Abstract
Leishmania (subgenus Viannia) braziliensis is the causative agent of mucocutaneous leishmaniasis (ML) in South America, and ML is characterized by excessive T- and B-cell responses to the parasite. We speculate that the unbalanced production of inflammatory mediators in response to L. braziliensis infection contributes to cell recruitment and disease severity. To test this hypothesis, we first examined the response of peripheral blood mononuclear cells (PBMCs) from healthy volunteers to L. braziliensis infection. We observed that while L. braziliensis infection induced the production of chemokine (C-X-C motif) ligand 10 (CXCL10) and interleukin-10 (IL-10) in human PBMCs and macrophages (MΦs), enhanced expression of CXCL10 and its receptor, chemokine CXC receptor (CXCR3), was predominantly detected in CD14+ monocytes. The chemoattractant factors secreted by L. braziliensis-infected cells were highly efficient in recruiting uninfected PBMCs (predominantly CD14+ cells) through Transwell membranes. Serum samples from American tegumentary leishmaniasis (ATL) patients (especially the ML cases) had significantly higher levels of CXCL10, CCL4, and soluble tumor necrosis factor (TNF) receptor II (sTNFRII) than did those of control subjects. Our results suggest that, following L. braziliensis infection, the production of multiple inflammatory mediators by the host may contribute to disease severity by increasing cellular recruitment.
Leishmaniasis is a tropical disease caused by infection with protozoan parasites from the genus Leishmania. Worldwide, the disease affects 12 million people, with 350 million people currently considered at risk of infection (28). Leishmania parasites are transmitted by infected female sand flies, which inoculate the metacyclic promastigotes into the skin during a blood meal (17). Parasites are able to survive initial capture by neutrophils and later replicate or persist within macrophages (MΦs), monocytes, dendritic cells, and fibroblasts as intracellular amastigotes (7, 12, 25). Leishmania braziliensis is a New World Leishmania species that is prevalent in many geographical areas of Central and South America (21, 28). L. braziliensis-infected individuals initially develop cutaneous leishmaniasis (CL); however, in 5 to 10% of the patients, the disease further develops into mucocutaneous leishmaniasis (ML) (3). ML is a severe and disfiguring form of the disease, characterized by uncontrolled T- and B-cell responses to the parasite, and is caused almost exclusively by L. braziliensis infection (28). Currently, the host- and pathogen-associated factors that lead into ML progression are largely uncharacterized.
Pathogen interactions with components of the host immune response are crucial for the establishment of protective immunity and pathological responses. Given that monocytes and MΦs serve as the main reservoir for Leishmania parasites during active infection (1), it is of interest to study the early events associated with parasite uptake. Uptake of Leishmania parasites can induce monocytes and MΦs to produce different chemokines, which in turn can skew immune responses by recruiting and inducing different components of the immune system (29). Chemokines are small (8- to 10-kDa) chemoattractant cytokines that play important roles during immune responses by triggering integrin activation and inducing the recruitment of antigen-specific lymphocytes to peripheral tissues in response to inflammation (36). At present, approximately 50 human chemokines and 20 chemokine receptors have been characterized (44). Chemokine (C-X-C motif) ligand 10 (CXCL10) (also known as the 10-kDa gamma interferon [IFN-γ]-inducible protein [IP-10]) is a small molecule that is secreted mainly by monocytes, fibroblasts, and endothelial cells in response to stimuli, such as viral infection, lipopolysaccharide (LPS), interleukin-1β (IL-1β), and IFN-γ (11, 19). The biological function of CXCL10 involves the recruitment of monocytes, MΦs, and T cells to sites of inflammation (11). Furthermore, it is known that during active leishmaniasis, lesion cells from self-healing CL patients produce Th1-mobilizing chemokines, such as CXCL10 and CXCL9 (29). Recruitment of IFN-γ-producing Th1 cells favors MΦ activation and parasite clearance; however, excessive production of these molecules could potentially lead to uncontrolled inflammation and tissue destruction (2, 29).
In the present study, we used an in vitro infection system to analyze the early events associated with L. braziliensis infection of human peripheral blood mononuclear cells (PBMCs). We found that L. braziliensis infection selectively induced the transcription of CXCL10 and chemokine (C-C motif) ligand 3 (CCL3). Furthermore, we showed that within PBMCs, Leishmania parasites preferentially infected monocytes and upregulated CXCL10 production and chemokine CXC receptor (CXCR3) expression on the surfaces of monocytes. Interestingly, only L. braziliensis infection induced the production of CXCL10, as PBMCs infected with Leishmania amazonensis (another New World Leishmania species and the causative agent of diffuse cutaneous leishmaniasis) failed to do so. To further validate and extend our results, we measured the presence of these inflammatory mediators in serum samples from American tegumentary leishmaniasis (ATL) patients from a region where L. braziliensis is endemic and found that the levels of CXCL10, CCL4, and soluble tumor necrosis factor (TNF) receptor II (sTNFRII) were significantly increased in ML patients. Collectively, our results indicate that immune responses against New World species of Leishmania are differentially regulated and that the excessive production of inflammatory mediators could potentially exacerbate disease severity in L. braziliensis-infected individuals.
MATERIALS AND METHODS
Sample collection and preparation.
