weakly positive for CD14 on their cell surface. (B) CD14 facilitates production of cytokines following in vivo infection with F. tularensis SchuS4. Mice were infected with F. tularensis SchuS4 in the presence of sCD14. Uninfected, treated animals served as negative controls. Additional controls consisted of SchuS4-infected and uninfected mice that received PBS. Two days after infection, cytokines present in lung and spleen homogenate were quantified. Administration of sCD14 did not significantly change the amount of cytokine detected in lungs or spleens from uninfected mice. SchuS4-infected mice treated with PBS also failed to produce any significantly different concentrations of cytokines compared to uninfected controls. In contrast, SchuS4-infected animals treated with sCD14 had significantly more TNF-α (P = 0.0034) and IL-6 (P = 0.0346) in their lungs compared to the PBS-treated, SchuS4-infected controls. No significant difference in cytokine production among any groups was noted in samples obtained from the spleen. Data are representative of two experiments of similar design. The symbols show the values for individual mice, and the mean value for a group of mice is indicated by a short horizontal line.
Although CD14 did not have an effect on the replication of F. tularensis SchuS4 in hDC or human monocytes in vitro, it was possible that in the complex environment of the lung, the presence of sCD14 might have a greater impact on the local replication and dissemination of SchuS4. To test this hypothesis, we compared bacterial loads in the lungs and spleens of SchuS4-infected mice that had received sCD14 to infected mice that were treated with PBS. Indeed, mice treated with sCD14 had significantly fewer bacteria in their lungs than mock-treated controls (P = <0.05) 24, 48, and 72 h after infection (Fig. 7A). However, the greatest difference in bacterial loads in the lungs was detected 72 h after infection (Fig. 7A). Furthermore, animals treated with sCD14 had undetectable levels of SchuS4 in the spleens compared to approximately 104 and 106 bacteria in the spleens of mock-treated controls 48 and 72 h after infection, respectively (Fig. 7A). Changes in pulmonary and splenic cell populations were monitored by flow cytometry. In agreement with previous reports, no significant differences in monocyte, neutrophil, macrophage, or dendritic cell populations were observed among uninfected and infected mice, regardless of the presence of exogenous sCD14 (30) (data not shown). We next determined whether addition of sCD14 could enhance survival of SchuS4-infected mice. Surprisingly, despite the dramatic control of dissemination to the spleen we observed during the first few days of infection, addition of sCD14 failed to enhance the survival of mice with pulmonary SchuS4 infection (Fig. 7B). In the studies depicted herein, sCD14 was administered only at the time of challenge and the first day after infection. Therefore, it was possible that the continued administration of CD14 was required to aid survival of mice infected with SchuS4. Thus, we also tested the ability of sCD14 to provide protection against SchuS4 infection when administered for the first 3 days after infection. Continuous administration of sCD14 also failed to significantly enhance survival of mice infected with SchuS4 (data not shown).
FIG. 7.
sCD14 aids in control of F. tularensis SchuS4 replication and dissemination, but not in survival of infection. Mice were infected with F. tularensis SchuS4 in the presence of sCD14. SchuS4-infected mice that received PBS served as negative controls. (A) At the time points indicated, the bacterial loads in their lungs and spleens were determined. Mice treated with sCD14 had significantly fewer bacteria in their lungs compared to PBS controls at each time point assessed. In contrast to PBS-treated controls, sCD14-treated mice did not have any detectable bacteria in their spleens 48 and 72 h after infection. Values that were significantly different (P < 0.05) from the value for the PBS-treated control are indicated (*). (B) sCD14 failed to enhance survival following SchuS4 infection. Values are means plus SEM (error bars). Data are representative of two experiments of similar design.
Administration of exogenous sCD14 into the airways of F. tularensis SchuS4-infected mice was required for production of proinflammatory cytokines and control of bacterial replication and dissemination within the first few days of infection, but it had little impact on the survival of SchuS4-infected mice. However, the cytokine production and control of SchuS4 replication observed in conjunction with delivery of sCD14 was likely due to activation of specific effector cells already present in the lung rather than recruitment of cells from the periphery.
The absence of CD14 does not exacerbate pulmonary SchuS4 infections.