Blood samples were collected by venipuncture of healthy volunteers into sodium heparin-coated collection tubes (BD Biosciences), and PBMCs were obtained by centrifugation on Accuprep gradients (Accurate Chemical). Cells were washed thoroughly and resuspended at 1 × 106 cells/ml in RPMI 1640 medium (Invitrogen), supplemented with 10% fetal bovine serum (FBS) (HyClone), 1% nonessential amino acids, 1 mM sodium pyruvate, 50 μg/ml kanamycin, and 2 mM l-glutamine (all from Sigma). Human monocytes were obtained by positive selection by using CD14 microbeads (Miltenyi Biotec). To differentiate MΦs, monocytes were cultured for 5 days in complete RPMI 1640 medium supplemented with 20 ng/ml recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech). Serum samples from 27 ATL patients (13 CL patients and 14 ML patients) were collected after clinical evaluation and upon admittance into the Leishmaniasis Working Group at the Tropical Medicine Institute “Alexander von Humboldt” in Universidad Peruana Cayetano Heredia in Lima, Peru. Parasitological diagnosis (either by smear microscopy, PCR, or parasite culture) was confirmed in 70% of CL cases and 65% of ML cases, respectively (see Table S1 in the supplemental material for patient demographic information). Inflammatory mediators in serum were detected by using a custom Quantibody array (RayBiotech). Serum samples obtained from noninfected individuals (n = 13) were used as controls for the Quantibody array. All cell and serum samples used in this study were collected after informed consent and approved by the Institutional Review Board at University of Texas Medical Branch (UTMB) and the Ethics Committee at Universidad Peruana Cayetano Heredia.
Parasite culture.
Infectivity of L. braziliensis (MHOM/BR/79/LTB111) and L. amazonensis (MHOM/BR/77/LTB0016) was maintained by regular passage through golden Syrian hamsters and BALB/c mice (Harlan Sprague Dawley), respectively. Promastigotes were cultured at 23°C in Schneider's Drosophila medium (Invitrogen), pH 7.0, supplemented with 20% FBS, 2 mM l-glutamine, and 50 μg/ml gentamicin (Sigma). Stationary promastigote cultures of fewer than five passages were used for cell infection. Between these two species of parasites, there were no major differences in promastigote growth rates in culture medium or in parasite infectivity in murine and human macrophages at 24 to 48 h of infection in vitro (see Fig. S1A to C in the supplemental material). Nevertheless, L. amazonensis-infected cells contained more parasites per cell at 24 h (see Fig. S1D in the supplemental material) or showed higher infection rates at 72 h of infection (see Fig. S1B in the supplemental material), suggesting that L. amazonensis parasites generally grew better in macrophages than did L. braziliensis parasites.
Cell infection and Transwell assays.
PBMCs and MΦs were infected at a 5:1 and 10:1 parasite-to-cell ratio, respectively. Infected cells were incubated at 33°C for 4 h and then at 37°C for another 20 h. LPS (100 ng/ml) of Salmonella enterica serovar Typhimurium (Sigma) and recombinant human IFN-γ (100 ng/ml; Leinco Technologies) were used as positive controls. At 24 h postinfection (p.i.), culture supernatants were harvested for cytokine detection, and cells were collected and stained for fluorescence-activated cell sorting (FACS) analysis. For cell migration experiments, 5-μm Transwell systems were used (24-well; Costar). Briefly, 1 × 106 infected PBMCs, monocytes (5:1 parasite-to-cell ratio) were seeded in the basal chamber, and 1.5 × 106 noninfected, donor-matched, carboxyfluoroscein succinimidyl ester (CFSE)-labeled (2.5 μM; Sigma) PBMCs were seeded into the apical chambers. After incubation for 4 h at 33°C and then at 37°C for 10 h, cells in the basal chamber were collected, counted, and stained for FACS analysis. Recombinant human CXCL10 (20 ng/ml; eBiosciences) was used as a positive control for cell migration.
Cytokine ELISA.
The cytokine levels in culture supernatants were measured by using enzyme-linked immunosorbent assay (ELISA) kits purchased from eBiosciences (IFN-γ and IL-10) or RayBiotech (CXCL10). Detection limits were 4 pg/ml for IFN-γ, 2 pg/ml for IL-10, and 8 pg/ml for CXCL10.
Reverse transcription-PCR (RT-PCR).
At 4 h p.i., total RNA was extracted from 2 × 106 infected PBMCs (5:1 parasite-to-cell ratio) by using the RNeasy system (Qiagen), and genomic DNA was digested with the on-column RNase-free DNase (Qiagen). cDNA was synthesized from 2 μg of total RNA by using the Superscript III first-strand system (Invitrogen) primed with random hexamers. All PCRs were performed by using 2 μl of cDNA, the Platinum Taq DNA polymerase system and specific primer pairs (see Table S2 in the supplemental material). For CXCL10, CCL4, and β-actin, PCRs included 30 cycles, with 1 cycle consisting of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min, with a final 7-min extension step at 72°C. For CCL2 and CCL3, the same reaction conditions were used, but with 58°C as the annealing temperature. PCR products (8 μl) were subjected to electrophoresis on 1% agarose gels and visualized by staining with ethidium bromide. Results were quantified using the AlphaEase FC densitometry analysis software (v4.0; Alpha Innotech) and normalized to the expression of the β-actin gene and the nonstimulated controls.
Flow cytometry.
Unless specified, all human-specific monoclonal antibodies(MAbs) and staining reagents were purchased from BD Biosciences: Alexa Fluor 488-conjugated anti-CXCR3 (1C6/CXCR3) and mouse IgG1(κ) and phycoerythrin (PE)-conjugated anti-CXCL10 (6D4/D6/G2) and mouse IgG2a. The following reagents were purchased from eBioscience: allophycocyanin (APC)-Cy7-conjugated anti-CD3 (SK7) and mouse IgG1(κ), PE-Cy5.5-conjugated anti-CD56 (MEM188) and mouse IgG2a, APC-conjugated anti-IFN-γ (4S.B3) and mouse IgG1(κ), and PE-Cy7-conjugated anti-CD14 (61D3) and mouse IgG1(κ). Briefly, cells were washed, stained for specific surface molecules, fixed/permeabilized with a Cytofix/Cytoperm kit, and then stained for specific intracellular molecules. To detect intracellular cytokines, we added 1 μl/ml of GolgiStop for the last 6 h of culture. Cells were read on a FACSCanto flow cytometer (BD Biosciences) and analyzed by using FlowJo v8.5 software (TreeStar).
Statistical analysis.
Unless otherwise specified, all data shown in figures were pooled from the number of healthy volunteers indicated in the figure legend. Results are shown as means ± standard errors of the means. Differences between individual treatment groups were all determined by using one-way analysis of variance (ANOVA). A P value of ≤0.05 was considered statistically significant (Prism v4.0; GraphPad software).
RESULTS
Early activation of chemokine genes following L. braziliensis infection.
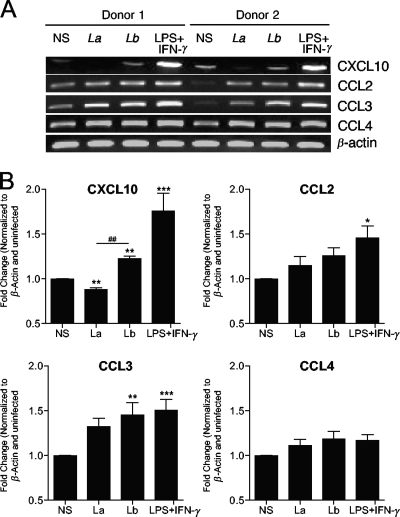
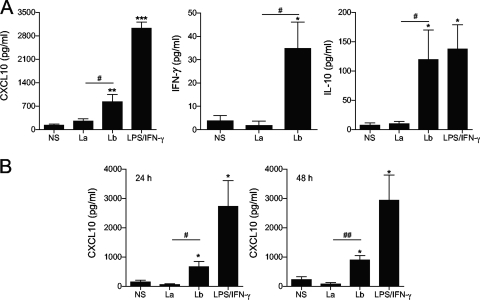
We have previously reported that L. braziliensis infection in mice leads to the development of a robust Th1 immune response, which includes the expansion of IFN-γ- and IL-17-producing CD4+ T cells (39). Furthermore, L. braziliensis, but not L. amazonensis, infection in mice induced the expression of several chemokines and chemokine receptors, such as CXCL10, CXCR3, CCL4, CCR1, CCR2, CCR3, and CCR5 at 3 weeks of infection (see Fig. S2 in the supplemental material). Given that L. braziliensis and L. amazonensis infections in mice represent self-healing and nonhealing CL, respectively, these findings support the view that early events involved in parasite recognition have a significant impact on disease outcome (38, 39). Human infections caused by L. braziliensis are rarely self-healing, and disease in 5 to 10% of patients can further complicate into ML (28). Although many reports have described the clinical characteristics of ML (28, 42), very few have focused on the initial interaction between human cells and L. braziliensis parasites. We addressed this issue by analyzing chemokine gene expression following infection of PBMCs from healthy volunteers with L. braziliensis parasites for 4 h and found increased transcription of CXCL10 and CCL3 in response to L. braziliensis infection (Fig. 1). We also included control cells infected with L. amazonensis, another New World species, and confirmed the specific responses triggered by L. braziliensis infection. Since CXCL10 is an important chemokine for cell recruitment and Th1 induction (19), and its transcription was observed only in L. braziliensis-infected PBMCs (Fig. 1B), we decided to investigate the potential role for CXCL10 during L. braziliensis infection. As shown in Fig. 2A, PBMCs infected with L. braziliensis parasites secreted higher levels of CXCL10, IFN-γ, and IL-10 than PBMCs infected with L. amazonensis (P < 0.05). Similarly, human MΦs also produced higher levels of CXCL10 at 24 and 48 h of infection with L. braziliensis, but not L. amazonensis, parasites (Fig. 2B).
FIG. 1.
Activation of chemokine gene transcription by human PBMCs in response to L. braziliensis infection. Human PBMCs were isolated from healthy donors (n = 8) and infected with L. braziliensis (Lb and Lb) or L. amazonensis (La and La) promastigotes at a 5:1 parasite-to-cell ratio. At 4 h p.i, total RNA was extracted for RT-PCR analysis. Human PBMCs were isolated from nonstimulated controls (NS). LPS plus IFN-γ were used as a positive control. (A) Gel images of CXCL10, CCL2, CCL3, and CCL4 transcripts from two donors. (B) Densitometry data obtained from all donors were pooled and shown in the plots. represents Values that are statistically significantly different (P < 0.001) between the compared groups are indicated (##). Values that are statistically significantly different between the nonstimulated control and infected groups are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG. 2.
L. braziliensis infection induces CXCL10 production in human PBMCs and macrophages. (A) PBMCs were isolated from healthy donors (n = 10) and infected with L. braziliensis (Lb) or L. amazonensis (La) promastigotes at a 5:1 parasite-to-cell ratio for 24 h. (B) CD14+ monocytes were isolated from healthy donor PBMCs (n = 3) and differentiated into MΦs by cultivation in conditioned medium for 5 days. MΦs were infected with promastigotes at a 10:1 parasite-to-cell ratio for 24 and 48 h. Cells stimulated with LPS/IFN-γ (100 ng/ml each) were used as positive controls. The level of the indicated molecule in culture supernatants was assayed by ELISA. Values that are statistically significantly different between the compared groups are indicated as follows: #, P < 0.05; ##, P < 0.01. Values that are statistically significantly different between the nonstimulated control (NS) and infected groups are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
CXCL10 production and CXCR3 expression are induced by L. braziliensis infection.