Since addition of sCD14 aided in early control of F. tularensis SchuS4 infections, it was possible that the complete absence of CD14 may result in even further diminished recognition of SchuS4 in the host, which in turn may exacerbate disease. To determine how the absence of CD14 might affect pulmonary SchuS4 infections, we evaluated the kinetics of bacterial replication, host response to infection, and survival in wild-type and CD14−/− mice following i.n. SchuS4 infection. There was no difference in bacterial loads in the lung and spleen at 2 or 4 days after infection in CD14−/− mice compared to wild-type controls (Table 1). There was no difference in survival in mice with intranasal SchuS4 infection; all 10 wild-type control mice and 10 CD14−/− mice died. The wild-type controls survived for 4.60 ± 0.16 days, and the CD14−/− mice survived for 4.55 ± 0.15 days. Furthermore, no difference in cytokine production, overall survival, or cellular infiltrate among CD14−/− mice compared to wild-type controls following SchuS4 infection were observed (Table 2 and data not shown). Thus, in agreement with our in vitro data, CD14 was not required for uptake and replication of strain SchuS4 by host cells in vivo. Additionally, complete absence of CD14 did not significantly alter the outcome of infection. However, our data clearly demonstrate that supplementation of sCD14 to the airways plays an important role in initial recognition and control of SchuS4 replication and dissemination during the early stages of pneumonic tularemia.
TABLE 1.
Absence of CD14 does not affect replication of F. tularensis SchuS4 in vivoa
| Organ | Mice (n = 4) | DPIb | No. of CFU/organ (105) (mean ± SEM) |
|---|---|---|---|
| Lung | WTc | 2 | 1.31 ± 0.263 |
| 4 | 39.8 ± 9.34 | ||
| CD14−/− | 2 | 2.89 ± 1.64 | |
| 4 | 35.7 ± 4.45 | ||
| Spleen | WT | 2 | 0.408 ± 0.320 |
| 4 | 518 ± 114 | ||
| CD14−/− | 2 | 0.101 ± 0.0327 | |
| 4 | 619 ± 63 |
Mice were challenged with 50 CFU of F. tularensis SchuS4 intranasally.
DPI, day postinfection.
WT, wild type.
TABLE 2.
Production of cytokines in the lungs and spleens of wild-type and CD14−/− mice following F. tularensis SchuS4 infectiona
| Organ | Cytokine | Mice | Infection | Cytokine concn (pg/ml) (mean ± SEM) |
|---|---|---|---|---|
| Spleen | IFN-γ | WTb | NIc | 3.52 ± 1.803 |
| CD14−/− | NI | 3.46 ± 0.685 | ||
| WT | SchuS4 | 1,107 ± 341 | ||
| CD14−/− | SchuS4 | 2,245 ± 158 | ||
| MCP-1 | WT | NI | 65.48 ± 6.96 | |
| CD14−/− | NI | 59.38 ± 8.16 | ||
| WT | SchuS4 | 4,965 ± 1,317 | ||
| CD14−/− | SchuS4 | 7,196 ± 837 | ||
| MIP-1α | WT | NI | 4.650 + 4.653 | |
| CD14−/− | NI | BLDd | ||
| WT | SchuS4 | 359.8 ± 131 | ||
| CD14−/− | SchuS4 | 626.0 ± 32.43 | ||
| IL-6 | WT | NI | BLD | |
| CD14−/− | NI | BLD | ||
| WT | SchuS4 | 5,566 ± 3,231 | ||
| CD14−/− | SchuS4 | 11,814 ± 1,910 | ||
| TNF-α | WT | NI | 121.7 ± 21.61 | |
| CD14−/− | NI | 76.87 ± 23.05 | ||
| WT | SchuS4 | 1,302 ± 773 | ||
| CD14−/− | SchuS4 | 4,054 ± 1,783 | ||
| Lung | IFN-γ | WT | NI | BLD |
| CD14−/− | NI | BLD | ||
| WT | SchuS4 | 513.4 ± 45.57 | ||
| CD14−/− | SchuS4 | 1,280 ± 396.8 | ||
| MCP-1 | WT | NI | 35.28 ± 8.57 | |
| CD14−/− | NI | 40.49 ± 5.719 | ||
| WT | SchuS4 | 3,440 ± 166 | ||
| CD14−/− | SchuS4 | 2,731 ± 992 | ||
| MIP-1α | WT | NI | BLD | |
| CD14−/− | NI | BLD | ||
| WT | SchuS4 | 210.2 ± 22.5 | ||
| CD14−/− | SchuS4 | 310.6 ± 79.3 | ||
| IL-6 | WT | NI | BLD | |
| CD14−/− | NI | BLD | ||
| WT | SchuS4 | 966.0 ± 170 | ||
| CD14−/− | SchuS4 | 3,848 ± 1,833 | ||
| TNF-α | WT | NI | 46.93 ± 3.70 | |
| CD14−/− | NI | 46.50 ± 3.581 | ||
| WT | SchuS4 | 364.4 ± 59.95 | ||
| CD14−/− | SchuS4 | 446.7 ± 97.18 |
Mice were infected with 50 CFU of F. tularensis SchuS4. The organs were collected 4 days after infection.