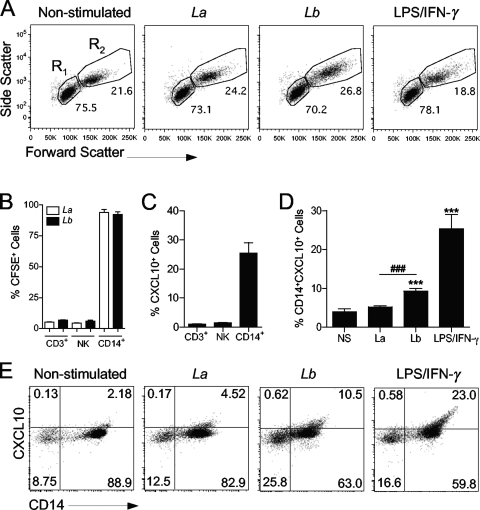
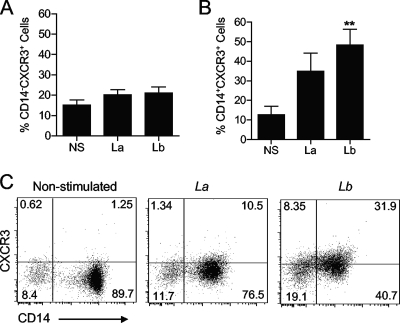
To determine the cell type that is susceptible to L. braziliensis infection and the origin of CXCL10 production, we used CFSE-labeled parasites in the following experiments. We observed that Leishmania infection was confined to CD14+ cells within the monocyte gate because >95% of cells in the R2 gate of infected groups were CD14+ CFSE+ (Fig. 3B). Using LPS plus IFN-γ as a control, we observed that only CD14+ cells (within the monocyte gate) produced CXCL10 (Fig. 3C). Consistent with ELISA results (Fig. 2A), our FACS studies confirmed that marked CXCL10 production was observed only in response to L. braziliensis infection and by CD14+ cells (Fig. 3D and E). Since cell responsiveness to CXCL10 chemotactic gradients depends on CXCR3 expression on the cell surface (19), we investigated whether parasite infection had any effect on surface expression of this receptor. We consistently observed that L. braziliensis infection upregulated CXCR3 expression only on the surfaces of CD14+ cells, not on CD14− cells (Fig. 4A and B).
FIG. 3.
Monocytes are the major producers of CXCL10 following L. braziliensis infection. Human PBMCs were isolated from healthy donors (n = 6) and infected with CFSE-labeled L. braziliensis (Lb) or L. amazonensis (La) promastigotes at a 5:1 parasite-to-cell ratio for 24 h. (A) Representative forward scatter versus side scatter plots of PBMCs cultured under different conditions are shown. Gates used to analyze lymphocytes (R1) and monocytes (R2) are indicated. 50K, 50,000. (B) CFSE intensity was used to determine the infection rates among T lymphocytes (CD3+), NK cells (CD3− CD56+), and monocytes (CD14+ cells within the monocyte gate). (C) CXCL10 production in different cell types in response to LPS/IFN-γ (100 ng/ml each) stimulation was assayed by FACS. (D and E) Intracellular CXCL10 in infected monocytes (CD14+ CFSE+) was measured by FACS. Values that are statistically significantly different (P < 0.001) between the compared groups are indicated (###). Values that are statistically significantly different (P < 0.001) between the nonstimulated control and infected groups are indicated (***). Numbers shown in panel E represent the percentage of cells in each quadrant.
FIG. 4.
Monocyte-specific upregulation of CXCR3 after Leishmania infection. PBMCs were isolated from healthy donors (n = 6) and infected with L. braziliensis (Lb) or L. amazonensis (La) promastigotes at a 5:1 parasite-to-cell ratio for 24 h. The levels of CXCR3 expression on CD14− (A) and CD14+ cells (B and C) were measured by FACS. Values that are statistically significantly different (P < 0.01) between the nonstimulated control (NS) and infected groups are indicated (**). Numbers shown in panel C represent the percentage of cells in each quadrant.
L. braziliensis-infected cells efficiently recruit monocytes through chemotactic gradients.
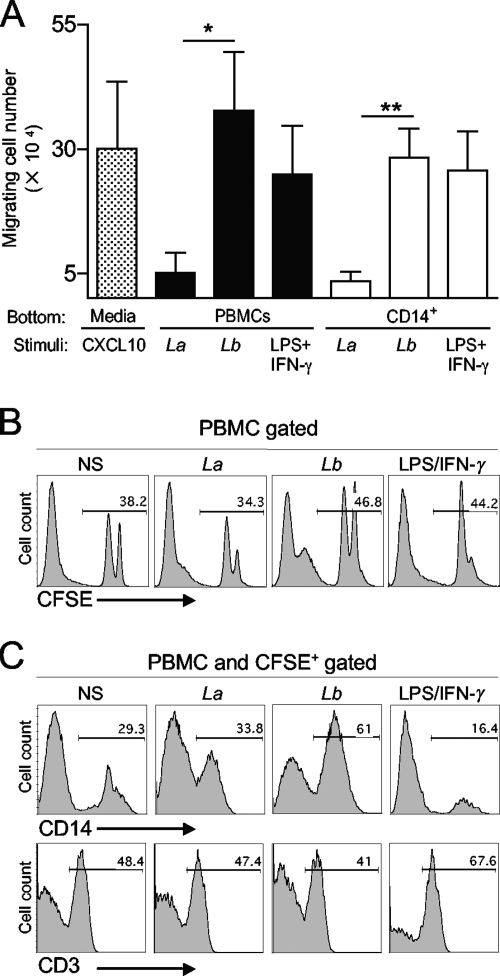
To investigate whether the increased production of chemokines following L. braziliensis infection had an impact on cell recruitment, we assessed the chemotactic properties of L. braziliensis-infected cells by using a 5-μm Transwell system. Our results showed that both L. braziliensis-infected PBMCs and monocytes induced higher numbers of cell migration than did L. amazonensis-infected or noninfected cells (Fig. 5A). The use of CFSE-labeled PBMCs in the apical chamber allowed us to quantify and type the responding cells. As shown in Fig. 5B, chemoattractant factors released by L. braziliensis-infected cells induced migration of higher numbers (∼46.8%) of CFSE-labeled cells compared to L. amazonensis-infected (∼34.3%) or noninfected (∼38.2%) cells. Further analysis of the migrated CFSE+ cells revealed that L. braziliensis-infected cells preferentially induced the migration of CD14+ monocytes (Fig. 5C, 61% compared to 29.3% in the control), whereas soluble factors released after LPS/IFN-γ treatment preferentially induced the migration of CD3+ T cells. These data suggest a selective recruitment of monocytes by L. braziliensis-infected cells.
FIG. 5.
L. braziliensis-infected PBMCs and monocytes efficiently induce migration of uninfected PBMCs. PBMCs and CD14+ cells were isolated from healthy donors (n = 4) and infected with L. braziliensis (Lb) or L. amazonensis (La) promastigotes at a 5:1 parasite-to-cell ratio and placed in the basal chambers of Transwell plates. Noninfected, donor-matched PBMCs were labeled with CFSE, placed in the apical chamber, and allowed to migrate in response to chemotactic factors released from the basal chamber for 14 h. Cells stimulated with LPS/IFN-γ (100 ng/ml each) were used as positive controls. (A) The total number of migrating cells present in the basal chamber was counted after the incubation period. Data were normalized by subtracting the migration observed in nonstimulated controls. Values that are statistically significantly different by one-way ANOVA are indicated as follows: *, P < 0.05; **, P < 0.01. (B) The percentage of migrating CFSE+ PBMCs in the basal chamber was measured by FACS. (C) Percentages of monocytes (CD14+) and T lymphocytes (CD3+) within CFSE+ migrating cells. (B and C) Histograms from one of four representative experiments are shown. NS, nonstimulated controls.
Increased levels of inflammatory mediators in serum samples from ATL patients.
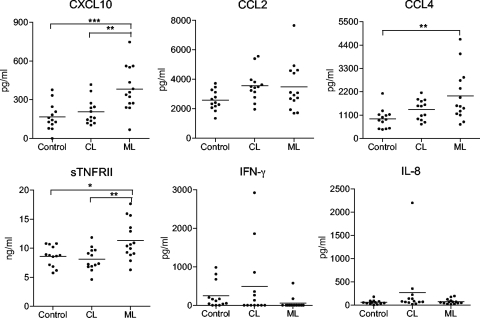
To validate and extend our in vitro observations, we analyzed the presence of inflammatory mediators in serum samples from ATL patients. Using the RayBiotech human inflammation antibody array in an initial screening of inflammatory factors, we found high levels of CXCL10, CCL2, CCL4, CCL15, sTNFRI, sTNFRII, and IL-18 in serum samples from CL and ML patients (see Fig. S3 in the supplemental material). We then used a custom Quantibody array and quantified the levels of 10 inflammatory factors in all of our samples. In accordance with our preliminary results (see Fig. S3 in the supplemental material), we detected significantly higher levels of CXCL10, CCL4, and sTNFRII in serum samples from ML patients compared to CL patients and control samples (Fig. 6). Our results suggest that responses to New World species of Leishmania are differentially regulated and that the excessive production of inflammatory mediators during active leishmaniasis may have an adverse impact on disease outcome.
FIG. 6.
Profiles of inflammatory chemokines and cytokines in serum samples from American tegumentary leishmaniasis patients. Serum samples were collected from a total of 13 CL patients and 14 patients with active ML. Samples from 13 healthy volunteers were used as controls. The levels of the indicated molecules were measured with a RayBiotech custom Quantibody array. Each circle shows the value for an individual patient or healthy volunteer, and the short horizontal bar shows the mean value for that group. Values that are statistically significantly different between the compared groups by one-way ANOVA are indicated by bars and asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Infection of PBMCs from healthy donors with Leishmania parasites can lead to induction or suppression of T-cell proliferation, monocyte/dendritic cell (DC) maturation, and cytokine production, depending on the parasite species tested (10, 12, 15, 30). Most of these studies, however, focus on changes that occurred at or after 48 h of infection in vitro. During host-pathogen interactions, the initial production of chemokines and cytokines can influence both innate and adaptive immune responses (29). In this study, we investigated the early events following infection with two New World species of Leishmania parasites, paying special attention to the inflammatory mediators involved in cell recruitment. Our studies clearly indicate a rapid and robust induction of several chemokines/cytokines during L. braziliensis, but not L. amazonensis, infection in human cells in vitro, as well as in C57BL/6 mice (see Fig. S2 in the supplemental material) (39) Although the in vivo function of CXCL10/CXCR3 in human CL remains unclear at this stage, our findings of elevated serum levels of CXCL10 and other proinflammatory factors in patients with active skin and mucosal lesions suggest a possible involvement of these molecules in disease outcome.
Infection of human PBMCs with Leishmania parasites can trigger the expression of different sets of inflammatory chemokines. For example, Leishmania major-treated PBMCs upregulated the transcription of CCL2 (22), whereas Leishmania donovani infection triggers the production of tumor necrosis factor alpha (TNF-α) and IL-18 (16). In our hands, L. braziliensis infection preferentially induced strong levels of expression of CXCL10 and CCL3, as judged by RT-PCR and ELISA (Fig. 1 and 2). A regulated production of these chemokines is required for pathogen clearance and wound healing due to their chemotactic potential in recruiting immune cells, such as T cells, monocytes, DCs, neutrophils and NK cells (9, 27, 33, 34). Of note, the CXCL10 transcription levels in L. amazonensis-infected cells were even lower than those observed in nonstimulated controls (P < 0.05) (Fig. 1). This finding was consistent with previous reports in human monocytes (12) and studies of murine DCs and MΦs (32, 43), indicating an impaired host innate immune response to L. amazonensis infection (24, 35).