WT, wild type.
NI, not infected (uninfected controls treated with PBS).
BLD, below the level of detection.
DISCUSSION
Francisella tularensis infections can be characterized by a biphasic inflammatory response. At the outset of infection, when the bacteria are primarily confined to the site of inoculation, there is little evidence of inflammation or production of detectable proinflammatory cytokines. However, shortly after the bacteria have disseminated and have begun to replicate in peripheral tissues, inflammation becomes evident and, in the setting of lethal disease, contributes to the death of infected hosts (20, 27). The mechanisms by which Francisella evades initiating early proinflammatory responses are not fully understood. Similarly, the direct contribution of Francisella in the induction of inflammation at the end of infection is equally poorly characterized.
In this report, we demonstrate that evasion of induction of early inflammatory responses by virulent F. tularensis in both primary target cells in vitro and pulmonary tissues in vivo is partially dependent on the absence of CD14. Supplementation of CD14 in vitro to target cells most likely to be present at the site of infection, i.e., dendritic cells, or in vivo directly at the site of inoculation, i.e., the lung, resulted in increased production of several proinflammatory cytokines. Importantly, CD14-mediated production of proinflammatory cytokines in vivo correlated with modest, but significant, control of bacterial replication and dissemination. The lack of control of bacterial replication in vitro may be explained by the absence of additional effector cells capable of responding to the initial wave of proinflammatory cytokines via production of other soluble mediators that stimulate killing in target cells. For example, preliminary data generated in our laboratory suggest that F. tularensis SchuS4 inhibits the ability of infected cells to respond to certain proinflammatory cytokines (e.g., TNF-α) and undergo activation. Thus, control of F. tularensis SchuS4 may rely on indirect activation via other, uninfected effector cells. In support of this hypothesis, previous reports suggest that efficient killing of intracellular F. tularensis requires production of IFN-γ and that the source of IFN-γ during the critical early stages of infection is NK cells (42). However, optimal production of IFN-γ by NK cells typically requires IL-12p70 secreted from resident macrophages and/or DC (reviewed in reference 57). Thus, it is reasonable to speculate that while production of proinflammatory cytokines, such as TNF-α and IL-12, following addition of CD14 to cells or tissues is the first step toward killing SchuS4, it is not sufficient on its own. Rather, early control of SchuS4 infection requires induction of IFN-γ by other, uninfected host cells responding to IL-12. Our in vitro culture consisted only of DC or monocytes and lacked IFN-γ-producing “effector” cells. Thus, although CD14 facilitated increased production of several proinflammatory cytokines, cells capable of responding to these cytokines via secretion of more potent stimulating cytokines were not present and no control of SchuS4 replication was observed. In vivo, the full complement of cells, both target and effector, are present at the site of infection. Therefore, it is possible that the IL-12 present in CD14-treated, SchuS4-infected mice activated resident NK cells to produce IFN-γ, resulting in modest control of SchuS4 replication.
The production of cytokines following pulmonary F. tularensis SchuS4 infection is tightly correlated with the recruitment of effector cells, such as granulocytes and monocytes (21, 30). However, this recruitment does not typically occur until 3 days after infection (21, 30). Rather, at the outset of infection, the primary pulmonary cell types infected are alveolar macrophages and dendritic cells, both of which lack CD14 (11, 30) (Fig. 6). In fact, in one report, SchuS4 was not even detected in resident granulocytes during the first day of infection (30). Thus, an additional explanation for the early control of SchuS4 in the lungs and spleens of sCD14-treated animals is that there was early recruitment of effector cells, such as granulocytes and monocytes, into these organs. However, we did not observe recruitment of granulocytes or monocytes following administration of sCD14 into either the lung or spleen. Thus, our data indicate that the cells responsible for producing proinflammatory cytokines in the presence of CD14 are resident alveolar macrophages and dendritic cells.