The role of CXCL10 in human leishmaniasis is not clear, although CXCL10 is well-known for its involvement in recruiting monocytes, MΦs, T cells, and NK cells (29) during human viral and bacterial infections (18, 26) and organ transplantation (8). By using a murine model of L. amazonensis infection, our group has previously shown that exogenous CXCL10 promotes parasite killing in MΦ cultures in vitro (40) and enhances the antigen-presenting function in infected DCs (41) and that local injection of CXCL10 significantly delays the onset of cutaneous lesions (40). By using an in vitro infection system, we observed a rapid induction of CXCL10 (4 h for RNA and 24 h for protein) in response to L. braziliensis infection, even though the levels of IFN-γ were relatively low and somewhat variable (34.83 ± 25.3 pg/ml [Fig. 2]). Several studies have reported an alternative or IFN-γ-independent mechanism for the induction of CXCL10 production, since CXCL10 can be induced in human monocytes after viral infection by autocrine and/or paracrine action of type I IFNs (18).
The biological effect of CXCL10 is also regulated by the inducible expression of CXCR3 on the surfaces of target cells (monocytes and T cells) (27). The necessity of CXCR3 for host defense against L. major infection in mice is supported by findings that CXCR3−/− mice fail to control L. major infection, even in the presence of an efficient Th1 immune response (6, 31). In a self-healing infection model with L. major, it has been reported that approximately 20% of lesion-derived cells expressed CXCR3 and that the majority of these CXCR3-expressing cells were neither CD4+ nor CD8+ T cells (31). With regard to human leishmaniasis, expression of CXCR3 on lesion-derived cells has yet to be determined. Our in vitro infection data provide solid evidence that CD14+ monocytes are the major target cells for Leishmania infection, the predominant producers of CXCL10 (Fig. 3), and the principal cell type expressing CXCR3 (Fig. 4). Given that over 95% of monocytes contained intracellular (CFSE+) parasites, but only a portion of cells expressed CXCR3 (on average 33% in L. amazonensis infection and 47% in L. braziliensis infection, respectively [Fig. 4B]), studies are ongoing in the lab to examine the expression of CXCL10/CXCR3 in Leishmania-infected tissues.
A functional readout for CXCL10/CXCR3 in host responses is cell migration in the Transwell system. Different from previously reported studies that have examined cell migration within short periods of time (23), our assay analyzed migrated cells after 14 h of host-parasite interaction in order to provide L. braziliensis-infected cells the necessary time to produce chemoattractant proteins. Our findings that L. braziliensis-infected cells can induce the migration of donor-matched naïve cells through Transwell membranes suggest a selective cellular recruitment during active infection. The importance of our findings is that, within the cells that migrated in response to chemotactic gradients, L. braziliensis-induced attractant factors favored the recruitment of CD14+ monocytes (Fig. 5B). Given that blood monocytes are the precursors of tissue MΦs (5) and that MΦs are the ultimate host cell for Leishmania survival and replication (13), this selective recruitment of monocytes may lead to the increased inflammatory response associated with ML.
The implication of CXCL10-mediated cell recruitment in leishmaniasis warrants further investigation. In an attempt to encourage this direction of investigation, we examined the serum levels of this and other proinflammatory mediators in 27 ATL patients. ATL is prevalent in most countries of Latin America and can be caused by up to 11 different species of Leishmania (28). In Peru, although three species of the Viannia subgenus [Leishmania (Viannia) braziliensis, Leishmania (Viannia) peruviana, and Leishmania (Viannia) guyanensis] account for 93% of all the CL cases, L. braziliensis is the principal etiological agent for ML (3, 20). Our quantification of inflammatory factors in serum samples from ATL patients revealed increased amounts of CXCL10, CCL4, and sTNFRII, especially in serum samples from ML patients. These results, together with those obtained from in vitro infection in PBMCs (Fig. 1 and 2) and in vivo studies of mice (see Fig. S2 in the supplemental material), led us to propose that the elevated production of CXCL10, CCL4, and sTNFRII may contribute to extensive cell recruitment/activation and tissue damage. In support of our view, Hailu et al. reported that patients with active visceral leishmaniasis had significantly higher serum levels of CXCL10, IL-15, IL-8, IFN-γ, and IL-12p40 than in asymptomatic Leishmania-infected subjects, malaria patients, or healthy controls (14). Similarly, a recent study of pulmonary tuberculosis revealed a positive correlation between increased serum levels of chemokines (CXCL9, CXCL10, CCL5, and IL-8) and the active status of the disease, as well as a positive correlation between the high levels of CCR1, CCR2, and CXCR2 on the surfaces of T and NK cells and disease severity (26). Overproduction of CXCL10 has also been associated with other pathological conditions, such as chronic obstructive pulmonary disease (37), human rhinovirus-induced respiratory infections (18), and HIV-associated dementia (4). In the future, it will be important to further evaluate intralesional expression of CXCL10 and CXCR3 in L. braziliensis-infected ML patients. Moreover, further studies for the correlation of time of disease versus the concentration of inflammatory mediators in serum samples from L. braziliensis-infected CL and ML patients will provide a definitive link between the levels of expression of these molecules and the severity of disease. Given that exogenous CXCL10 can promote killing of L. amazonensis parasites in murine macrophages and in mice (40) and that L. braziliensis infection is self-limited in vitro (see Fig. S1 in the supplemental material) and in mice (39), it is possible to hypothesize that the CXCL10 induced by L. braziliensis infection may activate an autocrine leishmanicidal pathway. Therefore, it would be of interest to examine the effects of CXCL10 neutralization on parasite survival in vitro and in animal infection and to test whether L. braziliensis-induced CXCL10 production contributes to protective or pathogenic immune responses in leishmaniasis patients.