Our studies also suggest that the dependence on CD14 for elicitation of early inflammatory responses in vivo may be unique to F. tularensis. Previous reports examining the role of CD14 in other pulmonary bacterial infections have suggested that the presence of this receptor plays a deleterious role in control of bacterial replication and dissemination. For example, mice lacking CD14 controlled pulmonary Burkholderia pseudomallei infections significantly better than wild-type mice did (63). Similarly, CD14 was found to contribute to mortality and development of pulmonary pathology observed in mice infected with Mycobacterium tuberculosis (62). However, it should be noted that in both of these examples the bacteria typically elicit a strong inflammatory response during the early stages of infection and that this response is thought to contribute to the overall pathology of disease mediated by these microorganisms. To better assess the contribution of CD14 in infections mediated by Gram-negative bacteria that do not typically elicit strong inflammatory responses, we examined the effects CD14 has on production of cytokines and chemokines by human dendritic cells and monocytes following infection with B. abortus. In contrast to F. tularensis SchuS4, addition of sCD14 to human DC infected with B. abortus failed to elicit production of cytokines and chemokines from these mammalian cells (see Fig. S1 in the supplemental material). However, the presence of CD14 did appear to play a role in the recognition of B. abortus by human monocytes (see Fig. 1 in the supplemental material). Thus, the role of CD14 in recognition of B. abortus is cell type dependent. This suggests that the requirement for CD14 in the recognition of strain SchuS4 by both dendritic cells and monocytes is unique to this virulent bacterium.
Given the effect CD14 had on the induction of proinflammatory cytokines during the first few days of infection, it is possible that this receptor contributes to the overwhelming production of cytokines that is typically associated with mortality in F. tularensis SchuS4-infected hosts (20, 27). However, SchuS4-infected CD14−/− mice were not more susceptible to SchuS4 infection and did not have significant differences in the amount of cytokine detected in target tissues at the end stage of disease compared to wild-type controls (Tables 1 and 2). The disparity of these data with results examining the role of CD14 at the outset of infection is similar to a phenomenon described in other bacterial infections. Earlier reports examining the roles for cell surface receptors in the recognition of soluble and particulate bacterial antigens demonstrated that CD14 was capable of recognizing both soluble and particulate antigens. In contrast, another cell surface receptor complex known to interact with bacterial antigens, CD11b/CD18, responded only to particulate antigen (26). These observations were extended by examining the contribution of CD14 and CD11b/CD18 during different stages of infection with Neisseria meningitidis. In that study, stimulation of host cells by small numbers of bacteria (representing the early phase on infection) was dependent on CD14. In contrast, activation of host cells by large numbers of bacteria (representing the terminal stage of disease) was CD14 independent (31). Additional reports have shown that CD11b/CD18 enhances responses against intact bacteria and can compensate for the absence of CD14 in the recognition of these microorganisms (43). Thus, while CD14 appears to be critical for recognition of F. tularensis SchuS4 at the outset of infection, production of proinflammatory cytokines at the end of disease, when high numbers of bacteria are present in a variety of tissues, was CD14 independent and may require signaling through other receptors, such as CD11b/CD18.
CD14 acts as a coreceptor for both TLR2 and TLR4/MD-2 (25, 37, 65). Thus, it is likely that the CD14-dependent secretion of cytokines from human and mouse cells following F. tularensis SchuS4 infection is due to enhanced interaction of SchuS4 antigens with either TLR2 or TLR4/MD-2. Further, since the contribution CD14 makes in elicitation of cytokine production occurs early after infection with SchuS4, it was also likely that the antigens interacting with this molecule are present on the surface of the bacterium. Specific TLR agonists present in SchuS4 have not been formally reported. However, recent reports examining the ability of intact, attenuated subspecies of Francisella, e.g., F. tularensis LVS, and their associated antigens to stimulate TLR-dependent responses have revealed several TLR2 and TLR4/MD-2 agonists in the membranes of these bacteria.
The two TLR4/MD-2 agonists found in F. tularensis LVS are LPS and the heat shock protein, DnaK (HSP70) (4, 23). LPS associated with both F. tularensis LVS and SchuS4 is tetraacylated and, therefore, is a very poor TLR4 agonist (46). Initially, it was assumed that poorly acylated LPS failed to interact with CD14, ultimately interfering with efficient delivery of LPS to the TLR4/MD-2 complex. However, it has been shown that the number of acyl groups present on LPS molecules does not impact their ability to interact with CD14 (44). Rather, the presence of six or more acyl groups is essential for interaction of LPS with the MD-2 portion of the TLR4/MD-2 complex (44). Thus, the weak activity tetraacylated LVS and SchuS4 LPS have in TLR4/MD-2 stimulation is not dependent on CD14 but on the absence of acylation required for triggering TLR4/MD-2 responses. Therefore, a predominate role for Francisella LPS in mediating inflammatory responses via CD14 and TLR4 seems unlikely.
The other TLR4/MD-2 agonist present in attenuated subspecies of Francisella tularensis is the heat shock protein DnaK, also known as HSP70 (4). Heat shock proteins, e.g., HSP60 and HSP70, are present in both pro- and eukaryotic systems and have been widely recognized as TLR4/MD-2 and TLR2 agonists that require CD14 for efficient delivery to the TLRs (53, 59-61). Both HSP60 and HSP70 are secreted by F. tularensis into culture medium, but only HSP60 has been found to be secreted during intracellular infection (41). Further, HSP60, but not HSP70, is located in the periplasmic membrane of F. tularensis (32). Thus, although HSP70 has been formally identified as a TLR4 agonist, the absence of secretion of this antigen during intracellular infections suggest that its role in elicitation of early inflammatory responses may be fairly minimal. Rather, HSP60 represents a more likely candidate antigen for stimulation of CD14-dependent TLR4/MD-2 and/or TLR2 responses following infection with viable, intact bacteria.
Earlier studies have shown that live, intact F. tularensis LVS induces inflammatory responses primarily through TLR2 (19). Since that initial observation, several lipoproteins from F. tularensis LVS have been identified as TLR2 agonists (55). These lipoproteins include Tul4 and FTT1103 and appear to require heterodimerization of TLR2 and TLR1 for optimal elicitation of proinflammatory cytokines from mammalian cells. Interestingly, these studies did not address the requirement for CD14 in elicitation of cytokines by Francisella lipoproteins. Thus, both Tul4 and FTT1103 could contribute to the production of cytokines and chemokines we observed herein, but the specific contribution of CD14 will need to be determined.
The failure of F. tularensis SchuS4 to elicit production of proinflammatory cytokines and chemokines early after infection is believed to contribute to the overall virulence of this bacterium. The mechanisms by which F. tularensis SchuS4 evades this early detection are not well characterized and represent a significant hurdle in the development of novel therapeutics and vaccines. Data presented herein demonstrate, for the first time, that elicitation of early inflammatory responses in the lung strongly correlates with modest control of SchuS4 replication and dissemination. Furthermore, we show that induction of these early proinflammatory responses in vitro and in vivo was dependent on CD14. Together, our data suggest that identification of host molecules, such as CD14, that contribute toward the early detection of F. tularensis SchuS4 may aid in development of novel therapeutics for treatment of this aggressive, virulent bacterium.
Supplementary Material
Acknowledgments
We thank Steven Holland, Eugene Howerton, and the Department of Transfusion Medicine at NIH for assistance in providing human monocytes; Aaron Carmody for assistance in performing the cytometric bead array assays; and Tregei Starr for assistance with the B. abortus experiments.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 19 October 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alcorn, J. F., L. M. Rinaldi, E. F. Jaffe, M. van Loon, J. H. Bates, Y. M. Janssen-Heininger, and C. G. Irvin. 2007. Transforming growth factor-beta1 suppresses airway hyperresponsiveness in allergic airway disease. Am. J. Respir. Crit. Care Med. 176:974-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano, H., H. Yamamoto, M. Senba, K. Oishi, S. Suzuki, K. Fukushima, N. Mukaida, K. Matsushima, K. Eguchi, and T. Nagatake. 2000. Impairment of endotoxin-induced macrophage inflammatory protein 2 gene expression in alveolar macrophages in streptozotocin-induced diabetes in mice. Infect. Immun. 68:2925-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, H., B. Hartmanova, R. Kuolee, P. Ryden, W. Conlan, W. Chen, and A. Sjostedt. 2006. Transcriptional profiling of host responses in mouse lungs following aerosol infection with type A Francisella tularensis. J. Med. Microbiol. 55:263-271. [DOI] [PubMed] [Google Scholar]