In summary, this study highlights the potential importance of CXCL10/CXCR3 expression during Leishmania infection. For a better understanding of the roles of CXCL10 and CXCR3 during active leishmaniasis, further studies are under way to analyze the expression profiles of these molecules in blood samples and lesion-derived cells obtained from ATL patients. Collectively, our data suggest that L. braziliensis infection induces the production of several proinflammatory mediators in human monocytes and that these factors can efficiently recruit potential target cells for propagating the infection. Moreover, the observation of a similar increase of inflammatory mediators in serum samples from patients with active ML suggests a potential role of these molecules in promoting cell recruitment, tissue damage, and disease severity.
Supplementary Material
Acknowledgments
This study was supported in part by an NIH grant AI043003 (to L.S.), institutional funds (to L.S. and J.J.E.), and the James W. McLaughlin Fellowship Fund (D.A.V.-I. and A.E.H.).
We thank Gary Klimpel for helpful discussions and critical review of the manuscript, Braulio M. Valencia for assisting with collection of clinical information from ATL patients, and Mardelle Susman for assisting in manuscript preparation.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 9 November 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alexander, J., A. R. Satoskar, and D. G. Russell. 1999. Leishmania species: models of intracellular parasitism. J. Cell Sci. 112:2993-3002. [DOI] [PubMed] [Google Scholar]
- 2.Amato, V. S., H. F. de Andrade, and M. I. Duarte. 2003. Mucosal leishmaniasis: in situ characterization of the host inflammatory response, before and after treatment. Acta Trop. 85:39-49. [DOI] [PubMed] [Google Scholar]
- 3.Arevalo, J., L. Ramirez, V. Adaui, M. Zimic, G. Tulliano, C. Miranda-Verastegui, M. Lazo, R. Loayza-Muro, S. De Doncker, A. Maurer, F. Chappuis, J. C. Dujardin, and A. Llanos-Cuentas. 2007. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J. Infect. Dis. 195:1846-1851. [DOI] [PubMed] [Google Scholar]
- 4.Asensio, V. C., J. Maier, R. Milner, K. Boztug, C. Kincaid, M. Moulard, C. Phillipson, K. Lindsley, T. Krucker, H. S. Fox, and I. L. Campbell. 2001. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J. Virol. 75:7067-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auffray, C., M. H. Sieweke, and F. Geissmann. 2009. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27:669-692. [DOI] [PubMed] [Google Scholar]
- 6.Barbi, J., F. Brombacher, and A. R. Satoskar. 2008. T cells from Leishmania major-susceptible BALB/c mice have a defect in efficiently up-regulating CXCR3 upon activation. J. Immunol. 181:4613-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdan, C., N. Donhauser, R. Doring, M. Rollinghoff, A. Diefenbach, and M. G. Rittig. 2000. Fibroblasts as host cells in latent leishmaniasis. J. Exp. Med. 191:2121-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crescioli, C., A. Buonamano, S. Scolletta, M. Sottili, M. Francalanci, P. Giomarelli, B. Biagioli, G. Lisi, F. Pradella, M. Serio, P. Romagnani, and M. Maccherini. 2009. Predictive role of pretransplant serum CXCL10 for cardiac acute rejection. Transplantation 87:249-255. [DOI] [PubMed] [Google Scholar]
- 9.Del Prete, A., M. Locati, K. Otero, E. Riboldi, A. Mantovani, A. Vecchi, and S. Sozzani. 2006. Migration of dendritic cells across blood and lymphatic endothelial barriers. Thromb. Haemost. 95:22-28. [PubMed] [Google Scholar]
- 10.Donovan, M. J., A. Jayakumar, and M. A. McDowell. 2007. Inhibition of groups 1 and 2 CD1 molecules on human dendritic cells by Leishmania species. Parasite Immunol. 29:515-524. [DOI] [PubMed] [Google Scholar]
- 11.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 12.Favali, C., N. Tavares, J. Clarencio, A. Barral, M. Barral-Netto, and C. Brodskyn. 2007. Leishmania amazonensis infection impairs differentiation and function of human dendritic cells. J. Leukoc. Biol. 82:1401-1406. [DOI] [PubMed] [Google Scholar]
- 13.Grazia Cappiello, M., F. S. Sutterwala, G. Trinchieri, D. M. Mosser, and X. Ma. 2001. Suppression of IL-12 transcription in macrophages following Fcγ receptor ligation. J. Immunol. 166:4498-4506. [DOI] [PubMed] [Google Scholar]
- 14.Hailu, A., T. van der Poll, N. Berhe, and P. A. Kager. 2004. Elevated plasma levels of interferon (IFN)-γ, IFN-γ inducing cytokines, and IFN-γ inducible CXC chemokines in visceral leishmaniasis. Am. J. Trop. Med. Hyg. 71:561-567. [PubMed] [Google Scholar]
- 15.Hviid, L., A. L. Sorensen, A. Kharazmi, and T. G. Theander. 1990. Functional and phenotypic changes in human lymphocytes after coincubation with Leishmania donovani in vitro. Infect. Immun. 58:3163-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iannello, D., A. Arena, C. Buemi, M. Calapai, G. Stassi, D. Gazzara, and P. Mastroeni. 2003. Differential induction of TNF-α and IL-18 in human peripheral blood mononuclear cells infected with Leishmania major or Leishmania donovani. New Microbiol. 26:399-404. [PubMed] [Google Scholar]
- 17.Kimblin, N., N. Peters, A. Debrabant, N. Secundino, J. Egen, P. Lawyer, M. P. Fay, S. Kamhawi, and D. Sacks. 2008. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc. Natl. Acad. Sci. U. S. A. 105:10125-10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korpi-Steiner, N. L., M. E. Bates, W. M. Lee, D. J. Hall, and P. J. Bertics. 2006. Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J. Leukoc. Biol. 80:1364-1374. [DOI] [PubMed] [Google Scholar]