- 4.Ashtekar, A. R., P. Zhang, J. Katz, C. C. Deivanayagam, P. Rallabhandi, S. N. Vogel, and S. M. Michalek. 2008. TLR4-mediated activation of dendritic cells by the heat shock protein DnaK from Francisella tularensis. J. Leukoc. Biol. 84:1434-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Haim, E., O. Gat, G. Markel, H. Cohen, A. Shafferman, and B. Velan. 2008. Interrelationship between dendritic cell trafficking and Francisella tularensis dissemination following airway infection. PLoS Pathog. 4:e1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernardo, J., A. M. Billingslea, R. L. Blumenthal, K. F. Seetoo, E. R. Simons, and M. J. Fenton. 1998. Differential responses of human mononuclear phagocytes to mycobacterial lipoarabinomannans: role of CD14 and the mannose receptor. Infect. Immun. 66:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billard, E., J. Dornand, and A. Gross. 2007. Brucella suis prevents human dendritic cell maturation and antigen presentation through regulation of tumor necrosis factor alpha secretion. Infect. Immun. 75:4980-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billard, E., J. Dornand, and A. Gross. 2007. Interaction of Brucella suis and Brucella abortus rough strains with human dendritic cells. Infect. Immun. 75:5916-5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biondi, A., T. H. Rossing, J. Bennett, and R. F. Todd III. 1984. Surface membrane heterogeneity among human mononuclear phagocytes. J. Immunol. 132:1237-1243. [PubMed] [Google Scholar]
- 10.Borron, P. J., E. A. Mostaghel, C. Doyle, E. S. Walsh, M. G. McHeyzer-Williams, and J. R. Wright. 2002. Pulmonary surfactant proteins A and D directly suppress CD3+/CD4+ cell function: evidence for two shared mechanisms. J. Immunol. 169:5844-5850. [DOI] [PubMed] [Google Scholar]
- 11.Bosio, C. M., H. Bielefeldt-Ohmann, and J. T. Belisle. 2007. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178:4538-4547. [DOI] [PubMed] [Google Scholar]
- 12.Bosio, C. M., and S. W. Dow. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 175:6792-6801. [DOI] [PubMed] [Google Scholar]
- 13.Bosio, C. M., and K. L. Elkins. 2001. Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect. Immun. 69:194-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosio, C. M., B. D. Moore, K. L. Warfield, G. Ruthel, M. Mohamadzadeh, M. J. Aman, and S. Bavari. 2004. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology 326:280-287. [DOI] [PubMed] [Google Scholar]
- 15.Brinker, K. G., H. Garner, and J. R. Wright. 2003. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 284:L232-L241. [DOI] [PubMed] [Google Scholar]
- 16.Butchar, J. P., T. J. Cremer, C. D. Clay, M. A. Gavrilin, M. D. Wewers, C. B. Marsh, L. S. Schlesinger, and S. Tridandapani. 2008. Microarray analysis of human monocytes infected with Francisella tularensis identifies new targets of host response subversion. PLoS One 3:e2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chase, J. C., J. Celli, and C. M. Bosio. 2009. Direct and indirect impairment of human dendritic cell function by virulent Francisella tularensis Schu S4. Infect. Immun. 77:180-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Checroun, C., T. D. Wehrly, E. R. Fischer, S. F. Hayes, and J. Celli. 2006. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. U. S. A. 103:14578-14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole, L. E., K. A. Shirey, E. Barry, A. Santiago, P. Rallabhandi, K. L. Elkins, A. C. Puche, S. M. Michalek, and S. N. Vogel. 2007. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect. Immun. 75:4127-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlan, J. W., W. Chen, H. Shen, A. Webb, and R. KuoLee. 2003. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb. Pathog. 34:239-248. [DOI] [PubMed] [Google Scholar]
- 21.Conlan, J. W., X. Zhao, G. Harris, H. Shen, M. Bolanowski, C. Rietz, A. Sjostedt, and W. Chen. 2008. Molecular immunology of experimental primary tularemia in mice infected by respiratory or intradermal routes with type A Francisella tularensis. Mol. Immunol. 45:2962-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubin, W., T. R. Martin, P. Swoveland, D. J. Leturcq, A. M. Moriarty, P. S. Tobias, E. R. Bleecker, S. E. Goldblum, and J. D. Hasday. 1996. Asthma and endotoxin: lipopolysaccharide-binding protein and soluble CD14 in bronchoalveolar compartment. Am. J. Physiol. 270:L736-L744. [DOI] [PubMed] [Google Scholar]
- 23.Duenas, A. I., M. Aceves, A. Orduna, R. Diaz, M. Sanchez Crespo, and C. Garcia-Rodriguez. 2006. Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than E. coli LPS. Int. Immunol. 18:785-795. [DOI] [PubMed] [Google Scholar]
- 24.Elkins, K. L., T. R. Rhinehart-Jones, S. J. Culkin, D. Yee, and R. K. Winegar. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64:3288-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenton, M. J., and D. T. Golenbock. 1998. LPS-binding proteins and receptors. J. Leukoc. Biol. 64:25-32. [DOI] [PubMed] [Google Scholar]
- 26.Flo, T. H., L. Ryan, L. Kilaas, G. Skjak-Braek, R. R. Ingalls, A. Sundan, D. T. Golenbock, and T. Espevik. 2000. Involvement of CD14 and beta2-integrins in activating cells with soluble and particulate lipopolysaccharides and mannuronic acid polymers. Infect. Immun. 68:6770-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forestal, C. A., M. Malik, S. V. Catlett, A. G. Savitt, J. L. Benach, T. J. Sellati, and M. B. Furie. 2007. Francisella tularensis has a significant extracellular phase in infected mice. J. Infect. Dis. 196:134-137. [DOI] [PubMed] [Google Scholar]
- 28.Guth, A. M., W. J. Janssen, C. M. Bosio, E. C. Crouch, P. M. Henson, and S. W. Dow. 2009. Lung environment determines unique phenotype of alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 296:L936-L946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall, J. D., R. R. Craven, J. R. Fuller, R. J. Pickles, and T. H. Kawula. 2007. Francisella tularensis replicates within alveolar type II epithelial cells in vitro and in vivo following inhalation. Infect. Immun. 75:1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall, J. D., M. D. Woolard, B. M. Gunn, R. R. Craven, S. Taft-Benz, J. A. Frelinger, and T. H. Kawula. 2008. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect. Immun. 76:5843-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellerud, B. C., J. Stenvik, T. Espevik, J. D. Lambris, T. E. Mollnes, and P. Brandtzaeg. 2008. Stages of meningococcal sepsis simulated in vitro, with emphasis on complement and Toll-like receptor activation. Infect. Immun. 76:4183-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huntley, J. F., P. G. Conley, K. E. Hagman, and M. V. Norgard. 2007. Characterization of Francisella tularensis outer membrane proteins. J. Bacteriol. 189:561-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huynh, M. L., V. A. Fadok, and P. M. Henson. 2002. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 109:41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen, W. J., K. A. McPhillips, M. G. Dickinson, D. J. Linderman, K. Morimoto, Y. Q. Xiao, K. M. Oldham, R. W. Vandivier, P. M. Henson, and S. J. Gardai. 2008. Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am. J. Respir. Crit. Care Med. 178:158-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang, Q., S. Akashi, K. Miyake, and H. R. Petty. 2000. Lipopolysaccharide induces physical proximity between CD14 and Toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J. Immunol. 165:3541-3544. [DOI] [PubMed] [Google Scholar]
- 36.Kirby, A. C., M. C. Coles, and P. M. Kaye. 2009. Alveolar macrophages transport pathogens to lung draining lymph nodes. J. Immunol. 183:1983-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirschning, C. J., H. Wesche, T. Merrill Ayres, and M. Rothe. 1998. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kusunoki, T., E. Hailman, T. S. Juan, H. S. Lichenstein, and S. D. Wright. 1995. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J. Exp. Med. 182:1673-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landmann, R., B. Muller, and W. Zimmerli. 2000. CD14, new aspects of ligand and signal diversity. Microbes Infect. 2:295-304. [DOI] [PubMed] [Google Scholar]
- 40.Landmann, R., M. Wesp, and J. P. Obrecht. 1991. Cytokine regulation of the myeloid glycoprotein CD14. Pathobiology 59:131-135. [DOI] [PubMed] [Google Scholar]
- 41.Lee, B. Y., M. A. Horwitz, and D. L. Clemens. 2006. Identification, recombinant expression, immunolocalization in macrophages, and T-cell responsiveness of the major extracellular proteins of Francisella tularensis. Infect. Immun. 74:4002-4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez, M. C., N. S. Duckett, S. D. Baron, and D. W. Metzger. 2004. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell. Immunol. 232:75-85. [DOI] [PubMed] [Google Scholar]
- 43.Moore, K. J., L. P. Andersson, R. R. Ingalls, B. G. Monks, R. Li, M. A. Arnaout, D. T. Golenbock, and M. W. Freeman. 2000. Divergent response to LPS and bacteria in CD14-deficient murine macrophages. J. Immunol. 165:4272-4280. [DOI] [PubMed] [Google Scholar]
- 44.Park, B. S., D. H. Song, H. M. Kim, B. S. Choi, H. Lee, and J. O. Lee. 2009. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191-1195. [DOI] [PubMed] [Google Scholar]