- 19.Lee, E. Y., Z. H. Lee, and Y. W. Song. 2009. CXCL10 and autoimmune diseases. Autoimmun. Rev. 8:379-383. [DOI] [PubMed] [Google Scholar]
- 20.Llanos-Cuentas, A., G. Tulliano, R. Araujo-Castillo, C. Miranda-Verastegui, G. Santamaria-Castrellon, L. Ramirez, M. Lazo, S. De Doncker, M. Boelaert, J. Robays, J. C. Dujardin, J. Arevalo, and F. Chappuis. 2008. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin. Infect. Dis. 46:223-231. [DOI] [PubMed] [Google Scholar]
- 21.Lucas, C. M., E. D. Franke, M. I. Cachay, A. Tejada, M. E. Cruz, R. D. Kreutzer, D. C. Barker, S. H. McCann, and D. M. Watts. 1998. Geographic distribution and clinical description of leishmaniasis cases in Peru. Am. J. Trop. Med. Hyg. 59:312-317. [DOI] [PubMed] [Google Scholar]
- 22.Meddeb-Garnaoui, A., H. Zrelli, and K. Dellagi. 2009. Effects of tropism and virulence of Leishmania parasites on cytokine production by infected human monocytes. Clin. Exp. Immunol. 155:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreland, J. G., J. S. Hook, G. Bailey, T. Ulland, and W. M. Nauseef. 2009. Francisella tularensis directly interacts with the endothelium and recruits neutrophils with a blunted inflammatory phenotype. Am. J. Physiol. Lung Cell. Mol. Physiol. 296:L1076-L1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira, B. A., and C. R. Alves. 2008. Immunological characteristics of experimental murine infection with Leishmania (Leishmania) amazonensis. Vet. Parasitol. 158:239-255. [DOI] [PubMed] [Google Scholar]
- 25.Peters, N. C., J. G. Egen, N. Secundino, A. Debrabant, N. Kimblin, S. Kamhawi, P. Lawyer, M. P. Fay, R. N. Germain, and D. Sacks. 2008. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321:970-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pokkali, S., and S. D. Das. 2009. Augmented chemokine levels and chemokine receptor expression on immune cells during pulmonary tuberculosis. Hum. Immunol. 70:110-115. [DOI] [PubMed] [Google Scholar]
- 27.Qin, S., J. B. Rottman, P. Myers, N. Kassam, M. Weinblatt, M. Loetscher, A. E. Koch, B. Moser, and C. R. Mackay. 1998. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Invest. 101:746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reithinger, R., J.-C. Dujardin, H. Louzir, C. Pirmez, B. Alexander, and S. Brooker. 2007. Cutaneous leishmaniasis. Lancet Infect. Dis. 7:581-596. [DOI] [PubMed] [Google Scholar]
- 29.Ritter, U., and H. Korner. 2002. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol. 24:295-301. [DOI] [PubMed] [Google Scholar]
- 30.Rogers, K. A., and R. G. Titus. 2004. The human cytokine response to Leishmania major early after exposure to the parasite in vitro. J. Parasitol. 90:557-563. [DOI] [PubMed] [Google Scholar]
- 31.Rosas, L. E., J. Barbi, B. Lu, Y. Fujiwara, C. Gerard, V. M. Sanders, and A. R. Satoskar. 2005. CXCR3−/− mice mount an efficient Th1 response but fail to control Leishmania major infection. Eur. J. Immunol. 35:515-523. [DOI] [PubMed] [Google Scholar]
- 32.Ruhland, A., and P. E. Kima. 2009. Activation of PI3K/Akt signaling has a dominant negative effect on IL-12 production by macrophages infected with Leishmania amazonensis promastigotes. Exp. Parasitol. 122:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salazar-Mather, T. P., and K. L. Hokeness. 2003. Calling in the troops: regulation of inflammatory cell trafficking through innate cytokine/chemokine networks. Viral Immunol. 16:291-306. [DOI] [PubMed] [Google Scholar]
- 34.Serbina, N. V., T. Jia, T. M. Hohl, and E. G. Pamer. 2008. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 26:421-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soong, L. 2008. Modulation of dendritic cell function by Leishmania parasites. J. Immunol. 180:4355-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 37.Torvinen, M., H. Campwala, and I. Kilty. 2007. The role of IFN-γ in regulation of IFN-γ-inducible protein 10 (IP-10) expression in lung epithelial cell and peripheral blood mononuclear cell co-cultures. Respir. Res. 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vargas-Inchaustegui, D. A., W. Tai, L. Xin, A. E. Hogg, D. B. Corry, and L. Soong. 2009. Distinct roles for MyD88 and TLR2 during Leishmania braziliensis infection in mice. Infect. Immun. 77:2948-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vargas-Inchaustegui, D. A., L. Xin, and L. Soong. 2008. Leishmania braziliensis infection induces dendritic cell activation, ISG15 transcription, and the generation of protective immune responses. J. Immunol. 180:7537-7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasquez, R. E., and L. Soong. 2006. CXCL10/gamma interferon-inducible protein 10-mediated protection against Leishmania amazonensis infection in mice. Infect. Immun. 74:6769-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasquez, R. E., L. Xin, and L. Soong. 2008. Effects of CXCL10 on dendritic cell and CD4+ T-cell functions during Leishmania amazonensis infection. Infect. Immun. 76:161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Stebut, E. 2007. Cutaneous Leishmania infection: progress in pathogenesis research and experimental therapy. Exp. Dermatol. 16:340-346. [DOI] [PubMed] [Google Scholar]
- 43.Xin, L., K. Li, and L. Soong. 2008. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol. Immunol. 45:3371-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.