- 45.Passlick, B., D. Flieger, and H. W. Ziegler-Heitbrock. 1989. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74:2527-2534. [PubMed] [Google Scholar]
- 46.Phillips, N. J., B. Schilling, M. K. McLendon, M. A. Apicella, and B. W. Gibson. 2004. Novel modification of lipid A of Francisella tularensis. Infect. Immun. 72:5340-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regueiro, V., M. A. Campos, P. Morey, J. Sauleda, A. G. Agusti, J. Garmendia, and J. A. Bengoechea. 2009. Lipopolysaccharide-binding protein and CD14 are increased in the bronchoalveolar lavage fluid of smokers. Eur. Respir. J. 33:273-281. [DOI] [PubMed] [Google Scholar]
- 48.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107:702-714. [DOI] [PubMed] [Google Scholar]
- 50.Schiff, D. E., L. Kline, K. Soldau, J. D. Lee, J. Pugin, P. S. Tobias, and R. J. Ulevitch. 1997. Phagocytosis of gram-negative bacteria by a unique CD14-dependent mechanism. J. Leukoc. Biol. 62:786-794. [DOI] [PubMed] [Google Scholar]
- 51.Senft, A. P., T. R. Korfhagen, J. A. Whitsett, S. D. Shapiro, and A. M. LeVine. 2005. Surfactant protein-D regulates soluble CD14 through matrix metalloproteinase-12. J. Immunol. 174:4953-4959. [DOI] [PubMed] [Google Scholar]
- 52.Starr, T., T. W. Ng, T. D. Wehrly, L. A. Knodler, and J. Celli. 2008. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 9:678-694. [DOI] [PubMed] [Google Scholar]
- 53.Takenaka, R., K. Yokota, K. Ayada, M. Mizuno, Y. Zhao, Y. Fujinami, S. N. Lin, T. Toyokawa, H. Okada, Y. Shiratori, and K. Oguma. 2004. Helicobacter pylori heat-shock protein 60 induces inflammatory responses through the Toll-like receptor-triggered pathway in cultured human gastric epithelial cells. Microbiology 150:3913-3922. [DOI] [PubMed] [Google Scholar]
- 54.Tarnvik, A., and L. Berglund. 2003. Tularaemia. Eur. Respir. J. 21:361-373. [DOI] [PubMed] [Google Scholar]
- 55.Thakran, S., H. Li, C. L. Lavine, M. A. Miller, J. E. Bina, X. R. Bina, and F. Re. 2008. Identification of Francisella tularensis lipoproteins that stimulate the Toll-like receptor (TLR) 2/TLR1 heterodimer. J. Biol. Chem. 283:3751-3760. [DOI] [PubMed] [Google Scholar]
- 56.Thepen, T., E. Claassen, K. Hoeben, J. Breve, and G. Kraal. 1993. Migration of alveolar macrophages from alveolar space to paracortical T cell area of the draining lymph node. Adv. Exp. Med. Biol. 329:305-310. [DOI] [PubMed] [Google Scholar]
- 57.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 58.Ulmer, A. J., V. T. El-Salmalouti, E. T. Rietschel, H.-D. Flad, and R. Dziarsky. 1999. CD14, an innate immune receptor for various bacterial cell wall components, p. 463-472. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (ed.), Endotoxin in health and disease. Marcel Dekker, New York, NY.
- 59.Vabulas, R. M., P. Ahmad-Nejad, C. da Costa, T. Miethke, C. J. Kirschning, H. Hacker, and H. Wagner. 2001. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the Toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 276:31332-31339. [DOI] [PubMed] [Google Scholar]
- 60.Vabulas, R. M., P. Ahmad-Nejad, S. Ghose, C. J. Kirschning, R. D. Issels, and H. Wagner. 2002. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 277:15107-15112. [DOI] [PubMed] [Google Scholar]
- 61.Valentinis, B., A. Capobianco, F. Esposito, A. Bianchi, P. Rovere-Querini, A. A. Manfredi, and C. Traversari. 2008. Human recombinant heat shock protein 70 affects the maturation pathways of dendritic cells in vitro and has an in vivo adjuvant activity. J. Leukoc. Biol. 84:199-206. [DOI] [PubMed] [Google Scholar]
- 62.Wieland, C. W., G. J. van der Windt, W. J. Wiersinga, S. Florquin, and T. van der Poll. 2008. CD14 contributes to pulmonary inflammation and mortality during murine tuberculosis. Immunology 125:272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiersinga, W. J., A. F. de Vos, C. W. Wieland, M. Leendertse, J. J. Roelofs, and T. van der Poll. 2008. CD14 impairs host defense against gram-negative sepsis caused by Burkholderia pseudomallei in mice. J. Infect. Dis. 198:1388-1397. [DOI] [PubMed] [Google Scholar]
- 64.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 65.Yang, R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- 66.Yauch, L. E., M. K. Mansour, S. Shoham, J. B. Rottman, and S. M. Levitz. 2004. Involvement of CD14, Toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect. Immun. 72:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.